Abstract
Bis(2-hydroxybenzylidene)isophthalohydrazide (H4L) has been used to synthesize the dinuclear [Cu2(1κNO2:2κN′O′2-H2L)(NO3)2(H2O)2] (1) and the tetranuclear [Cu4(μ-1κNO2:2κN′O2-H2L)2(μ-NO3)2(H2O)4]·2C2H5OH (2) complexes. The solvent plays an important role in determining the ligand behaviour in the syntheses of the complexes. An ethanol-acetonitrile mixture of solvents favours partials enolization in the case of 2. Both complexes have been characterized by elemental analysis, infrared radiation (IR), single crystal X-ray crystallography and electrochemical methods. The variable temperature magnetic susceptibility measurements of 2 show strong antiferromagnetic coupling between the central nitrato-bridged Cu (II) ions. The catalytic activity of both 1 and 2 has been screened toward the solvent-free microwave-assisted oxidation of alcohols and the peroxidative oxidation of alkanes under mild conditions. Complex 1 exhibits the highest activity for both oxidation reactions, leading selectively to a maximum product yield of 99% (for the 1-phenylethanol oxidation after 1 h without any additive) and 13% (for the cyclohexane oxidation to cyclohexyl hydroperoxide, cyclohexanol and cyclohexanone after 3 h).
1. Introduction
The oxidation of alcohols to carbonyl-containing compounds [1,2,3] is one of the fundamental reactions in organic synthesis [4,5], with a great interest due to the application of carbonyl compounds in research and industrial manufacturing, e.g., in the production of new materials [6] and energy sources [7]. In view of their central role in synthetic chemistry and expected further applications, these reactions continue to attract great attention in order to develop environmentally benign processes [8,9] disclosing new efficient catalysts [10,11], substrates or oxidation agents which could successfully be used in the near future and make a difference in terms of efficiency, selectivity, economy and/or sustainability of the processes. The metal-catalyzed aerobic and peroxidative oxidations of alcohols, in particular of benzylic alcohols, are typical model reactions due to their importance and generality; inexpensive primary oxidants such as O2, H2O2 or tert-butylhydroperoxide (TBHP) and simple procedures are usually explored [1,2,3,4,5]. The accelerating effect of microwave (MW) irradiation in the synthesis of ketones from secondary alcohols with TBHP as an oxidant has been largely reported [12,13,14,15,16,17,18]; this technology is a useful alternative source in organic synthesis, with an environmentally friendly nature.
The mild oxidation of cycloalkanes by hydrogen peroxide to the corresponding alkyl hydroperoxides, alcohols and ketones, a highly significant reaction in terms of industrial interest, remains a challenge in modern catalysis [19,20,21,22,23,24].
It has been shown that copper (II) complexes are useful catalysts towards the MW assisted oxidation of alcohols and also the functionalization of inert alkanes into valuable organic products, using readily available and cheap oxidants [22,25,26,27,28]; however, such applications are still limited and the subject requires further exploration. Moreover, aroylhydrazone complexes are potentially important as oxidation catalyst and some of them also showed interesting magnetic properties [29,30,31,32].
Continuing our research on the syntheses of aroylhydrazone metal complexes and their application in catalysis, herein we report the syntheses and characterization of new dinuclear and tetranuclear Cu(II) complexes derived from the aroylhydrazone Schiff base bis(2-hydroxybenzylidene) isophthalohydrazide (H4L), their electrochemical behaviour and magnetic properties (2), as well as their catalytic activity in the solvent-free MW assisted peroxidative oxidation of alcohols and also in the oxidation of alkanes under mild conditions, towards the development of environmentally friendly catalytic systems.
2. Results and Discussion
2.1. Syntheses and Characterizations
The aroylhydrazone Schiff base bis(2-hydroxybenzylidene) isophthalohydrazide (H4L) has two potential tridentate coordination pockets which can bind two metal centres simultaneously. It has previously been observed [32,33,34] that the aroylhydrazone can act as ligand in two tautomeric (keto or enol) forms. At room temperature, deprotonation of the phenolic –OH group from the aroylhydrazone in the presence of a copper (II) source, in methanol, generates the H2L2− species and leads to the stable dinuclear complex 1 (Scheme 1). When the reaction is carried out in a 2:1 acetonitrile-ethanol solvent mixture, part of the aroylhydrazone remains in the keto form, the remaining undergoing enolization and deprotonation during complexation. As a result, a HL3− species is formed in solution and provides the tetranuclear complex 2 (Scheme 1). This compound 2 can be considered as a dimer of 1, where the two central Cu (II) ions are bridged by two nitrate ions and one of the phenoxido groups of the organic ligand. The IR spectra of complexes 1 and 2 contain all the characteristic bands of the corresponding coordinated tridentate anionic ligand viz., 3476, 3024, 1608, 1254 and 1159 cm−1 for 1 and 3388, 3226, 2978, 1611, 1252 and 1068 cm−1 for 2. The electrospray ionization mass spectrometry (ESI-MS) spectra of both compounds, in ethanol solution (see experimental section), display the molecular ion peaks at m/z = 672 [1 + H]+ (100%) and at m/z = 1158 [2 + H]+ (100%).
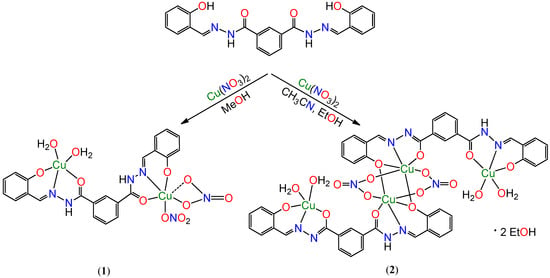
Scheme 1.
Syntheses of 1 and 2.
2.2. General Description of the Crystal Structures
X-ray low quality (low diffracting) crystals of 1 and 2 were obtained upon slow evaporation of a methanolic (for 1) or an acetonitrile-ethanol solution mixture (for 2), at room temperature. Crystallographic data are summarized in Table 1, representative plots are displayed in Figure 1 and Figure 2, and selected dimensions are presented in Table 2. Cif files for Compound 1 and Compound 2 are available as Supporting Information for this paper.

Table 1.
Crystal data and structure refinement details for complexes 1 and 2.
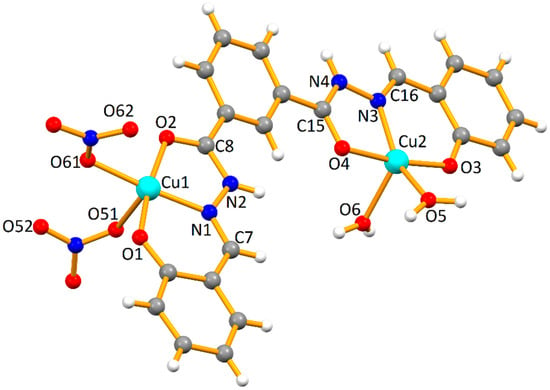
Figure 1.
