Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model
Abstract
1. Introduction
2. Results and Discussion
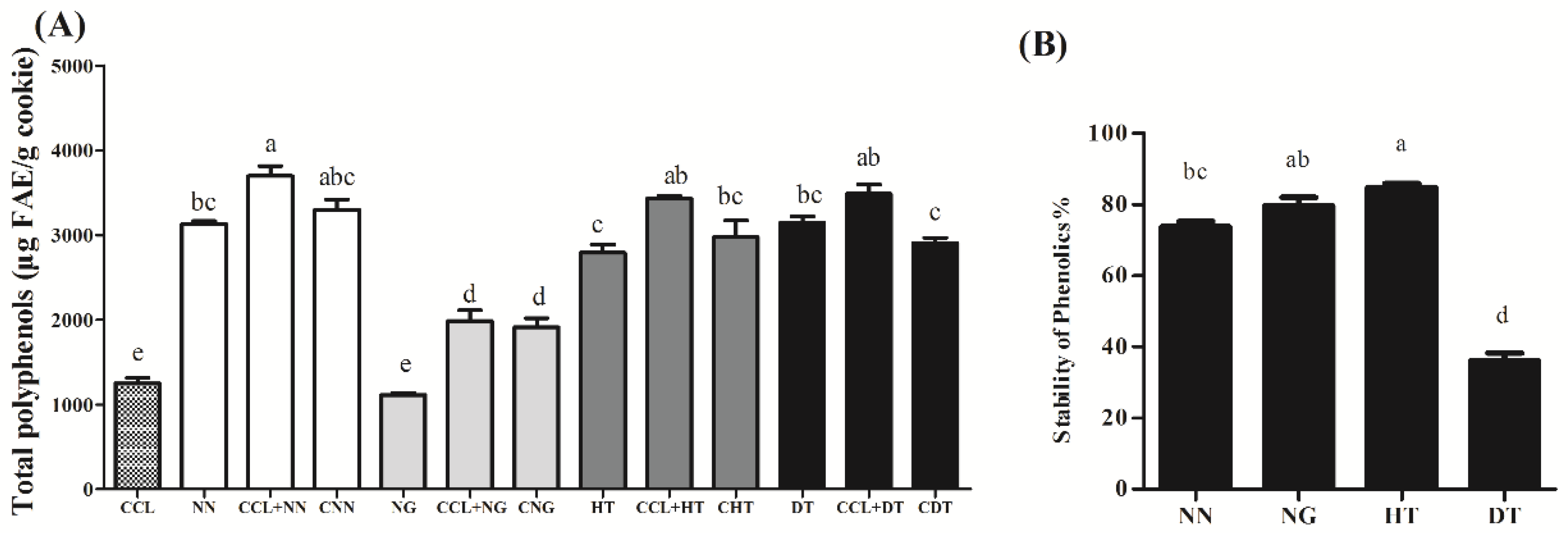
2.1. Stability of NN, NG, HT, and DT during Baking Process of Cookie Model
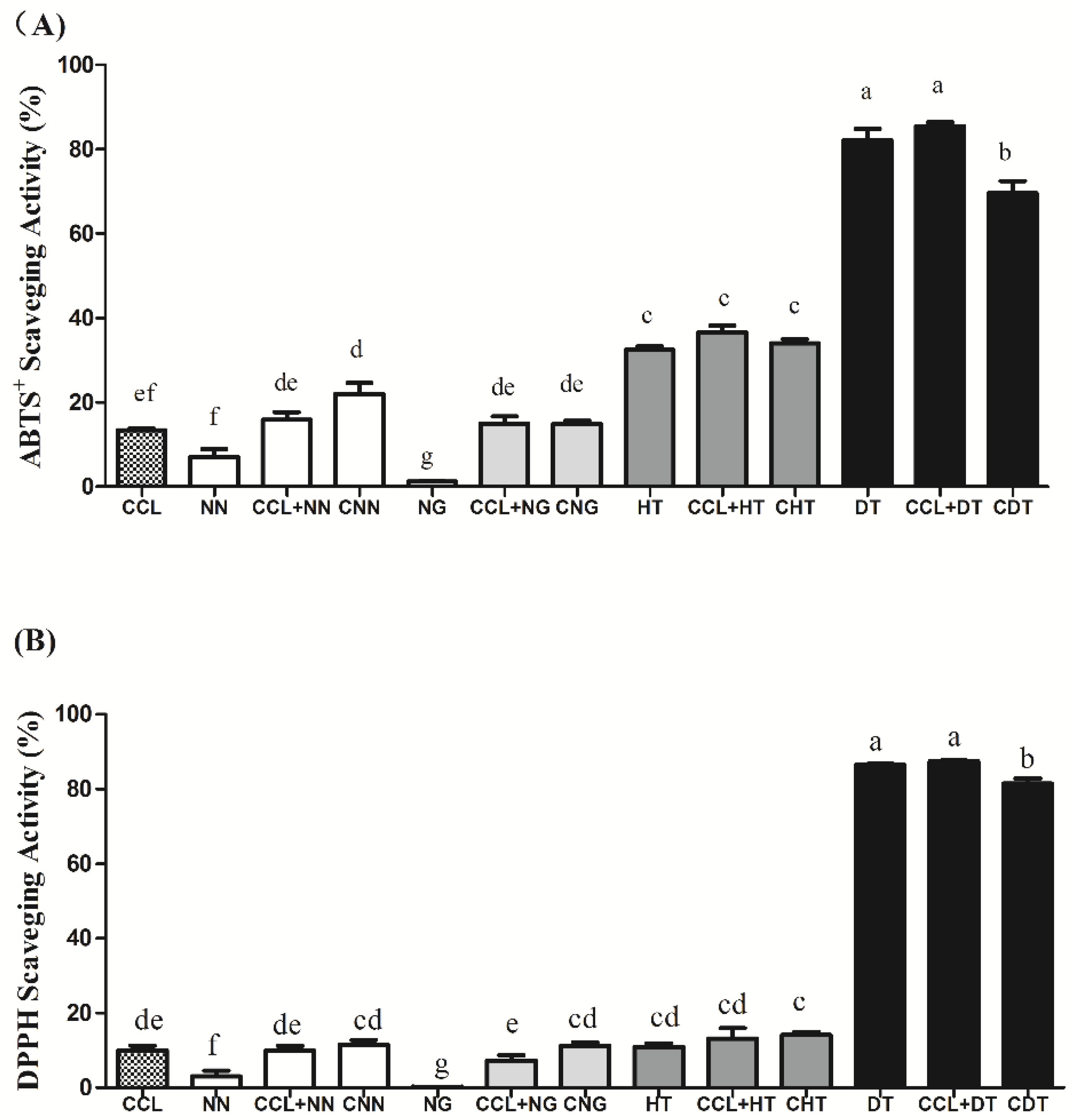
2.2. ABTS+ and DPPH Radical Scavenging Activity
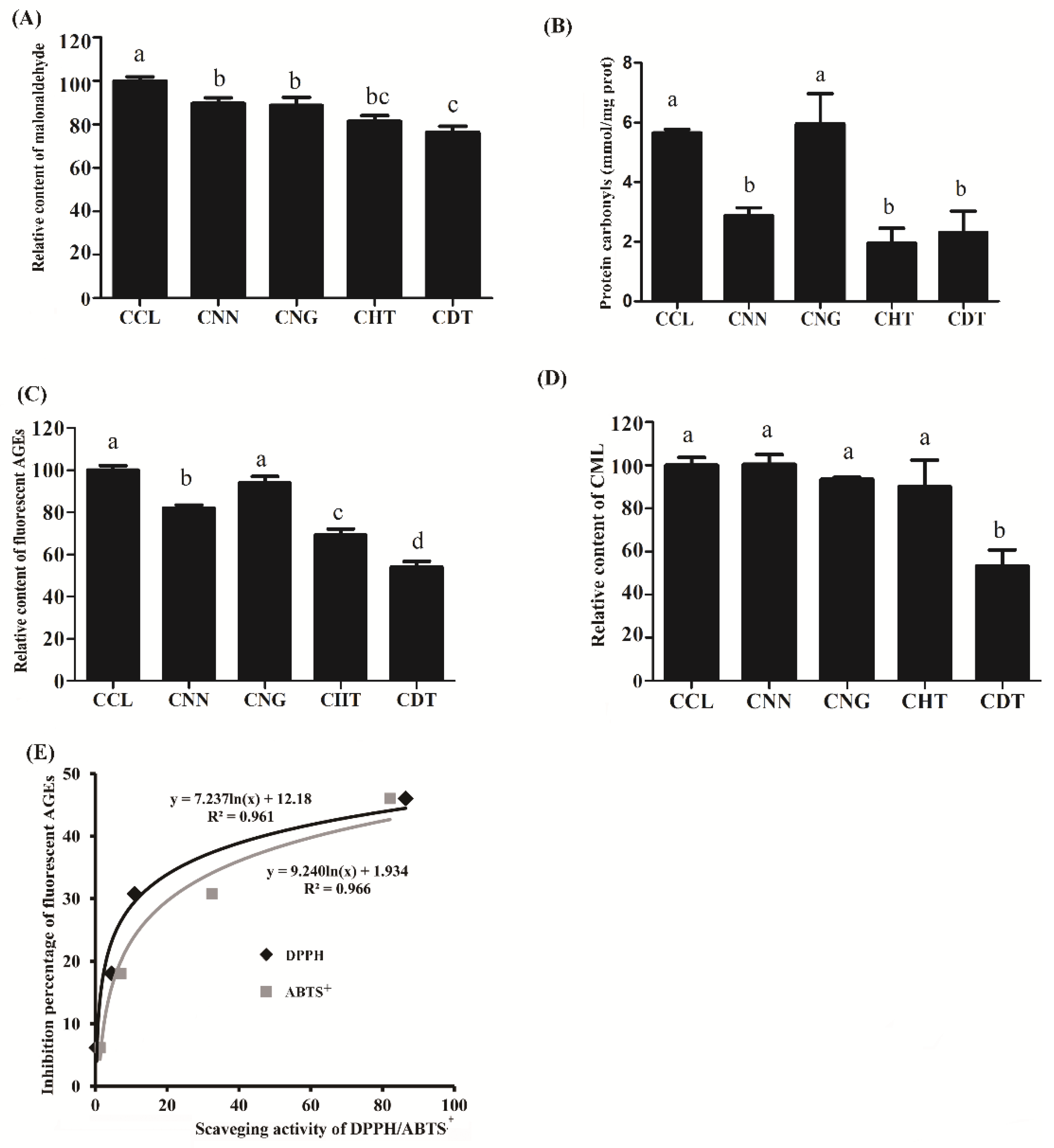
2.3. Impact of Selected Flavonoids on Toxicant Formation in Cookie Model
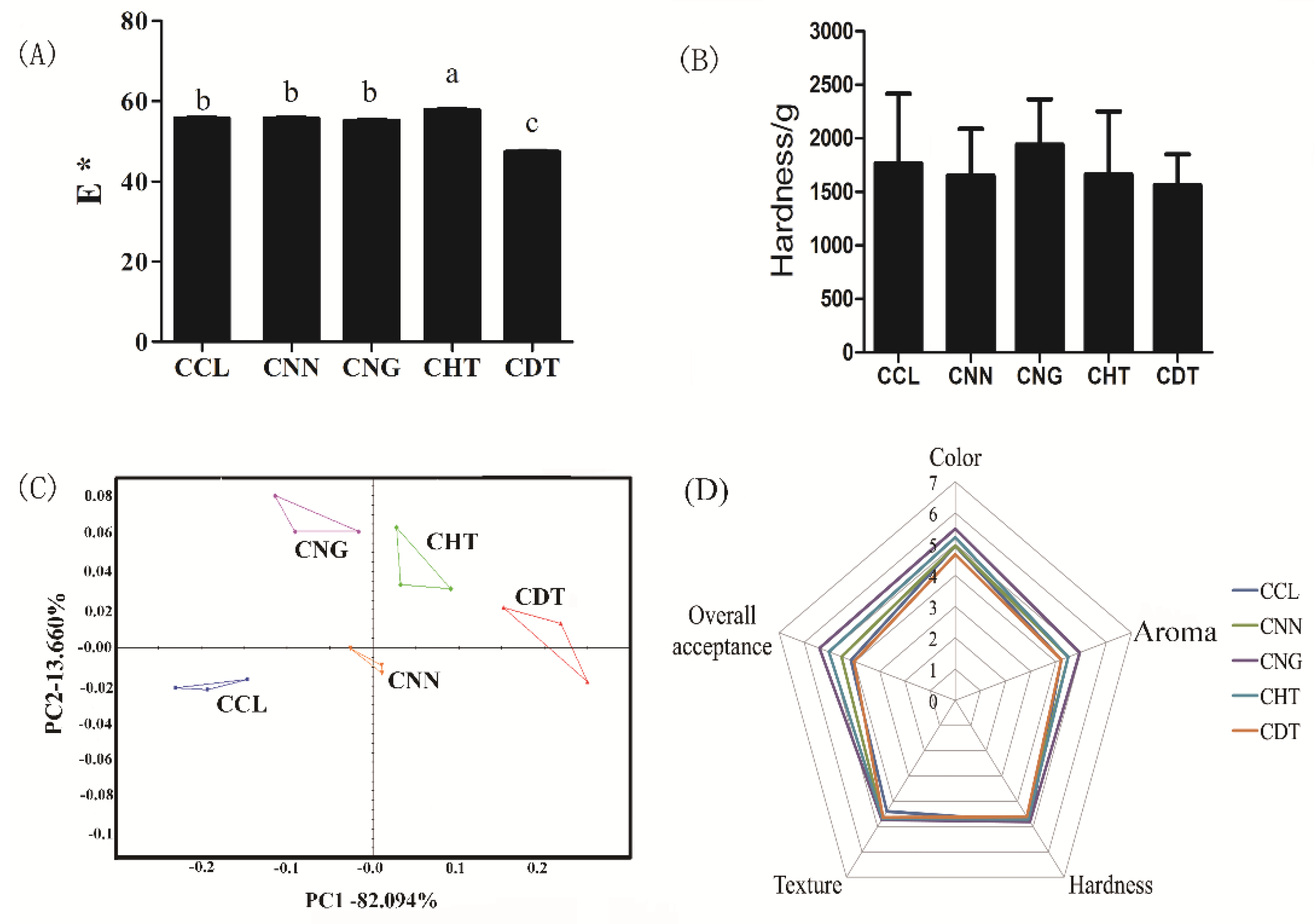
2.4. Quality Attributes for Cookies Fortified with Selected Flavonoids
3. Materials and Methods
3.1. Materials
3.2. Preparation of Cookie Model
3.3. Stability of Selected Flavonoids during Cookie Baking Proces
3.4. Determination of Total Polyphenol Content (TPC)
3.5. Antioxidant Capacity of Cookie Models
3.6. Measurement of Lipid Oxidation and Protein Carbonyl Content
3.7. Determination of Advanced Glycation Endproducts in Cookie Samples
3.8. Determination of Selected Quality Attributes of Cookie Samples
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, B.; Zhao, Y.; Wang, X.; Fan, D.; Cheng, K.; Wang, M. Unraveling the inhibitory effect of dihydromyricetin on heterocyclic aromatic amines formation. J. Sci. Food. Agric. 