Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study
Abstract
1. Introduction
2. Results
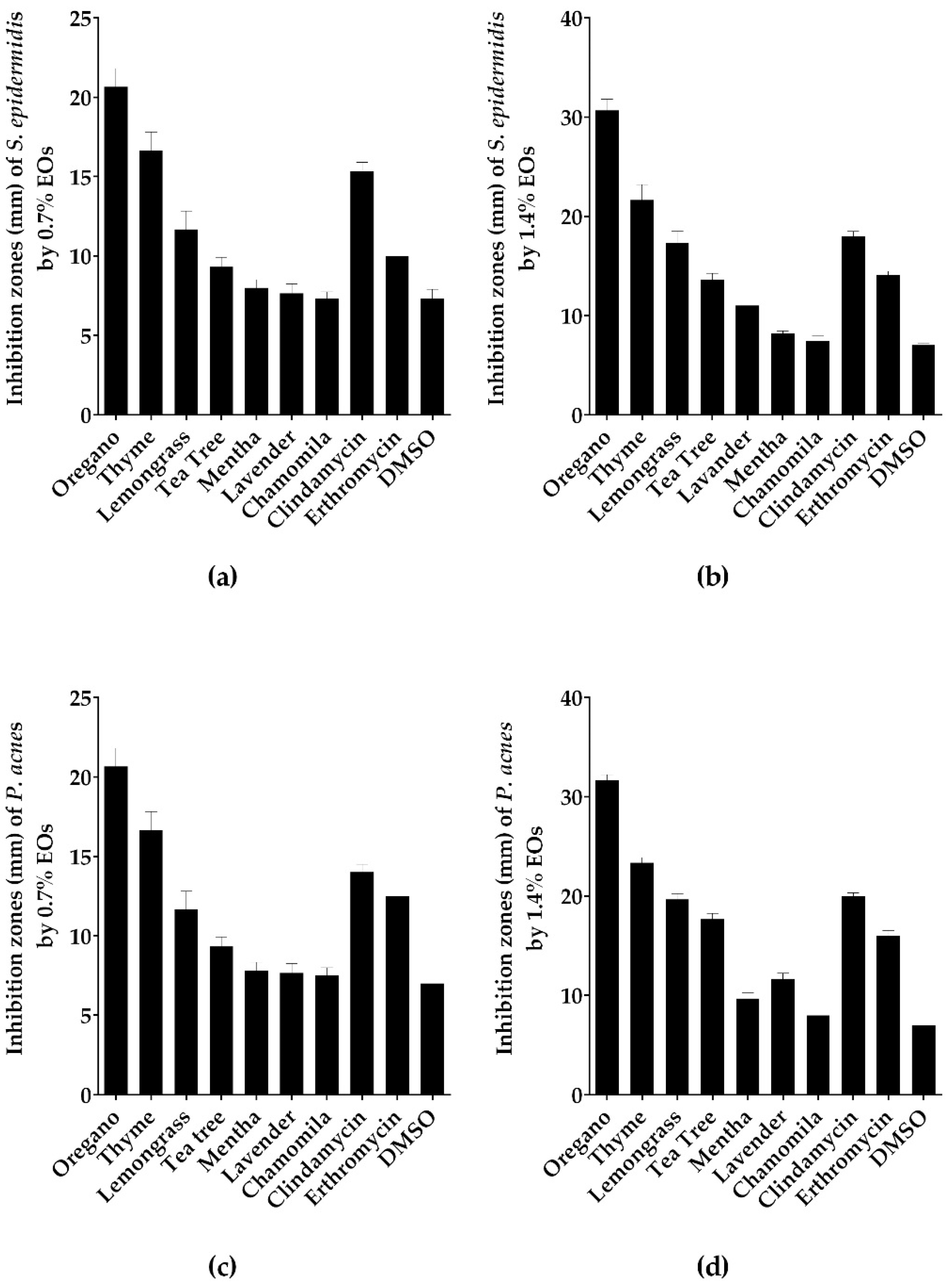
2.1. Screening of Antibacterial Activity of Tested EOs Using Agar Disc Diffusion Method
2.2. Antimicrobial Activity of the Tested EOs Using Minimal Inhibitory Concentration (MIC) Method
2.3. The Bactericidal and Anti-Biofilm Activities of the Tested EOs
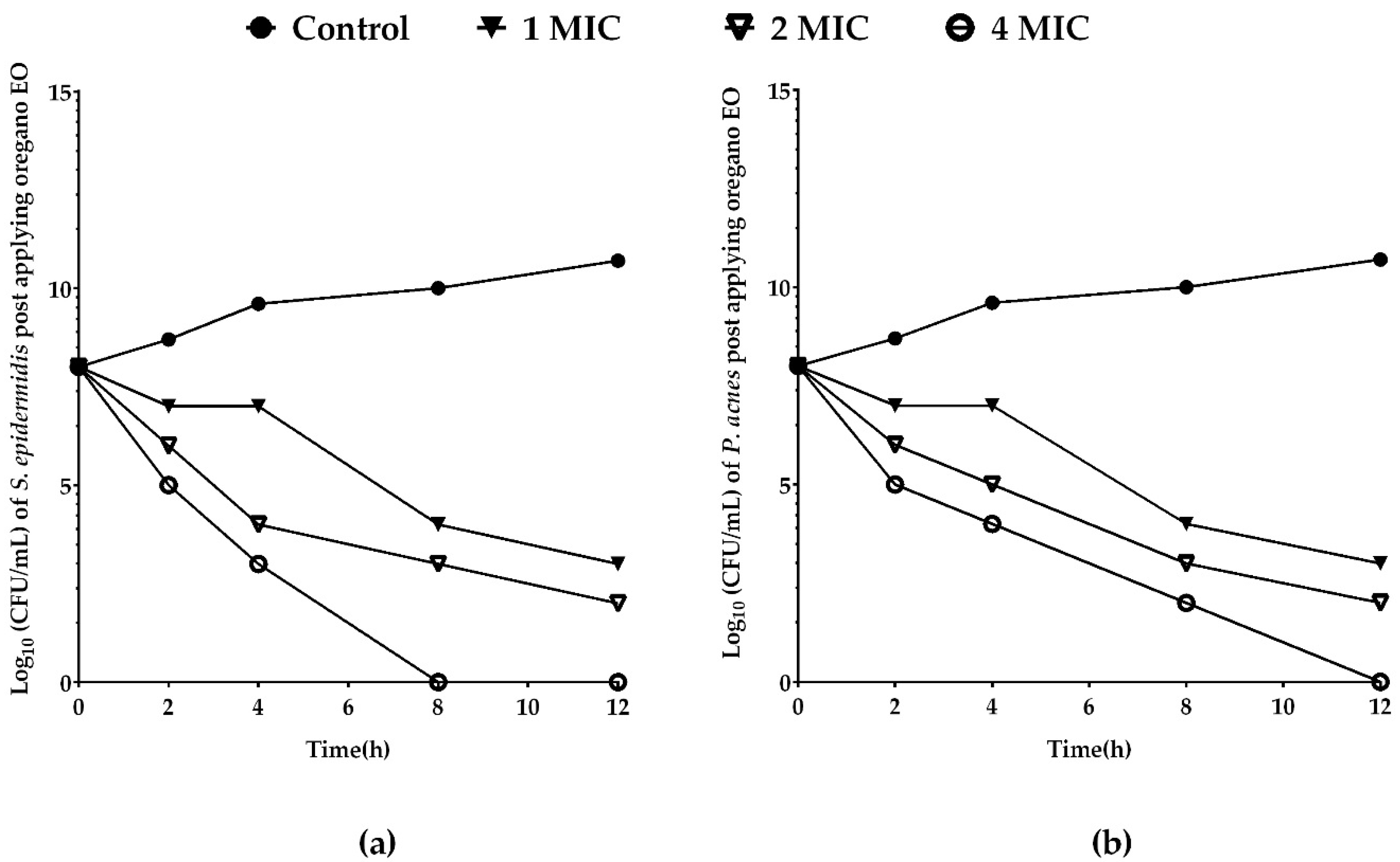
2.4. Killing Dynamics of Oregano EO against S. epidermidis and P. acnes
2.5. Chemical Composition of Oregano and Thyme EOs
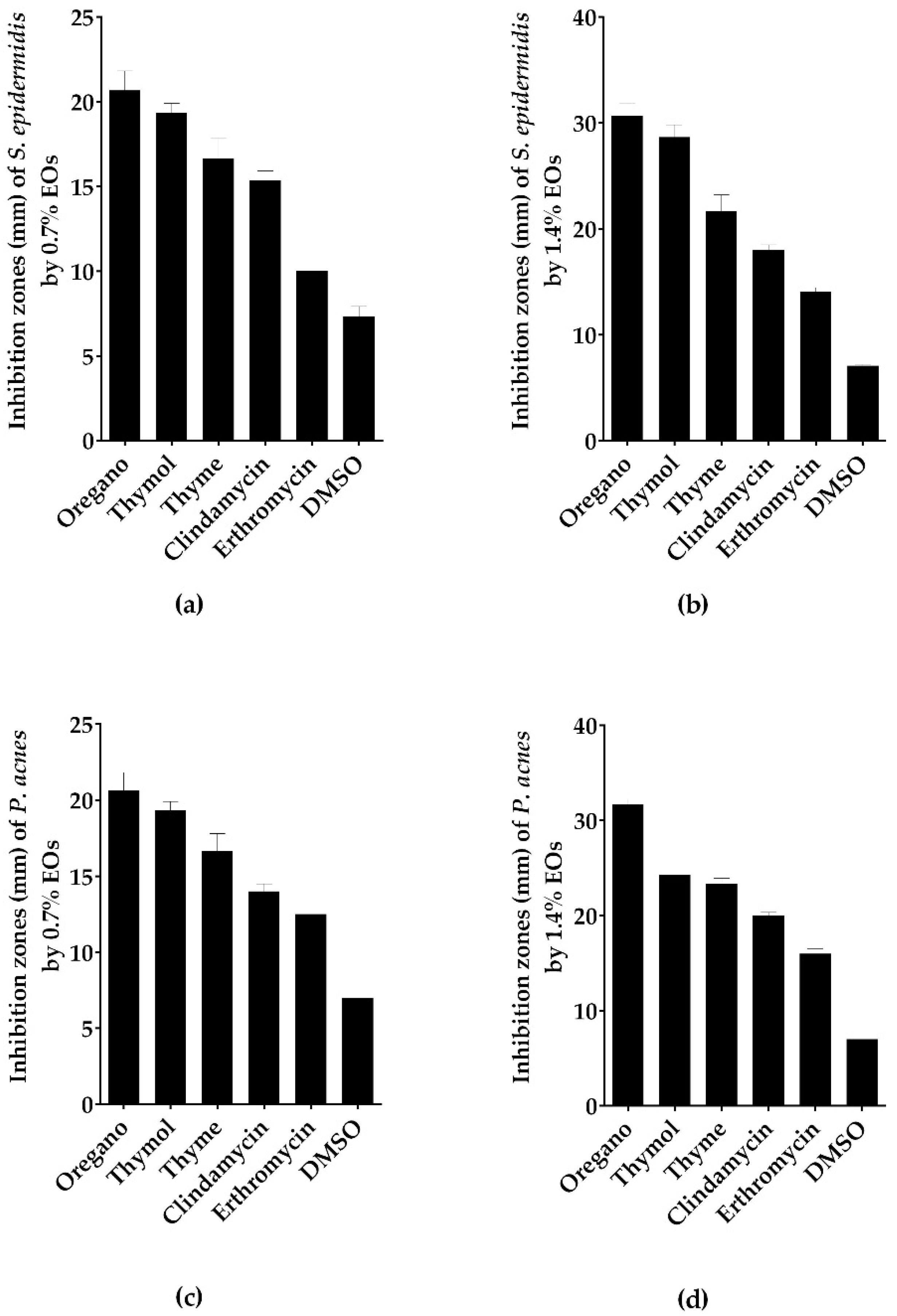
2.6. Antimicrobial Activity of Thymol Using In Vitro Disc Diffusion, MIC, MBC and MBIC Assays