Molecular structure of 1 with partial atom numbering scheme. The O-atoms from solvent water molecules are omitted for clarity.
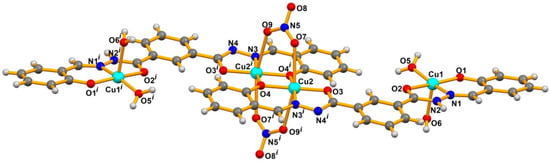
Figure 2.
Molecular structure of 2 with partial atom numbering scheme. The ethanol solvent molecule is omitted for clarity. Symmetry operation to generate equivalent atoms (i): 3−x, −y, 1−z.

Table 2.
Selected bond distances (Å) and angles (º) in complexes 1–2.
The organic ligand in 1 and 2 is almost planar, behaves as a hexadentate chelator and acts as a NO2 donor for each metal cation in 1 and for the outer ones in 2, while for the inner metals it behaves as an Ophenolate-bridging species as well. The copper cations exhibit square pyramidal [Cu1: τ5 = 0.08 (1) and 0.06 (2)], distorted penta-coordinate (Cu2 in 1, τ5 = 0.38) or distorted octahedral geometries (Cu2 in 2; quadratic elongation = 1.078, angle variance = 77.60 º2). The relative orientation of one of the nitrate ligands of Cu1 in 1 caused a measured Cu-Onitrate bond distance of 2.69(1) Å which is similar to those of 2.680 (7) and 2.709 (5) Å in 2, well below the sum on the van der Waals radii of copper and oxygen (1.40 and 1.52 Å, respectively). Therefore, on this basis, the geometry of that cation in 1 may be thought as octahedral, although with a great distortion (quadratic elongation = 1.125, angle variance = 272.62 º2).
Compound 2 can be considered as a dimer of 1, where dimerization took place at the level of the metals bearing nitrates, leading to a central four-membered dicopper metalacycle. This process affected not only the dimensions involving the central copper cations (Cu(nitrate), see Table 2), but also the outer ones with water ligands bound to copper (Cu(water), see Table 2), which is probably related to the observed change of geometry (see above the values of τ5 descriptor for such metals). Thus, from 1 to 2, the Cu-Ophenolate increased in both types of metals, but the Cu-Oketone increased only in the metal with water ligands, and decreased slightly in the other. Concerning the Cu-Owater lengths, they differ markedly in 2 with the apical much longer than the equatorial one; in 1 the dissimilarity is not so significant, but still the longer distance pertains to the water molecule more distant from the least-square plane of the molecule. An influence has also been perceived in the Cu···Cu lengths; in the molecule of 2 the distance between the Cu(nitrate) and the Cu(water) is more than 0.100 Å shorter than in 1, but the shortest intermolecular distance between the metal cations is higher in the former. The effect on selected O-Cu-O and O-Cu-N angles in 1 and 2 (Table 2) is most probably related to the aforementioned differences in geometry.
Moreover, the enolate forms are more strongly coordinated to the metal centres with shorter M–O bond distances than the corresponding ones in the keto forms, as observed in other cases [32,33,34].
2.3. Electrochemical Properties
Cyclic voltammograms of 1 and 2 exhibit a first single-electron (per metal atom) irreversible reduction process (wave Ired) at IEpred −0.27 or −0.43 V vs. SCE, for 1 or 2, respectively, followed, at a lower potential, by a second single-electron (per metal atom) reduction (wave IIred) at IIEpred = −1.12 or −1.08 V vs. SCE, for 1 or 2, respectively, which are believed to correspond to the CuII → CuI (wave Ired) and CuI → Cu0 (wave IIred) cathodic processes, in this order. Upon scan reversal following the first reduction wave, an irreversible oxidation at IEpox −0.05 or 0.02 V vs. SCE, for 1 or 2, respectively, was detected and assigned to the oxidation of a novel Cu(I) species. By reversing the direction of the potential scan after the formation of wave IIred, an adsorption wave was formed at IIEpox 0.15 or 0.27 V vs. SCE for 1 or 2, respectively.
Expectedly, no genuine anodic waves have been detected for any of the complexes. Bis(2-hydroxybenzylidene) isophthalohydrazide (H4L) was not redox active under our experimental conditions, and hence the cyclic voltammetric waves of 1 and 2 can be due to metal-based electron transfer processes.
2.4. Magnetic Properties
The dc magnetic susceptibilities of complex 2 have been determined under 2000 Oe from 2 K to 300 K. As shown in Figure 3, the χMT value at 300 K is 1.48 cm3 K mol−1, slightly smaller than the expected value for four noninteracting Cu2+ ions (g = 2.0). The χMT product decreases with the decreased temperture until about 100 K, then drops slowly to about 10 K; below this temperature, χMT further decreases. These results indicate that there are antiferromagnetic interactions between copper(II) ions.
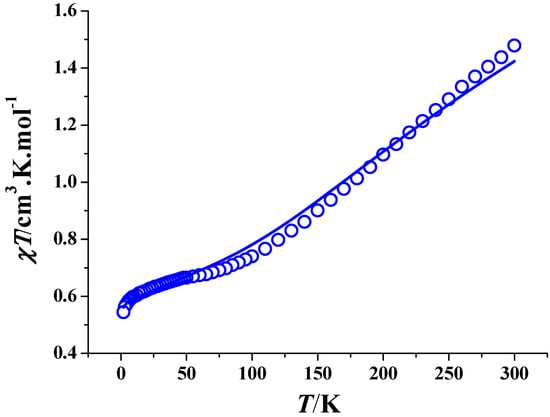
Figure 3.
Plot of χMT versus T for complex 2. The solid line represents the best theoretical fitting.
A linear tetranuclear copper (II) magnetic coupling model was adopted to analyze the magnetic interaction [35]. Owing to the crystallographic inversion symmetry, four exchange coupling constants J1, J2, J3 and J4 were used in the Hamiltonian, H = −2J1(S1S2+S1′S2′) − 2J2 S2S2′ − 2J3(S1S2′+S1′S2) − 2J4(S1S1′) [35]; they represent the magnetic interactions between Cu1 and Cu2 (or Cu1i and Cu2i), Cu2 and Cu2i, Cu1 and Cu2i (or Cu1i and Cu2), and between Cu1 and Cu1i, respectively. Given the high number of variables, fitting the magnetic data using all four coupling constants led to unreasonable results. Since the distance between Cu1 and Cu2i (or Cu1i and Cu2) and the distance between Cu1 and Cu1i are large enough, J3 and J4 could be considered as zero for a simplified model, and better results were thus achieved. The best fitting gave g = 2.02, J1 = −5.1 cm−1, J2 = −172.8 cm−1 and Nα = 4.8 × 10−5 with R = 5.5 × 10−4 (R = Σ[(χM)obs − (χM)calc]2/Σ[(χM)obs]2). The large negative value of J2 reveals that there is a strong antiferromagnetic exchange between the Cu (II) ions mediated by the Ophenolate and NO3─ bridges.