2018, 98, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Alipour, B.; Rashidkhani, B.; Edalati, S. Dietary flavonoid intake, total antioxidant capacity and lipid oxidative damage: A cross-sectional study of Iranian women. Nutrition 2015, 32, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, W.; Nguyen, T.A.; Zhao, J. Empirical, thermodynamic and quantum-chemical investigations of inclusion complexation between flavanones and (2-hydroxypropyl)-cyclodextrins. Food Chem. 2012, 134, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Jabbari, A. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf. A 2016, 489, 392–399. [Google Scholar] [CrossRef]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- Teng, J.; Li, Y.; Yu, W.; Zhao, Y.; Hu, X.; Tao, N.-P.; Wang, M. Naringenin, a common flavanone, inhibits the formation of AGEs in bread and attenuates AGEs-induced oxidative stress and inflammation in RAW264.7 cells. Food Chem. 2018, 269, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Y.; Niu, X.; Tang, H.; Meydani, S.N.; Wu, D. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. J. Nutr. Biochem. 2017, 54, 130. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, S.O.; Adelani, I.B.; Bankole, G.E.; Rotimi, O.A. Naringin enhances reverse cholesterol transport in high fat/low streptozocin induced diabetic rats. Biomed. Pharmacot. 2018, 101, 430. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Taléns-Visconti, R.; Magraner-Pardo, L.; Peris, J.E. Hesperetin induces melanin production in adult human epidermal melanocytes. Food Chem. Toxicol. 2015, 80, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Ma, Y.; Lin, W.; Xu, K.; Chen, M. Study on the structure–activity of dihydromyricetin and its new production. J. Therm. Anal. Calorim. 2014, 116, 241–248. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, F.; Thakur, K.; Ci, A.T.; Wang, H.; Zhang, J.G.; Wei, Z.J. Effect of natural polyphenol on the oxidative stability of pecan oil. Food Chem. Toxicol. 2018, 119, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S.S.; Santos, N.A.; Santos, I.M.G.; Souza, A.L.; Souza, A.G.; Queiroz, N. Caffeic and ferulic acids: An investigation of the effect of antioxidants on the stability of soybean biodiesel during storage. Fuel 2013, 107, 641–646. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kawahara, S. Malonaldehyde content in some breads and its change during storage. J. Food Hyg. Soc. Jpn. 2005, 46, 121. [Google Scholar] [CrossRef]
