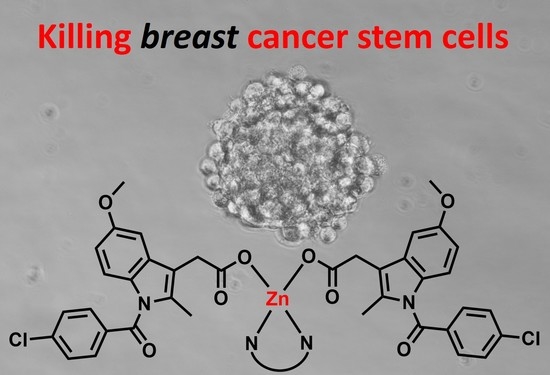
Polypyridyl Zinc(II)-Indomethacin Complexes with Potent Anti-Breast Cancer Stem Cell Activity
Abstract
1. Introduction
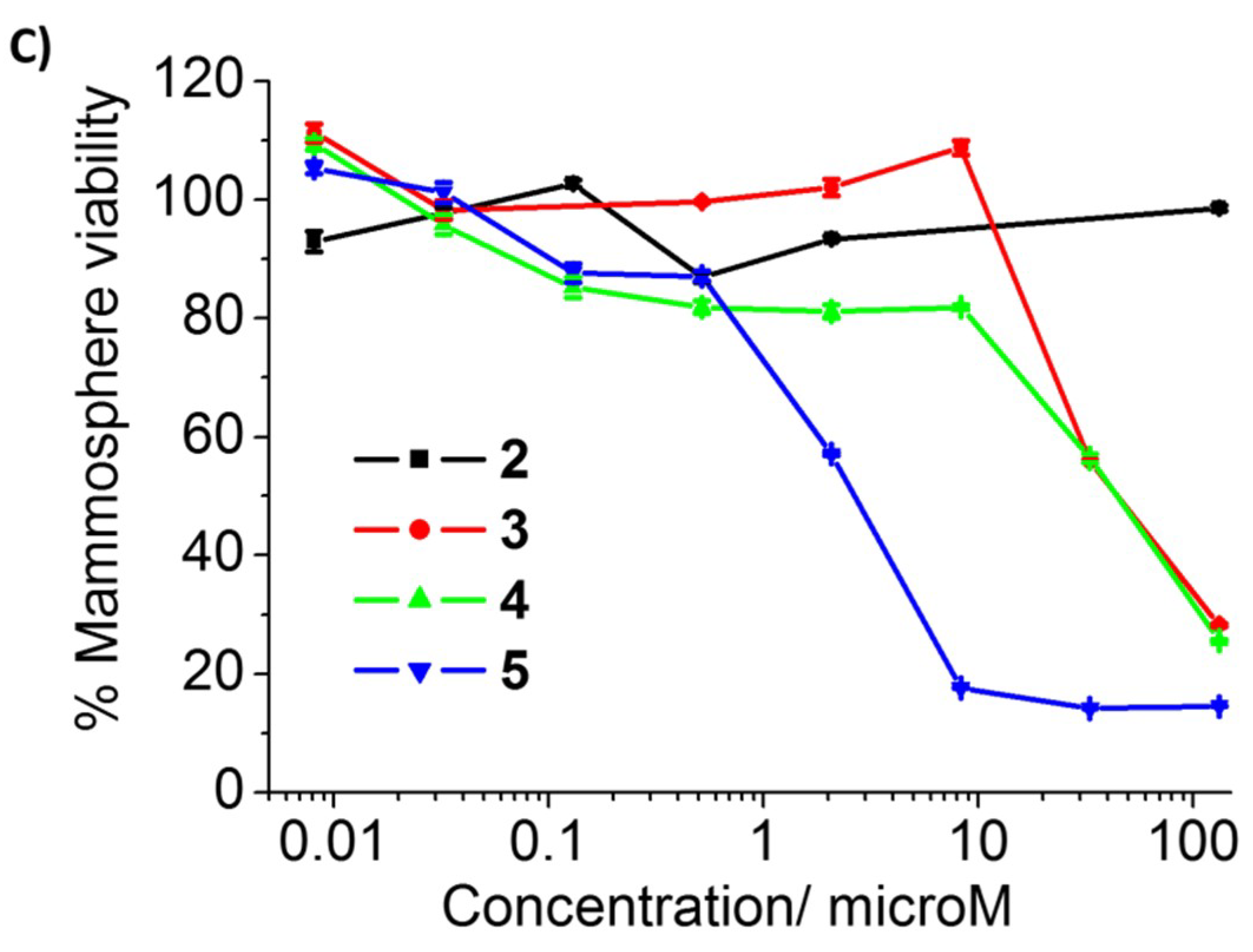
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
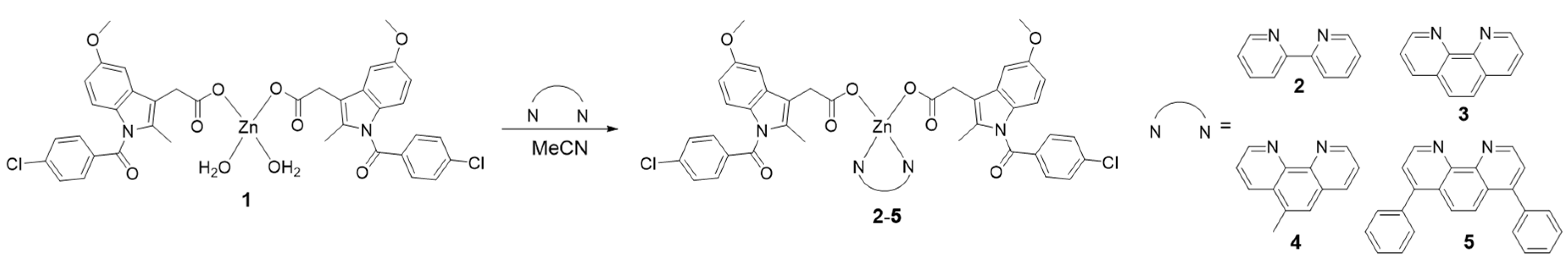
3.2. Synthesis of Zn(indomethacin)2(2,2′-bipyridine) (2)
3.3 Synthesis of Zn(indomethacin)2(1,10-phenanthroline) (3)
3.4. Synthesis of Zn(indomethacin)2(5-methyl-1,10-phenanthroline) (4)
3.5. Synthesis of Zn(indomethacin)2(4,7-diphenyl-1,10-phenanthroline) (5)
3.6. Determination of the LogP Values
3.7. Cell Lines and Cell Culture Conditions
3.8. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assay
3.9. Mammosphere Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, G.M. The Development and Causes of Cancer, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Marx, J. Cancer’s perpetual source? Science 2007, 317, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells-what challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ramena, G.; Elble, R.C. The role of cancer stem cells in relapse of solid tumors. Front. Biosci. 2012, 4, 1528–1541. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.N.; Chow, E.K. Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Iwatsuki, M.; Ieta, K.; Ohta, D.; Haraguchi, N.; Mimori, K.; Mori, M. Cancer stem cells and chemoradiation resistance. Cancer Sci. 2008, 99, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jeon, H.Y.; Kim, H. The molecular mechanisms underlying the therapeutic resistance of cancer stem cells. Arch. Pharm. Res. 2015, 38, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.N. Cancer stem cells in radiation resistance. Cancer Res. 2007, 67, 8980–8984. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. The cancer stem cell gamble. Science 2015, 347, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Laws, K.; Suntharalingam, K. The next generation of anticancer metallopharmaceuticals: Cancer stem cell-active inorganics. Chembiochem 2018. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Malina, J.; Kasparkova, J.; Brabec, V.; Natile, G. Chiral discrimination in platinum anticancer drugs. Environ. Health Perspect. 2002, 110 (Suppl. 5), 779–782. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Ducani, C.; Migoni, D.; Antonucci, D.; Vecchio, V.M.; Ciccarese, A.; Romano, A.; Verri, T.; Ciccarella, G.; Fanizzi, F.P. Experimental evidence that a DNA polymerase can incorporate N7-platinated guanines to give platinated DNA. Angew. Chem. Int. Ed. 2008, 47, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Lunetti, P.; Romano, A.; Carrisi, C.; Antonucci, D.; Verri, T.; De Benedetto, G.E.; Dolce, V.; Fanizzi, F.P.; Benedetti, M.; Capobianco, L. Platinated nucleotides are substrates for the human mitochondrial deoxynucleotide carrier (DNC) and DNA polymerase γ: Relevance for the development of new platinum-based drugs. ChemistrySelect 2016, 1, 4633–4637. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Lin, W.; Johnstone, T.C.; Bruno, P.M.; Zheng, Y.R.; Hemann, M.T.; Lippard, S.J. A breast cancer stem cell-selective, mammospheres-potent osmium(VI) nitrido complex. J. Am. Chem. Soc. 2014, 136, 14413–14416. [Google Scholar] [CrossRef] [PubMed]