2.5. Catalytic Studies
2.5.1. Solvent-Free Microwave (MW) Assisted Oxidation of Secondary Alcohols
Complexes 1 and 2 were tested as catalyst precursors for the homogeneous oxidation of secondary alcohols [1-phenylethanol (model substrate), cyclohexanol, 2-hexanol and 3-hexanol] to the corresponding ketones following our previously developed procedure [18,31,36,37,38,39] using tert-butylhydroperoxide (t-BuOOH, Equation (2), used as aq. 70%) as oxidizing agent, under typical conditions of 80–120 °C, microwave (MW) irradiation (5–20 W), 0.5–3 h reaction time and in the absence of any added solvent (Scheme 2 for 1-phenylethanol and cyclohexanol oxidation). Results are summarized in Table 3 and Table 4.
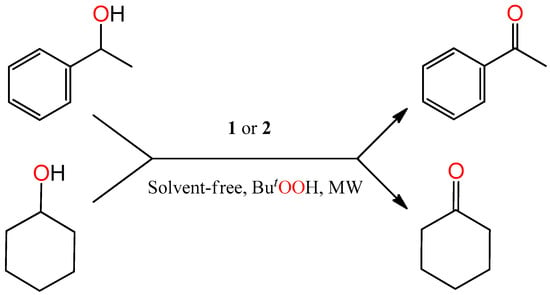
Scheme 2.
Microwave (MW)-assisted solvent-free oxidation of 1-phenylethanol and cyclohexanol to acetophenone and cyclohexanone, respectively, catalysed by 1 or 2.

Table 3.
Selected results for the MW-assisted oxidation of 1-phenylethanol by tert-butylhydroperoxide (TBHP) with 1 and 2 as catalyst precursors a.

Table 4.
MW-assisted oxidation of selected alcohols by TBHP with 1 and 2 as catalyst precursors a.
Under typical reaction conditions (120 °C and 0.5 h reaction time) ketone yields up to 37% (TOF = 264 h−1) are obtained for the oxidation of 1-phenylethanol (Table 3, entry 3) by the 1/TBHP/MW system (catalyst/substrate molar ratio of 0.2%) and in the absence of any additive. For a longer reaction time of 1 and 3 h, and for the same catalytic system, the oxidation by TBHP of 1-phenyletanol leads to 75 and 95% of acetophenone, respectively (Table 3, entries 4 and 5). The acetophenone yield value for 1 h reaction (Table 3, entry 4) is significantly higher than that obtained (17%, Table 3, entry 15) without MW assistance, for the same reaction time. Reactions performed at 120 °C with 20 W of MW irradiation and in the presence of complex 2 (2/TBHP/MW) resulted only in 28, 42 and 71% of acetophenone after 0.5, 1 and 3 h of oxidation reaction, respectively (Table 3, entries 16–18, respectively). The addition of the heteroaromatic 2-pyrazynecarboxylic acid (Hpca) or of trifluoroacetic acid (TFA) to the 1/TBHP/MW system [n (acid)/n (catalyst 1) = 10], slightly improved the yield from 11 to 14% (Table 3, entry 6) or to 18% (Table 3, entry 9), respectively, when the reaction was performed at 80 °C for 0.5 h.
A major effect was observed in the presence of 2,2,6,6-tetramethylpiperydil-1-oxyl (TEMPO) radical, an efficient promotor in aerobic oxidation of alcohols [11,40,41,42,43,44,45,46,47,48]. In fact, the oxidation of 1-phenylethanol by the 1/TBHP/TEMPO/MW system afforded substantial increase in the yield of acetophenone achieving 99% after 1 h at 120 °C (Table 3, entry 14) or 88% in only 0.5 h (Table 3, entry 13). Under the oxidation conditions used, the TEMPO additive could be oxidized to the oxoammonium species acting as oxidant, as well as acting as a hydrogen atom abstract from TBHP, enhancing the formation of tert-BuOO• and t-BuO• [40,41,42,43,44,45,46,47,48]. The 2/TBHP/TEMPO/MW system was not so effective (Table 3, entries 23 and 24). The blank tests in the absence of metal catalyst (with and without TEMPO) lead to very low conversion (up to 4% yield) of 1-phenylethanol to acetophenone (Table 3, entries 25 and 26).
Attempts to perform microwave-assisted oxidation of secondary alcohols in the presence of 1 or 2, in the absence of any additive and at room temperature, failed. Performing the reaction at 80 °C did not allow yields beyond 27% in 3 h (with catalyst 1), but at 120 °C the product was obtained in 95 (with 1) or 71% yield (with 2), in the same reaction time (Table 3, entries 5 and 20). The accelerating effect of the increase in temperature was also observed in the presence of the tested additives (Figure 4). Thus, for example, in the presence of the TEMPO radical, one observes a change in the conversion of 1-phenylethanol from 22 to 88% and in the TOF value from 168 to 1112 h−1 (Table 3, entries 12 and 13) when going from 80 or 120 °C, in 0.5 h. This feature also concerns the microwave power since at 80 °C the power went up to 10 W in the first 10 s but then, after reaching the desired temperature, it stabilized at ca. 5 W, while for the temperature of 120 °C, ca. 40 W were reached in the first 10 s followed by stabilisation at 10–15 W for the remaining time.
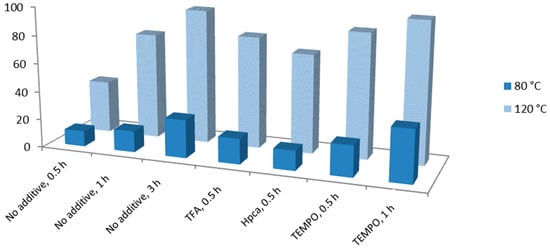
Figure 4.
Temperature effect on the yield of acetophenone from MW-assisted peroxidative oxidation of 1-phenylethanol by the 1/TBHP/MW catalytic system.
Complexes 1 and 2 were also tested towards the oxidation of aliphatic alcohols, namely cyclohexanol and the linear 2- and 3-hexanols. As expected, they were less reactive than 1-phenylethanol (benzylic alcohol derivative), leading to moderate yields in the 15–31% range (in 0.5 h reaction at 120 °C, without any additive (Table 4, entries 1, 4, 7, 10, 13 and 16). The efficiency of the oxidation of those alcohols could also be enhanced by using TEMPO radical and the yields increased to 29–58% in 0.5 h (Table 4, entries 2, 5, 8, 11, 14 and 17). Extending the reaction time to 1 h and in the presence of the same promoter, the conversions raised to 87% for cyclohexanol (Table 4, entry 3), 71% for 2-hexanol (Table 4, entry 6) and 59% for 3-hexanol (Table 4, entry 9) with 1 as catalyst. The position (2 or 3) of the OH group in the aliphatic chain of the linear alcohols (2-hexanol and 3-hexanol) appeared not to influence significantly the efficiency of the system, in view of the obtained yields (compare, e.g., entries 4 and 7 or 6 and 9, Table 4, for complex 1). Blank tests were performed for all aliphatic alcohols in the absence of any catalyst and residual conversions (up to 5%) were recorded.