- Masahiro, T.; Risa, M.; Harutaka, Y.; Kazuhiro, C. Novel antioxidants isolated from Perilla frutescens Britton var. crispa (Thunb.). Biosci. Biotechnol. Biochem. 1996, 60, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Teng, J.; El-Nezami, H.S.; Wang, M. Impact of resveratrol, epicatechin and rosmarinic acid on fluorescent AGEs and cytotoxicity of cookies. J. Funct. Foods 2018, 40, 44–50. [Google Scholar] [CrossRef]
- Hull, G.L.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl) lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Yu, P.; Xu, X.B.; Yu, S.J. Inhibitory effect of sugarcane molasses extract on the formation of Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine. Food Chem. 2017, 221, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Charissou, A.; Ait-Ameur, L.; Birlouez-Aragon, I. Kinetics of formation of three indicators of the Maillard reaction in model cookies: Influence of baking temperature and type of sugar. J. Agric. Food Chem. 2007, 55, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Wang, M. Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J. Agric. Food Chem. 2014, 62, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Padhi, E.M.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Zhu, F.; Sakulnak, R.; Wang, S. Effect of black tea on antioxidant, textural, and sensory properties of Chinese steamed bread. Food Chem. 2016, 194, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.H.; Aziz, N.A.A.; Azahari, B. Physico-chemical characteristics and sensory evaluation of wheat bread partially substituted with banana (Musa acuminata X balbisiana cv. Awak) pseudo-stem flour. Food Chem. 2013, 139, 532–539. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, J.; Liu, X.; Hu, X.; Zhao, Y.; Tao, N.-P.; Wang, M. Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model. Molecules 2018, 23, 2184. https://doi.org/10.3390/molecules23092184
Teng J, Liu X, Hu X, Zhao Y, Tao N-P, Wang M. Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model. Molecules. 2018; 23(9):2184. https://doi.org/10.3390/molecules23092184
Chicago/Turabian StyleTeng, Jing, Xuduo Liu, Xiaoqian Hu, Yueliang Zhao, Ning-Ping Tao, and Mingfu Wang. 2018. "Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model" Molecules 23, no. 9: 2184. https://doi.org/10.3390/molecules23092184
APA StyleTeng, J., Liu, X., Hu, X., Zhao, Y., Tao, N.-P., & Wang, M. (2018). Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model. Molecules, 23(9), 2184. https://doi.org/10.3390/molecules23092184