- Tomao, F.; Papa, A.; Rossi, L.; Strudel, M.; Vici, P.; Lo Russo, G.; Tomao, S. Emerging role of cancer stem cells in the biology and treatment of ovarian cancer: Basic knowledge and therapeutic possibilities for an innovative approach. J. Exp. Clin. Cancer Res. 2013, 32, 48. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.P.; Gray, S.G.; Hoffmann, A.C.; Hilger, R.A.; Thomale, J.; O’Flaherty, J.D.; Fennell, D.A.; Richard, D.; O’Leary, J.J.; O’Byrne, K.J. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS ONE 2013, 8, e54193. [Google Scholar] [CrossRef] [PubMed]
- Rackham, O.; Nichols, S.J.; Leedman, P.J.; Berners-Price, S.J.; Filipovska, A. A gold(I) phosphine complex selectively induces apoptosis in breast cancer cells: Implications for anticancer therapeutics targeted to mitochondria. Biochem. Pharmacol. 2007, 74, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Trevisan, A.; Giovagnini, L.; Fregona, D. Synthesis of a new platinum(II) complex: Anticancer activity and nephrotoxicity in vitro. Toxicol. In Vitro 2002, 16, 413–419. [Google Scholar] [CrossRef]
- Gismondi, A.; Nanni, V.; Reina, G.; Orlanducci, S.; Terranova, M.L.; Canini, A. Nanodiamonds coupled with 5,7-dimethoxycoumarin, a plant bioactive metabolite, interfere with the mitotic process in B16F10 cells altering the actin organization. Int. J. Nanomed. 2016, 11, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Liu, L.; Guo, J.; Wu, D.; Xu, G.; Wang, X.; Jia, D. Anticancer activity, structure, and theoretical calculation of N-(1-phenyl-3-methyl-4-propyl-pyrazolone-5)-salicylidene hydrazone and its copper(II) complex. Inorg. Chim. Acta 2010, 363, 289–293. [Google Scholar] [CrossRef]