The catalytic mechanism may proceed through the metal-assisted generation of t-BuOO● and t-BuO● radicals [40,41,42,43,44,45,46,47,48], upon oxidation or reduction of t-BuOOH by a CuII or CuI centre (Equations (1) and (2)), respectively, and is summed up in Equations (1)–(6).
Cu(II) + t-BuOOH → Cu(I) + t-BuOO• + H+
Cu(I) + t-BuOOH → Cu(II)-OH + t-BuO•
Cu(II)-OH + t-BuOOH → Cu(II)-OO-t-Bu + H2O
t-BuO• + R2CHOH → t-BuOH + R2C•-OH
t-BuOO• + R2CHOH → t-BuOOH + R2C•-OH
Cu(II)-OO-t-Bu + R2C•-OH → R2C=O + t-BuOOH + Cu(I)
2.5.2. Peroxidative Oxidation of Cyclohexane
Compounds 1 and 2 were also tested as catalyst precursors in the oxidation of cyclohexane by H2O2 (Equation (2), used as 50% aqueous solution) at 50 °C in MeCN/H2O medium (Scheme 3). The reaction was monitored by Gas Chromatography (GC) to determine the amount of cyclohexanol and cyclohexanone formed, typically after treatment with PPh3 (to reduce cyclohexyl hydroperoxide to cyclohexanol) [29,30,49,50,51].
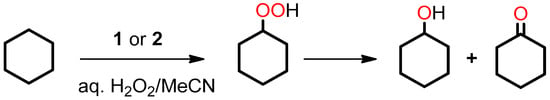
Scheme 3.
Oxidation of cyclohexane to cyclohexyl hydroperoxide, cyclohexanol and cyclohexanone.
The accumulation of oxygenated products (cyclohexanol and cyclohexanone) in the cyclohexane oxidation catalysed by 1 and 2, in the absence and in the presence of TFA, is given in Table 5. Both complexes were active for this reaction in the absence of any additive (Figure 5) with approximately 7 and 5% (for 1 and 2, respectively) of total product yield after ca. 0.5 h (Table 5, entries 3 and 15, for 1 and 2, respectively). Further increase of the reaction time to 2 h slightly improved the total yield of the products in the presence of 1 and almost had not effect when 2 was the catalyst. The presence of TFA improved the catalytic performance of both copper compounds, more markedly for 1 with the total yield of cyclohexanol and cyclohexanone achieving ca. 13% in 1 h (Table 5, entry 11). The promoting effect of an acid co-catalyst was already observed for other Cu-catalysed systems in the oxidative transformation of alkanes and can be related to (i) its involvement in proton transfer steps, (ii) catalyst activation by unsaturation of the Cu (II) centres upon ligand protonation or to the (iii) facilitation of the formation of peroxo complexes [27,52,53,54,55,56].

Table 5.
Total yield (cyclohexanol and cyclohexanone) with time in the oxidation of cyclohexane by H2O2 at 50 °C in CH3CN catalysed by 1 or 2 a.
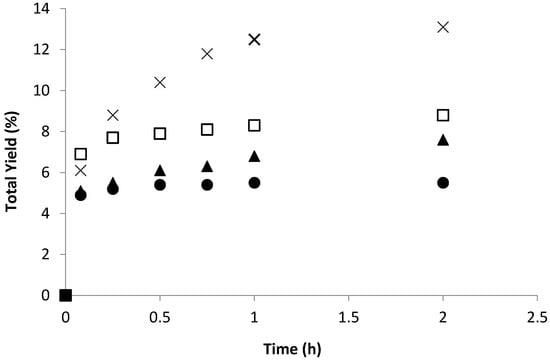
Figure 5.
Total yield (cyclohexanol and cyclohexanone) along the time in the oxidation of cyclohexane with aq. H2O2 catalyzed by 1 in the absence of any additive (□, squares) or in the presence of TFA (1:10) (×, cross), or by 2 in the absence of any additive (●, circles) or in the presence of TFA (1:10) (▲, triangles), at 50 °C in CH3CN.
The activity exhibited by compounds 1 and 2, even in the absence of TFA, is higher than that shown, e.g., by [Cu(OTf)2(Py2S2)] (Py2S2 = 1,6-bis(2′-pyridyl)-2,5-dithiahexane) (with 4.3% overall yield) [57] and is comparable to those of the complexes bearing azathia macrocycles, e.g., [Cu(OTf)2(L3)] (L3 = mixed 14-membered N2S2 azathia macrocycle) or [Cu(OTf)(L4)(H2O)](OTf) (L4 = nine-membered NS2 macrocyclic ligand with a 2-methylpyridyl pendant arm) (overall yield of ca. 8%) [58].
The formation of cyclohexyl hydroperoxide CyOOH was confirmed by GC-MS methods and accounts for a free radical reaction mechanism, which conceivably involves the presence of oxygen-centered radicals, HOO• and HO•, very reactive species, formed upon reaction of the catalyst with hydrogen peroxide (Equations (7) and (8)) [50,59,60,61,62] and enhanced by the presence of TEMPO radical. The HO• radical abstracts hydrogen from cyclohexane CyH to produce cyclohexyl radical Cy• (Equation (9)), which is then trapped by dioxygen to give CyOO• radical (Equation (10)). The latter may react with the oxidant to form CyOOH (Equation (11)). Metal-assisted decomposition of CyOOH to CyO• and CyOO• (Equations (12) and (13)) would then lead to cyclohexanol (CyOH) and cyclohexanone (Cy-H=O) products (Equations (14) and (15)) [61].
Cu(II) + HOOH → HOO• + H+ + Cu(I)
Cu(I) + HOOH → HO• + Cu(II) + HO−
HO• + CyH → ROH + Cy•
Cy• + O2 → CyOO•
CyOO• + HOOH → CyOOH + HOO•
CyOOH + Cu(I) → CyO• + Cu(II) + HO−
CyOOH + Cu(II) → CyOO• + H+ + Cu(I)
CyO• + CyH → CyOH + Cy•
2CyOO• → CyOH + Cy-H=O + O2
The coordination of the oxidant to the metal centre is dependent on the Lewis acidity of the latter. The more Lewis acid metal centre should be easier to reduce. To correlate the electrochemical and catalytic behaviours, the reduction potentials of both complexes were determined by cyclic voltammetry (see above). In fact, complex 1, the most active one in both oxidation reactions, is easier to reduce than 2 (reduction at a less negative potential, i.e., IEpred −0.27 V versus −0.43 V vs. SCE).
3. Experimental Section
3.1. General Materials and Equipment
All synthetic work was performed in air. Commercially available reagents and solvents were used as received, without further purification or drying. Cu(NO3)2·5H2O was used as a metal source for the synthesis of the complexes.