2.7. Nanoemulsion Formulation of Oregano EO
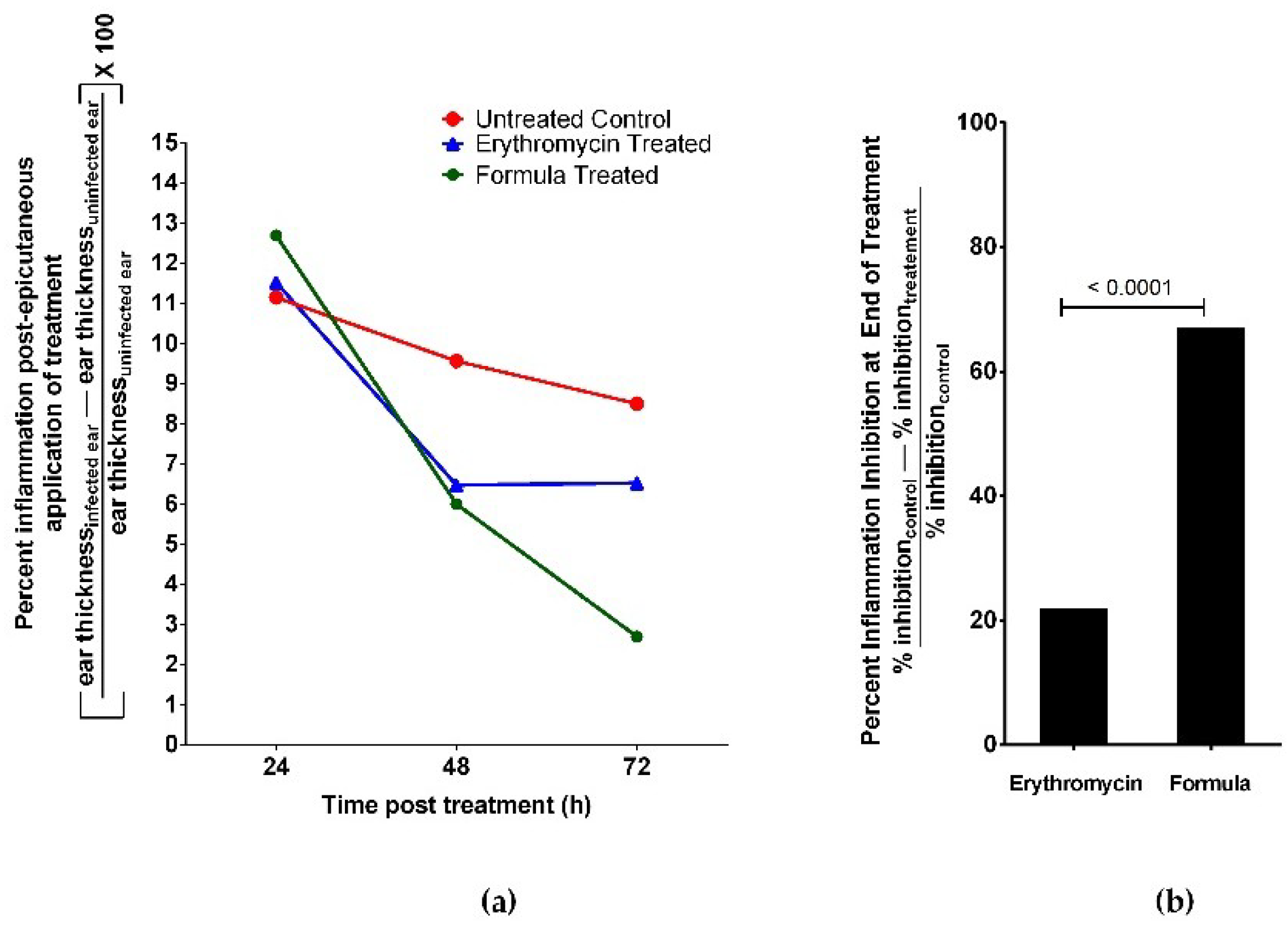
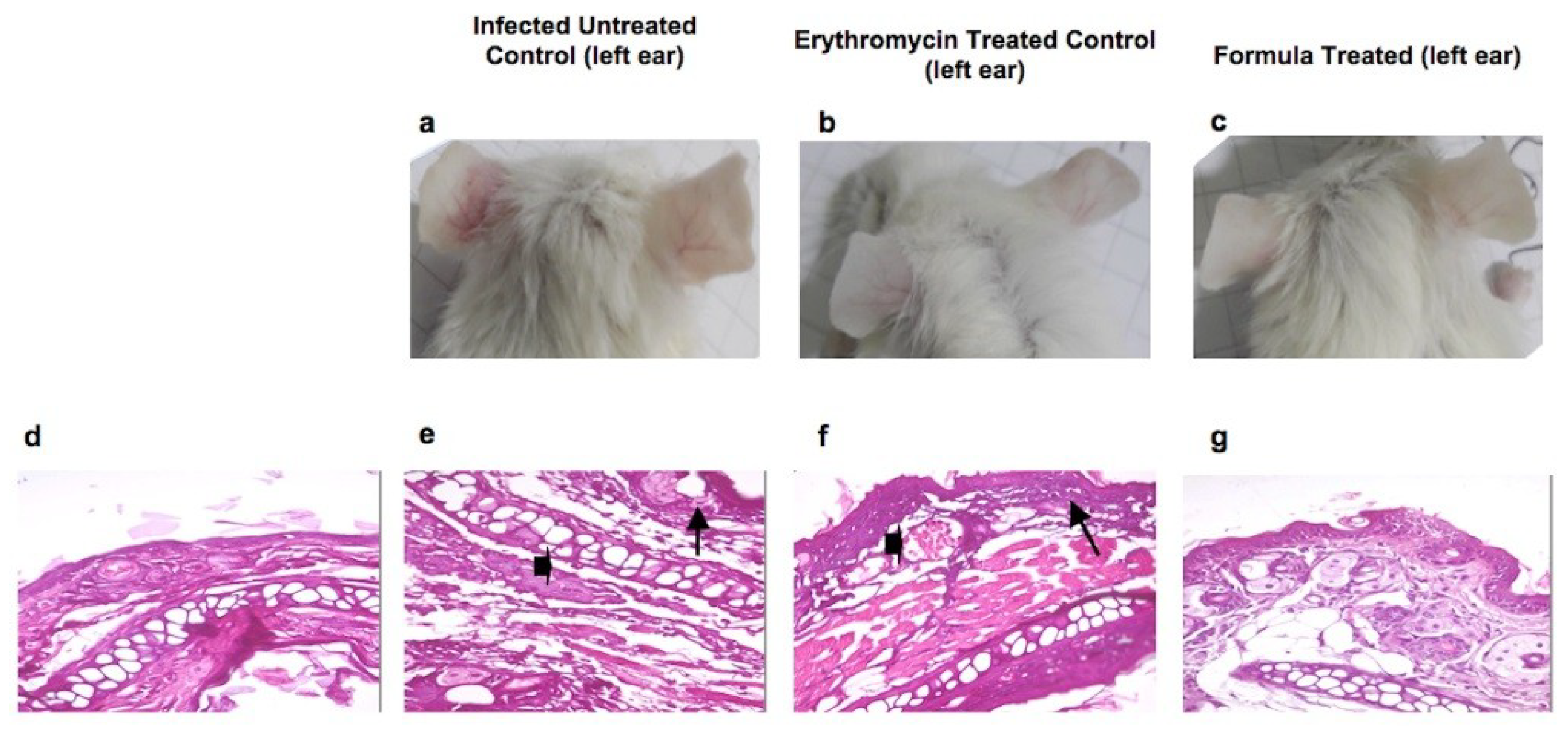
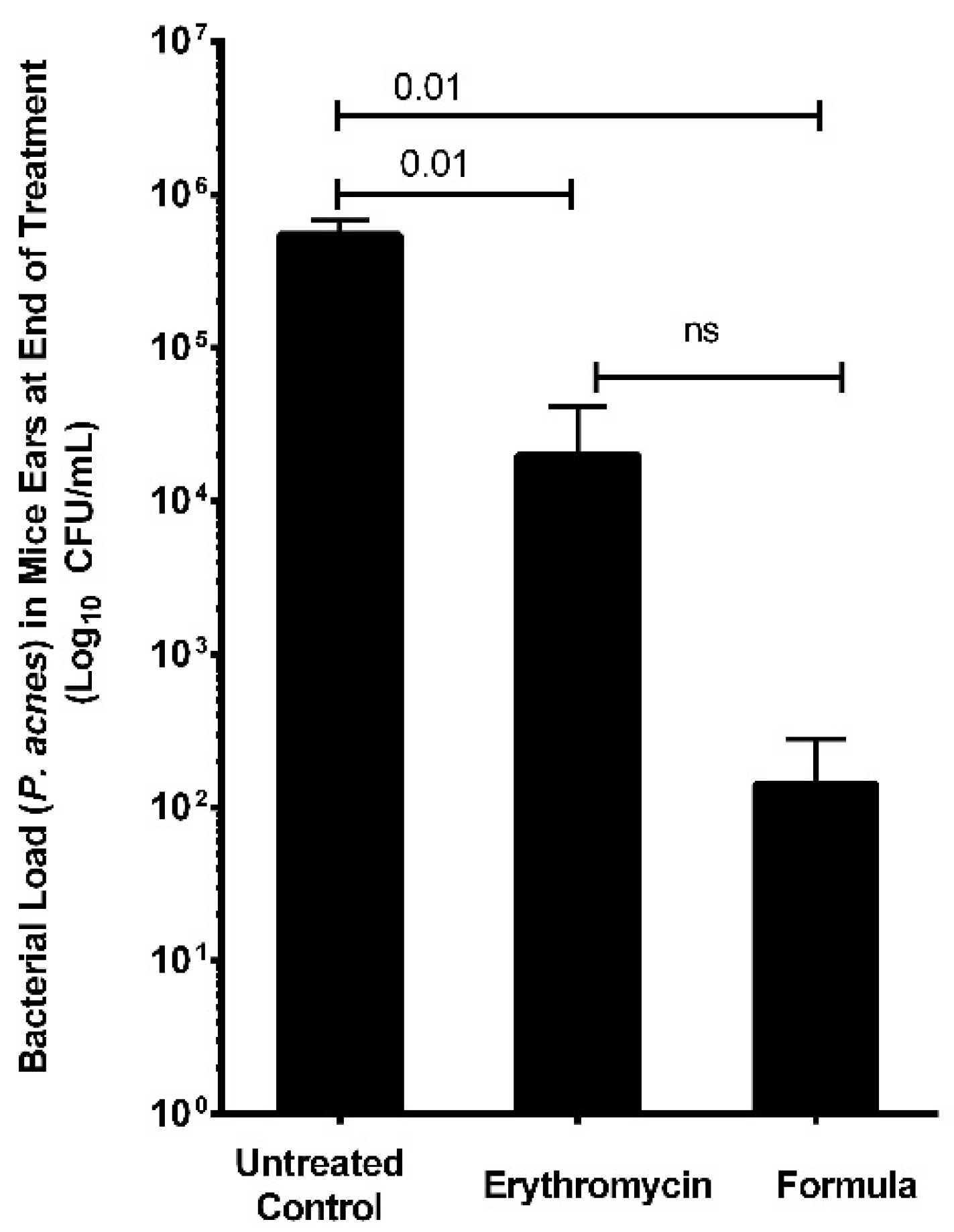
2.8. Assessment of In Vivo Antimicrobial and Healing Activities of Oregano EO Nanoemulsion Using In Vivo Acne Mouse Model
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Bacterial Strains and Culturing
4.3. Animals
4.4. Screening of Antibacterial Activities of EOs by Disc Diffusion Method
4.5. Determination of the MIC and MBC of the EOs
4.6. Minimum Biofilm Inhibitory Concentration (MBIC)
4.7. Determination of Kill Kinetics
4.8. Gas Chromatography-Mass Spectroscopy