- Hu, W.; Fang, L.; Hua, W.; Gou, S. Biotin-Pt (IV)-indomethacin hybrid: A targeting anticancer prodrug providing enhanced cancer cellular uptake and reversing cisplatin resistance. J. Inorg. Biochem. 2017, 175, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Boodram, J.N.; McGregor, I.J.; Bruno, P.M.; Cressey, P.B.; Hemann, M.T.; Suntharalingam, K. Breast cancer stem cell potent copper(II)-non-steroidal anti-inflammatory drug complexes. Angew. Chem. Int. Ed. 2016, 55, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Boodram, J.N.; Cressey, P.B.; Lu, C.; Bruno, P.M.; Hemann, M.T.; Suntharalingam, K. The breast cancer stem cell potency of copper(II) complexes bearing nonsteroidal anti-inflammatory drugs and their encapsulation using polymeric nanoparticles. Dalton Trans. 2016, 45, 17867–17873. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Berry, J.A.; Shoher, A.; Ramakrishnan, V.; Lucci, A. Cox-2 overexpression increases motility and invasion of breast cancer cells. Int. J. Oncol. 2005, 26, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Cook, K.R.; Vincent, L.; Hall, C.S.; Martin, C.; Lucci, A. Role of cox-2 in tumorospheres derived from a breast cancer cell line. J. Surg. Res. 2011, 168, e39–e49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Dixit, D.; Ghosh, S.; Sen, E. Cox-2 regulates the proliferation of glioma stem like cells. Neurochem. Int. 2011, 59, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Turner, P.; Warwick, B.; Biffin, J.R.; Regtop, H.L. Syntheses and characterization of anti-inflammatory dinuclear and mononuclear zinc indomethacin complexes. Crystal structures of [Zn2(indomethacin)4(L)2] (L = N,N-dimethylacetamide, pyridine, 1-methyl-2-pyrrolidinone) and [Zn(indomethacin)2(L1)2] (L1 = ethanol, methanol). Inorg. Chem. 2000, 39, 3742–3748. [Google Scholar] [PubMed]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Martinez, D.; Motevalli, M.; Watkinson, M. Is there really a diagnostically useful relationship between the carbon-oxygen stretching frequencies in metal carboxylate complexes and their coordination mode? Dalton Trans. 2010, 39, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Jabali, B.; Abu Ali, H. New zinc(II) complexes of the non-steroidal anti-inflammatory drug (indomethacin) and various nitrogen donor ligands. Synthesis, characterization and biological activity. Polyhedron 2016, 117, 249–258. [Google Scholar] [CrossRef]
- Lakshman, T.R.; Deb, J.; Paine, T.K. Anti-inflammatory activity and enhanced cox-2 selectivity of nitric oxide-donating zinc(II)-NSAID complexes. Dalton Trans. 2016, 45, 14053–14057. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Laws, K.; Eskandari, A.; Suntharalingam, K. A reactive oxygen species-generating, cyclooxygenase-2 inhibiting, cancer stem cell-potent tetranuclear copper(II) cluster. Dalton Trans. 2017, 46, 12785–12789. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2–5 are available from the authors. |
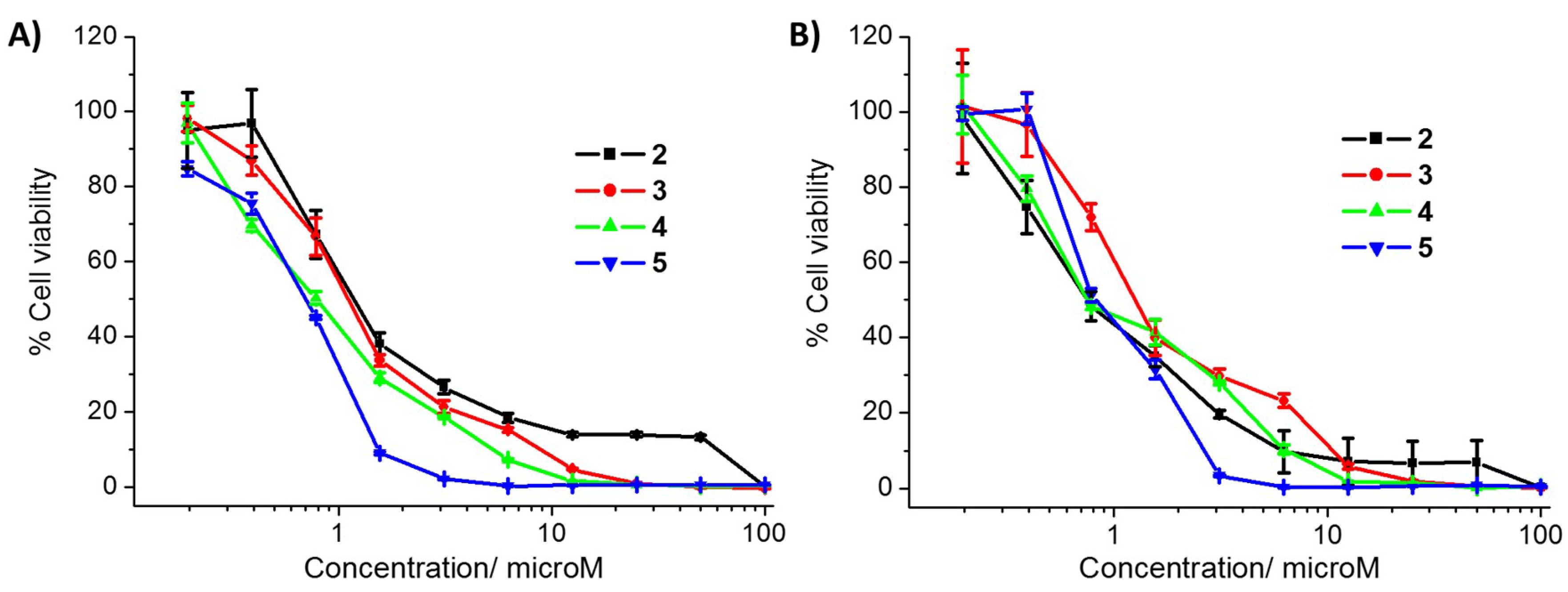