C, H, and N elemental analyses were carried out by the Microanalytical Service of Instituto Superior Técnico. Infrared spectra (4000–400 cm–1) were recorded on a Bruker Vertex 70 instrument in KBr pellets; wavenumbers are in cm–1. The 1H NMR spectra were recorded at room temperature on a Bruker Avance II + 400.13 MHz (UltraShieldTM Magnet, Rheinstetten, Germany) spectrometer. Tetramethylsilane was used as the internal reference and the chemical shifts are reported in ppm. Mass spectra were run in a Varian 500-MS LC Ion Trap Mass Spectrometer equipped with an electrospray (ESI) ion source. For electrospray ionization, the drying gas and flow rate were optimized according to the particular sample with 35 p.s.i. nebulizer pressure. Scanning was performed from m/z 100 to 1200 in methanol solution. The compounds were observed in the positive mode (capillary voltage = 80–105 V). The catalytic tests were performed under microwave (MW) irradiation using a focused Anton Paar Monowave 300 microwave fitted with a rotational system and an IR temperature detector, using a 10 mL capacity reaction tube with a 13 mm internal diameter. Gas chromatographic (GC) measurements were carried in a FISONS Instrument GC 8000 series gas chromatograph with a capillary DB-WAX column (30 m × 0.32 mm), a FID detector, helium as the carrier gas and using the Jasco-Borwin v.1.50 software. The magnetic susceptibility measurements were carried out on polycrystalline samples with a Quantum Design MPMS-XL5 SQUID magnetometer in the temperature range of 2–300 K and at an applied field of 2000 Oe. Diamagnetic corrections were estimated from Pascal’s constants for all constituent atoms [63]. The electrochemical experiments were performed in an EG&G PAR 273A potentiostat/galvanostat connected to a personal computer through a GPIB interface.
Syntheses of the pro-ligand H4L: The aroylhydrazone Schiff base pro-ligand bis(2-hydroxybenzylidene) isophthalohydrazide (H4L) (Scheme 1) was prepared by a reported method [64,65] upon condensation of the 2-hydroxybenzohydrazide with isophthalohydrazide.
Yield: 84%. Anal. Calcd for H4L C22H18N4O4: C, 65.66; H, 4.51; N, 13.92. Found: C, 65.60; H, 4.48; N, 13.87. IR (KBr pellet, cm−1): 3212 ν(OH), 3055 ν(NH), 1653 ν(C=O). 1H NMR (DMSO-d6, δ): 12.09 (s, 2H, OH), 11.18 (s, 2H, NH), 8.09 (s, 2H, -CH=N), 7.58–6.94 (m, 12H, C6H4).
Synthesis of [Cu2(1κNO2:2κN′O2-H2L)(NO3)2(H2O)2] (1): 0.402 g (1.0 mmol) of H4L was suspended in 25 mL of methanol and 0.525 g Cu(NO3)2·5H2O (2.05 mmol) were added. The resultant mixture was stirred at room temperature for 15 min; a dark green solution was obtained. The solution was then filtered and the solvent was allowed to evaporate slowly. After 3–4 days, single crystals suitable for X-ray diffraction were isolated, washed 3 times with cold methanol and dried in open air.
Yield: 0.443 g (66%, with respect to Cu). Anal. Calcd for C22H20Cu2N6O12: C, 38.43; H, 2.93; N, 12.22. Found: C, 38.36; H, 2.90; N, 12.14. IR (KBr; cm−1): 3448 ν(OH), 1612 ν(C=N), 1250 ν(C–O)enolic and 1159 ν( N–N). ESI-MS (+): m/z 672 [M+H]+ (100%).
Synthesis of [Cu4(μ-1κNO2:2κN′O2-H2L)2(μ-NO3)2(H2O)4]·2C2H5OH (2): To a 20 mL acetonitrile solution of H4L (0.402 g, 1.00 mmol), a 20 mL ethanol solution of Cu(NO3)2·5H2O (0.525 g, 2.05 mmol) was added and the reaction mixture was stirred for 20 min at room temperature. The resultant dark green solution was filtered and the filtrate was kept in open air. Dark green single crystals suitable for X-ray diffraction analysis were isolated after 4 days. Crystals were washed 3 times with cold ethanol and dried in open air.
Yield: 0.800 g (64%, with respect to Cu). Anal. Calcd for C48H52Cu4N10O20 (2): C, 42.92; H, 3.90; N, 10.43. Found: C, 42.87; H, 3.84; N, 10.39. IR (KBr; cm−1): 3456 ν(OH), 2986 ν(NH), 1609 ν(C=O), and 1168 ν(N–N). ESI-MS (+): m/z 1158 [(M-2EtOH)+H]+ (100%).
3.2. X-Ray Measurements
Good quality single crystals suitable for X-ray diffraction of 1 and 2 were immersed in cryo-oil, mounted in Nylon loops and measured at a temperature of 296 K. Intensity data were collected using a Bruker AXS PHOTON 100 diffractometer with graphite monochromated Mo-Kα (λ 0.71073) radiation. Data were collected using omega scans of 0.5° per frame and full spheres of data were obtained. Cell parameters were retrieved using Bruker SMART [66] software and refined using Bruker SAINT [66] on all the observed reflections. Absorption corrections were applied using SADABS [66]. Structures were solved by direct methods by using SIR97 [67] and refined with SHELXL2014 [68]. Calculations were performed using WinGX version 2014.1 [69]. All non-hydrogen atoms were refined anisotropically. The H-atoms bonded to carbons were included in the model at geometrically calculated positions and refined using a riding model. Uiso(H) values were defined as 1.2Ueq of the parent aromatic and methylene groups and 1.5Ueq of the parent methyl ones. The other hydrogen atoms (O–H and N–H) were located in the difference Fourier synthesis and refined, in some cases with the help of distance and angle restraints, their isotropic thermal parameter set at 1.5 times the average thermal parameter of the parent oxygen or nitrogen atom. The structure of 1 appears to have three water molecules per unit cell, but their hydrogen atoms could not be ascertained and the Calc-OH routine of WinGX led to unreasonable locations. The possibility of disordered solvent molecules in a void was taken into consideration and Platon/Squeeze [70] was used to establish a void of 158 Å3 with 56 electrons; removing such content in the void did not lead to any improved solution, the reason why such strategy was not followed. Least square refinements with anisotropic thermal motion parameters for all the non-hydrogen atoms and isotropic for the remaining atoms were employed.
3.3. Electrochemical Studies
Cyclic voltammetry (CV) and controlled-potential electrolyses (CPE) were undertaken in 0.2 M [nBu4N][BF4]/DMSO electrolyte solutions, saturated with N2 before each run, at room temperature. They were performed in a three-electrode cell equipped with a Luggin capillary connected to a silver wire pseudo-reference electrode. Platinum disc (for CV; d = 1 mm) or gauze (CPE) working electrodes were used, as well as platinum wire (CV) or gauze (CPE) counter electrodes. In CPE experiments, the working and counter compartments were separated by a sintered glass frit and the studies were monitored regularly by CV. The redox potentials were measured using ferrocene as internal standard, and their values are quoted relative to the SCE by using the [Fe(η5-C5H5)2]0/+ redox couple ( = 0.44 V vs. SCE for DMSO) [71,72,73,74,75,76].
3.4. Typical Procedures and Product Analysis for Catalysis
The microwave-assisted (MW) solvent-free peroxidative catalytic oxidation of 1-phenyethanol was performed in a focused Anton Paar Monowave 300 reactor using a 10 mL capacity cylindric Pyrex tube with a 13 mm internal diameter, fitted with a rotational system and an IR temperature detector. The tube was charged with the alcohol (2.5 mmol), 5 µmol catalyst precursor 1 or 2 (0.2 mol% vs. substrate) and a 70% aqueous solution of t-BuOOH (5 mmol), sealed, placed in the microwave reactor and the system left stirring under irradiation (5 or 20 W) at 80 or 120 °C for 0.5–3 h. After cooling to room temperature, 150 µL of benzaldehyde (internal standard) and 2.5 mL of MeCN (to extract the substrate and the organic products from the reaction mixture) were added. The obtained mixture was stirred for 10 min and then a sample (1 µL) was taken from the organic phase and analysed by GC.
The peroxidative oxidations of cyclohexane were performed in round bottom flasks with vigorous stirring and using MeCN as solvent (up to 5.0 mL total volume), under air. The catalyst precursors 1 and 2 and trifluoroacetic acid (TFA, optional), in the form of a stock solution in acetonitrile, were mixed, cyclohexane (0.25 mL, 2.3 mmol) was included and the reaction started when hydrogen peroxide was added (50% in H2O, 0.68 mL, 11 mmol). The concentrations of the reactants were thus as follows: catalyst precursor (5 × 10−4 mol L−1), TFA (0.005 mol L−1), substrate (0.46 mol L−1) and H2O2 (2.2 mol L−1). The reaction mixture was stirred at 50 °C for 2 h and at 5, 15, 30, 45, 60 and 120 min, an aliquot was taken and analysed by GC using nitromethane (50 µL) as internal standard. Before the GC analysis an excess of triphenylphosphine was added, in order to reduce the formed cyclohexyl hydroperoxide to the corresponding alcohol and hydrogen peroxide to water, following a method developed by Shul’pin [49,50,51]. Blank experiments were performed and confirmed that no product of cyclohexane oxidation was obtained unless the metal catalyst was used.
For the GC measurements, the temperature of injection was 240 °C. The initial temperature of the column was maintained at 100 °C (oxidation of cyclohexane) or 120 °C (oxidation of alcohol) for 1 min, then raised 10 °C/min up to 180 °C (oxidation of cyclohexane) or 200 °C (oxidation of alcohol), and held at this temperature for 1 min. Attribution of peaks was made by comparison with chromatograms of genuine samples and, in some cases, by GC-MS analyses using a Perkin Elmer Clarus 600 C instrument (He as the carrier gas), equipped with a 30 m × 0.22 mm × 25 μm BPX5 (SGE) capillary column.
4. Conclusions
The dinuclear [Cu2(1κNO2:2κN′O′2-H2L)(NO3)2(H2O)2] (1) and the tetranuclear [Cu4(μ-1κNO2:2κN′O′2-HL)2(μ-NO3)2(H2O)4] (2) complexes derived from bis(2-hydroxybenzylidene)isophthalohydrazide (H4L) have been synthesized and characterized. The electrochemical studies show two one-electron cathodic reductions [CuII → CuI and CuI → Cu0]. The variable temperature magnetic susceptibility measurements of 2 show strong antiferromagnetic exchange between the Cu (II) ions meadiated by the O-phenolate and NO3─ bridges.
Both complexes act as catalyst precursors towards the solvent-free microwave-assisted oxidation of alcohols and the peroxidative oxidation of alkanes under mild conditions. The highest activity and selectivity with a maximum product yield of 99% (for the 1-phenylethanol oxidation after 1 h without any additive) were observed with 1. In the case of oxidation of cyclohexane, 1 also exhibits a better activity (a maximum product yield of 13% after 3h) than 2.
The catalytic oxidations under microwave assisted and solvent-free conditions have significance for the development of environmentally friendly catalytic systems and deserve further investigations. Our study was an attempt to contribute to this aim.
Supplementary Materials
The following are available online, Cif: Compound 1 and Compound 2. CCDC 1858873 and 1858872 for 1 and 2 contain the supplementary crystallographic data for this paper. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Author Contributions
Conceptualization, M.S. and E.C.B.A.A.; Methodology, M.S. and E.C.B.A.A.; Software, M.S., M.F.C.G.d.S. and C.M.L.; Validation, M.S., M.F.C.G.d.S. and C.M.L.; Formal Analysis, M.S. and E.C.B.A.A.; Investigation, M.S.; Resources, M.S. and A.J.L.P.; Data Curation, M.F.C.G.d.S. and C.M.L.; Writing-Original Draft Preparation, M.S. and E.C.B.A.A.; Writing-Review & Editing, M.F.C.G.d.S., C.M.L.and A.J.L.P.; Visualization, M.S.; Supervision, M.S. and E.C.B.A.A. and M.F.C.G.d.S.; Project Administration, A.J.L.P.; Funding Acquisition, A.J.L.P.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia, Portugal grant number UID/QUI/00100/2013, PTDC/QEQ-ERQ/1648/2014 and PTDC/QEQ-QIN/3967/2014.