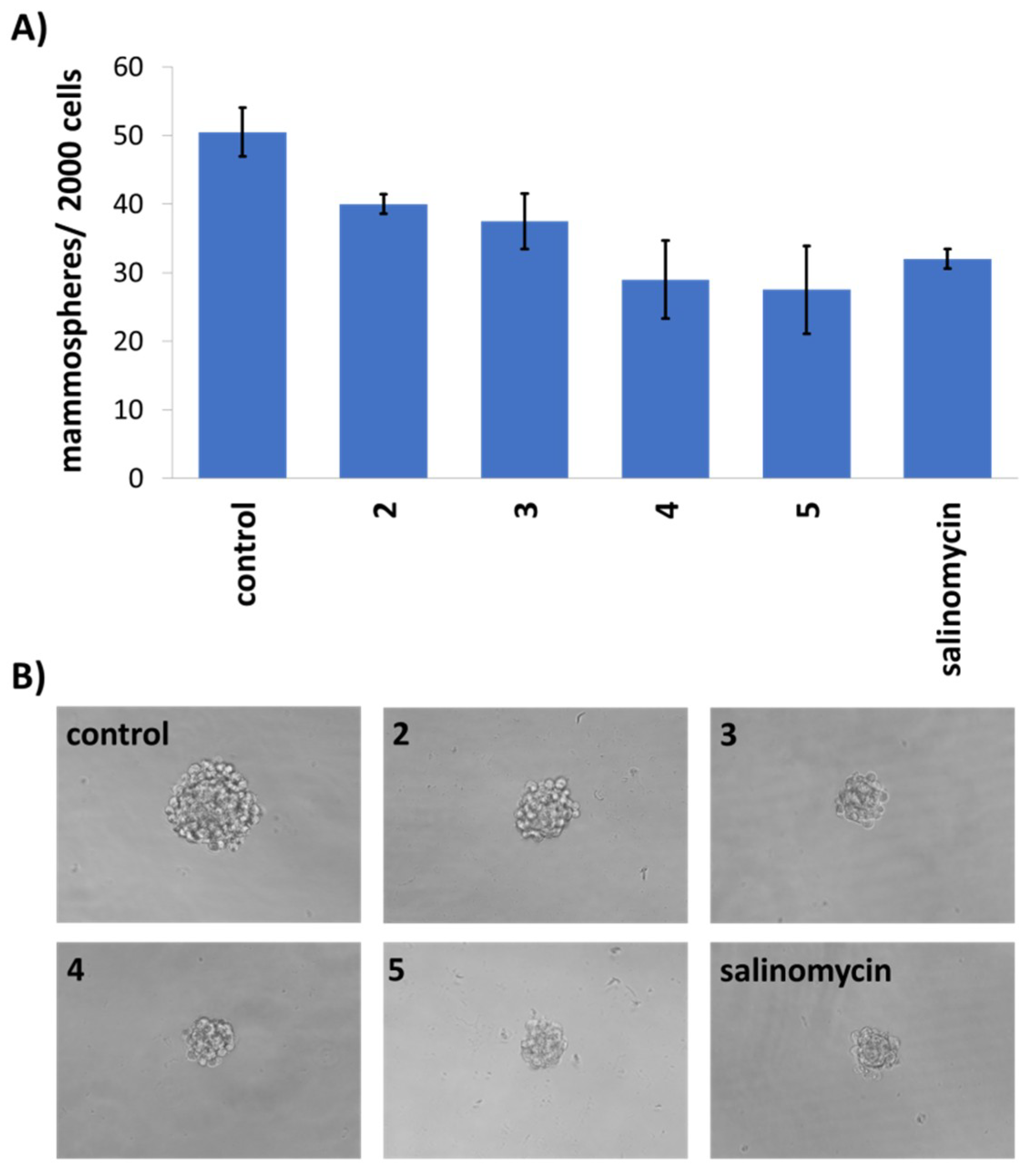
| Compound | HMLER IC50 (μM) | HMLER-shEcad IC50 (μM) | HEK 293T IC50 (μM) | Mammosphere IC50 (μM) |
|---|---|---|---|---|
| 2 | 1.2 ± 0.02 | 0.7 ± 0.04 | 20.0 ± 3.9 | >133 |
| 3 | 1.1 ± 0.05 | 1.1 ± 0.08 | 2.3 ± 0.1 | 44.1 ± 0.6 |
| 4 | 0.8 ± 0.03 | 0.7 ± 0.07 | 0.9 ± 0.2 | 44.2 ± 1.4 |
| 5 | 0.7 ± 0.03 | 0.9 ± 0.08 | 0.3 ± 0.02 | 2.7 ± 0.03 |
| cisplatin 1 | 3.4 ± 0.5 | 4.9 ± 0.4 | n.d. | n.d. |
| carboplatin 1 | 67.3 ± 2.8 | 72.3 ± 7.9 | n.d. | n.d. |
| salinomycin 1 | 11.4 ± 0.4 | 4.2 ± 0.3 | n.d. | 18.5 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rundstadler, T.K.; Eskandari, A.; Norman, S.M.; Suntharalingam, K. Polypyridyl Zinc(II)-Indomethacin Complexes with Potent Anti-Breast Cancer Stem Cell Activity. Molecules 2018, 23, 2253. https://doi.org/10.3390/molecules23092253
Rundstadler TK, Eskandari A, Norman SM, Suntharalingam K. Polypyridyl Zinc(II)-Indomethacin Complexes with Potent Anti-Breast Cancer Stem Cell Activity. Molecules. 2018; 23(9):2253. https://doi.org/10.3390/molecules23092253
Chicago/Turabian StyleRundstadler, Tiffany K., Arvin Eskandari, Sarah M. Norman, and Kogularamanan Suntharalingam. 2018. "Polypyridyl Zinc(II)-Indomethacin Complexes with Potent Anti-Breast Cancer Stem Cell Activity" Molecules 23, no. 9: 2253. https://doi.org/10.3390/molecules23092253
APA StyleRundstadler, T. K., Eskandari, A., Norman, S. M., & Suntharalingam, K. (2018). Polypyridyl Zinc(II)-Indomethacin Complexes with Potent Anti-Breast Cancer Stem Cell Activity. Molecules, 23(9), 2253. https://doi.org/10.3390/molecules23092253