Acknowledgments
Authors are grateful to the Fundação para a Ciência e a Tecnologia: FCT (projects UID/QUI/00100/2013, PTDC/QEQ-ERQ/1648/2014 and PTDC/QEQ-QIN/3967/2014), Portugal, for financial support. M.S. acknowledges the FCT, Portugal for a postdoctoral fellowship (SFRH/BPD/86067/2012). Authors are thankful to the Portuguese NMR Network (IST-UL Centre) for access to the NMR facility and the IST Node of the Portuguese Network of mass-spectrometry for the ESI-MS measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic oxidation of alcohols: Recent advances. Adv. Organomet. Chem. 2015, 63, 91–174. [Google Scholar]
- Karabach, Y.Y.; Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J.L. Microwave-assisted catalytic oxidation of alcohols to carbonyl compounds. In Advances in Organometallic Chemistry Catalysis; Pombeiro, A.J.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Chapter 18; pp. 233–245. [Google Scholar]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.W.C.E.; Dijksman, A. New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal. Today 2000, 57, 157–166. [Google Scholar] [CrossRef]
- Ryland, B.L.; Stahl, S.S. Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. Engl. 2014, 53, 8824–8838. [Google Scholar] [CrossRef] [PubMed]
- Trincado, M.; Banerjee, D.; Grutzmacher, H. Molecular catalysts for hydrogen production from alcohols. Energy Environ. Sci. 2014, 7, 2464–2503. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Ten Brink, G.J.; Dijksman, A.G. Catalytic Oxidations of Alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Dobereiner, G.E.; Crabtree, R.H. Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev. 2010, 110, 681–703. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N. Modern Heterogeneous Oxidation Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Sheldon, R.A.; Arends, I.W.C.E. Organocatalytic Oxidations Mediated by Nitroxyl Radicals. Adv. Synth. Catal. 2004, 346, 1051–1071. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Barman, T.R.; Scorcelletti, F.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enol aroylhydrazone Cu(II) complexes. Mol. Cat. 2017, 439, 224–232. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Fontolan, E.; Alegria, E.C.B.A.; Kopylovich, M.N.; Bertani, R.; Pombeiro, A.J.L. The influence of multiwalled carbon nanotubes and graphene oxide additives on the catalytic activity of 3d metal catalysts towards alcohol oxidation. J. Mol. Catal. Chem. 2017, 426, 557–563. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Alegria, E.C.B.A.; Sutradhar, M.; Cristiano, M.L.S.; Ismael, A.; Kopylovich, M.N.; Pombeiro, A.J.L. Copper(II) and cobalt(II) tetrazole-saccharinate complexes as effective catalysts for oxidation of secondary alcohols. J. Mol. Cat. Chem. 2016, 425, 283–290. [Google Scholar] [CrossRef]
- Timokhin, I.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel coordination polymers with (pyrazolato)-based tectons: Catalytic activity in the peroxidative oxidation of alcohols and cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxido vanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohol. Appl. Catal. Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Zaltariov, M.F.; Alexandru, M.; Cazacu, M.; Shova, S.; Novitchi, G.; Train, C.; Dobrov, A.; Kirillova, M.V.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; et al. Tetranuclear copper(II) complexes with macrocyclic and open-chain disiloxane ligands as catalyst precursors for hydrocarboxylation and oxidation of alkanes and 1-phenylethanol. Eur. J. Inorg. Chem. 2014, 29, 4946–4956. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mizar, A.; Silva, M.F.C.G.; MacLeod, T.C.O.; Mahmudov, K.T.; Pombeiro, A.J.L. Template syntheses of copper(II) complexes from arylhydrazones of malononitrile and their catalytic activity towards alcohol oxidations and the nitroaldol reaction: Hydrogen bond-assisted ligand liberation and E/Z isomerisation. Chem. Eur. J. 2013, 19, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 2016, 138, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Matias, I.A.S.; Duarte, T.A.G.; Pombeiro, A.J.L. Catalytic performance of Fe(II)-scorpionate complexes towards cyclohexane oxidation in organic, ionic liquid and/or supercritical CO2 media: A comparative study. Catalysts 2017, 7, 230. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. Toward functionalization of alkanes under environmentally benign conditions. In Advances. In Organometallic Chemistry and Catalysis; Pombeiro, A.J.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Chapter 2; pp. 15–25. [Google Scholar]
- Shul’pin, G.B. New trends in oxidative functionalization of carbon–hydrogen bonds: A review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon oxygenations with peroxides catalyzed by metal compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 2236–2252. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Nesterova, O.V.; Pombeiro, A.J.L. Copper(II) complex of the 2-pyridinecarbaldehyde aminoguanidine Schiff base: Crystal structure and catalytic behaviour in mild oxidation of alkanes. Inorg. Chem. Commun. 2017, 78, 85–90. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silv, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301-302, 200–239. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. A hexanuclear oxovanadium(IV,V) complex bearing N,O-ligand as a highly efficient alkane oxidation catalyst. Inorg. Chem. 2012, 51, 11229–11231. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Alegria, E.C.B.A.; Liu, C.M.; Pombeiro, A.J.L. Mn(II,II) complexes: Magnetic properties and microwave assisted oxidation of alcohols. Dalton. Trans. 2014, 43, 3966–3977. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Liu, C.M.; Pombeiro, A.J.L. Trinuclear Cu(II) structural isomers: Coordination, magnetism, electrochemistry and catalytic activity toward oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 3959–3969. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Aroylhydrazone Cu(II) complexes in keto form: Structural characterization and catalytic activity towards cyclohexane oxidation. Molecules 2016, 21, 425. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Alegria, E.C.B.A.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Iron(III) and cobalt(III) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: Catalysts for the microwave assisted oxidation of alcohols. RSC Adv. 2016, 6, 8079–8088. [Google Scholar] [CrossRef]
- Chiari, B.; Piovesana, O.; Tarantelli, T.; Zanazzi, P.F. Further neighbor magnetic exchange interaction in a novel pseudolinear tetramer of copper (II). Inorg. Chem. 1993, 32, 4834–4838. [Google Scholar] [CrossRef]
- Karabach, Y.Y.; Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J.L. Microwave-assisted Catalytic oxidation of Alcohols to carbonyl compounds. In Advances in Organometallic Chemistry and Catalysis; Pombeiro, A.J.L., Ed.; Wiley-VCH: Weinheim, Germany; Hoboken, NJ, USA, 2013; Chapter 22; pp. 285–294. [Google Scholar]
- Figiel, P.J.; Kopylovich, M.N.; Lasri, J.; Guedes da Silva, M.F.C.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Solvent-free microwave-assisted peroxidative oxidation of secondary alcohols to the corresponding ketones catalyzed by copper (II) 2,4-alkoxy-1,3,5-triazapentadienato complexes. Chem. Commun. 2010, 46, 2766–2768. [Google Scholar] [CrossRef] [PubMed]
- Figiel, P.J.; Kirillov, A.M.; Guedes da Silva, M.F.C.; Lasri, J.; Pombeiro, A.J.L. Self-assembled dicopper(II) diethanolaminate cores for mild aerobic and peroxidative oxidation of alcohols. Dalton Trans. 2010, 39, 9879–9888. [Google Scholar] [CrossRef] [PubMed]
- Lasri, J.; Rodriguez, M.J.F.; Guedes da Silva, M.F.C.; Smolenski, P.; Kopylovich, M.N.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Microwave synthesis of bis(tetrazolato)-PdII complexes with PPh3 and water-soluble 1,3,5-triaza-7-phosphaadamantane (PTA). The first example of C–CN bond cleavage of propionitrile by a PdII centre. J. Organomet. Chem. 2011, 696, 3513–3520. [Google Scholar] [CrossRef]
- Alexandru, M.; Cazacu, M.; Arvinte, A.; Shova, S.; Turta, C.; Simionescu, B.C.; Dobrov, A.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. μ-Chlorido-bridged dimanganese(II) complexes of the Schiff base derived from [2+2]condensation of 2,6-diformyl-4-methylphenol and 1,3-bis(3-aminopropyl)tetramethyldisiloxane: Structure, magnetism, electrochemical behaviour and catalytic oxidation of secondary alcohols. Eur. J. Inorg. Chem. 2014, 120–131. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Figiel, P.J.; Räisänen, M.T.; Leskelä, M.; Repo, T. Aerobic oxidation of benzylic alcohols with bis(3,5-di-tert-butylsalicylaldimine) copper (II) complexes. Appl. Catal. A 2009, 371, 17–21. [Google Scholar] [CrossRef]
- Figiel, P.J.; Kirillov, A.M.; Karabach, Y.Y.; Kopylovich, M.N.; Pombeiro, A.J.L. Mild aerobic oxidation of benzyl alcohols to benzaldehydes in water catalyzed by aqua-soluble multicopper(II) triethanolaminate compounds. J. Mol. Catal. Chem. 2009, 305, 178–182. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Copper(II) and iron(III) complexes with arylhydrazone of ethyl 2-cyanoacetate or formazan ligands as catalysts for oxidation of alcohols. New J. Chem. 2016, 40, 10071–10083. [Google Scholar] [CrossRef]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 29, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Belanzon, P.; Gamez, P.; Reedijk, J.; Baerends, E.J. Activation of the C−H bond by electrophilic attack: Theoretical study of the reaction mechanism of the aerobic oxidation of alcohols to aldehydes by the Cu(bipy)2+/2,2,6,6-tetramethylpiperidinyl-1-oxy cocatalyst system. Inorg. Chem. 2009, 48, 11909–11920. [Google Scholar] [CrossRef] [PubMed]
- Gamez, P.; Arends, I.W.C.E.; Sheldon, R.A.; Reedijk, J. Room temperature aerobic copper–catalysed selective oxidation of primary alcohols to aldehydes. Adv. Synth. Catal. 2004, 346, 805–811. [Google Scholar] [CrossRef]
- Gamez, P.; Arends, I.W.C.E.; Reedijk, J.; Sheldon, R.A. Copper(II)-catalysed aerobic oxidation of primary alcohols to aldehydes. Chem. Commun. 2003, 19, 2414–2415. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Karabach, Y.Y.; Guedes da Silva, M.F.C.; Figiel, P.J.; Lasri, J.; Pombeiro, A.J.L. Alkoxy-1,3,5-triazapentadien(e/ato) copper(II) complexes: Template formation and applications for the preparation of pyrimidines and as catalysts for oxidation of alcohols to carbonyl products. Chem. Eur. J. 2012, 18, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely efficient alkane oxidation by a new catalytic reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Kopylovich, M.N.; Nunes, A.C.C.; Mahmudov, K.T.; Haukka, M.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Complexes of copper(II) with 3-(ortho-substituted phenylhydrazo)pentane-2,4-diones: Syntheses, properties and catalytic activity for cyclohexane oxidation. Dalton Trans. 2011, 40, 2822–2836. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kozlov, Y.N.; Shul’pina, L.S.; Lyakin, Q.Y.; Kirillov, A.M.; Talsi, E.P.; Pombeiro, A.J.L.; Shul’pin, G.B. Remarkably fast oxidation of alkanes by hydrogen peroxide catalyzed by a tetracopper(II) triethanolaminate complex: Promoting effects of acid co-catalysts and water, kinetic and mechanistic features. J. Catal. 2009, 268, 26–38. [Google Scholar] [CrossRef]
- Gruenwald, K.R.; Kirillov, A.M.; Haukka, M.; Sanchiz, J.; Pombeiro, A.J.L. Mono-, di- and polynuclear copper(II) compounds derived from N-butyldiethanolamine: Structural features, magnetism and catalytic activity for the mild peroxidative oxidation of cyclohexane. Dalton Trans. 2009, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Karabach, Y.Y.; Kirillov, A.M.; Haukka, M.; Kopylovich, M.N.; Pombeiro, A.J.L. Copper(II) coordination polymers derived from triethanolamine and pyromellitic acid for bioinspired mild peroxidative oxidation of cyclohexane. J. Inorg. Biochem. 2008, 102, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, C.; Karabach, Y.Y.; Kirillov, A.M.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. Supramolecular assemblies of trinuclear triangular copper(II) secondary building units through hydrogen bonds. Generation of different metal−organic frameworks, valuable catalysts for peroxidative oxidation of alkanes. Inorg. Chem. 2007, 46, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.R.; Lasri, J.; Guedes da Silva, M.F.C.; da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Mild alkane C–H and O–H oxidations catalysed by mixed-N,S copper, iron and vanadium systems. Appl. Catal. Gen. 2011, 402, 110–120. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; Kirillov, A.M.; Guedes da Silva, M.F.C.; da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. New FeII and CuII complexes bearing azathia macrocycles—Catalyst precursors for mild peroxidative oxidation of cyclohexane and 1-phenylethanol. Eur. J. Inorg. Chem. 2011, 3781–3790. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Suss-Frank, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Pombeiro, A.J.L. Radical formation in the [MeReO3]-catalyzed aqueous peroxidative oxidation of alkanes: A theoretical mechanistic study. Inorg. Chem. 2009, 48, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, M.V.; Kuznetsov, M.L.; Kozlov, Y.N.; Shul’pina, L.S.; Kitaygorodskiy, A.; Pombeiro, A.J.L.; Shul’pin, G.B. Participation of oligovanadates in alkane oxidation with H2O2 catalyzed by vanadate anion in acidified acetonitrile: Kinetic and DFT studies. ACS Catal. 2011, 1, 1511–1520. [Google Scholar] [CrossRef]
- Kirilova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Sutradhar, M.; Mukherjee, G.; Drew, M.G.B.; Ghosh, S. Simple general method of generating non-oxo, non-amavadine model octacoordinated vanadium(IV) complexes of some tetradentate ONNO chelating ligands from various oxovanadium(IV/V) compounds and structural characterization of one of them. Inorg. Chem. 2007, 46, 5069–5075. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Roy Barman, T.; Mukherjee, G.; Drew, M.G.B.; Ghosh, S. Oxidoalkoxidovanadium(V) complexes: Synthesis, characterization and comparison of X-ray crystal structures. Polyhedron 2012, 34, 92–101. [Google Scholar] [CrossRef]
- Bruker. APEX2 & SAINT; AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystall. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Redox potential parameterization in coordination compounds with polydentate scorpionate and benzene ligands. Electrochim. Acta 2012, 82, 478–483. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L.; Guedes da Silva, M.F.C.; Lemos, M.A.N.D.A. Electron-transfer induced isomerizations of coordination compounds. Coord. Chem. Rev. 2001, 219, 53–80. [Google Scholar] [CrossRef]
- Silva, M.E.N.P.R.A.; Pombeiro, A.J.L.; Fraústo da Silva, J.J.R.; Herrmann, R.; Deus, N.; Castilho, T.J.; Guedes da Silva, M.F.C. Redox potential and substituent effects at ferrocene derivatives. Estimates of Hammett σp and Taft polar σ substituent constants. J. Organomet. Chem. 1991, 421, 75–90. [Google Scholar]
- Silva, M.E.N.P.R.A.; Pombeiro, A.J.L.; Fraústo da Silva, J.J.R.; Herrmann, R.; Deus, N.; Bozak, R.E. Redox potential and substituent effects in ferrocene derivatives: II. J. Organomet. Chem. 1994, 480, 81–90. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. Characterization of coordination compounds by electrochemical parameters. Eur. J. Inorg. Chem. 2007, 1473–1482. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. Electron-donor/acceptor properties of carbynes, carbenes, vinylidenes, allenylidenes and alkynyls as measured by electrochemical ligand parameters. J. Organomet. Chem. 2005, 690, 6021–6040. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).