4.9. Development of Nanoemulsion
4.10. In Vivo Antiacne Experiment
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Hull, P.R.; D’Arcy, C. Acne, depression, and suicide. Dermatol. Clin. 2005, 23, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Nishijima, S.; Kurokawa, I.; Katoh, N.; Watanabe, K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium acnes and Staphylococcus epidermidis isolated from acne lesions. J. Dermatol. 2000, 27, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Chularojanamontri, L.; Tuchinda, P.; Kulthanan, K.; Pongparit, K. Moisturizers for acne: What are their constituents? J. Clin. Aesthet. Dermatol. 2014, 7, 36–44. [Google Scholar] [PubMed]
- Patel, M.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: A review. J. Drugs Dermatol. JDD 2010, 9, 655–664. [Google Scholar] [PubMed]
- Kinney, M.A.; Yentzer, B.A.; Fleischer, A.B., Jr.; Feldman, S.R. Trends in the treatment of acne vulgaris: Are measures being taken to avoid antimicrobial resistance? J. Drugs Dermatol. JDD 2010, 9, 519–524. [Google Scholar] [PubMed]
- Högberg, L.D.; Heddini, A.; Cars, O. The global need for effective antibiotics: Challenges and recent advances. Trends Pharmacol. Sci. 2010, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Camele, I.; Elshafie, H.S.; de Martino, L.; Pellegrino, C.; Grulova, D.; de Feo, V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the Southern Apennines (Italy). Chem. Biodivers. 2014, 11, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Srivastava, S.; Mishra, N.; Yadav, N.P. New perspectives on antiacne plant drugs: Contribution to modern therapeutics. BioMed Res. Int. 2014, 2014, 301304. [Google Scholar] [CrossRef] [PubMed]
- González-Tejero, M.; Casares-Porcel, M.; Sánchez-Rojas, C.; Ramiro-Gutiérrez, J.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.; Censorii, E.; de Pasquale, C.; Della, A. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Budhiraja, A.; Dhingra, G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015, 22, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.; Pannowitz, D.; Barnetson, R. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [PubMed]
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar] [PubMed]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.; Horhat, F. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.-P.; Bégin, A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37, 155–162. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, antiviral and antifungal activity of essential oils: Mechanisms and applications. In Antimicrobial Compounds; Springer: Berlin, Germany, 2014; pp. 51–81. [Google Scholar]
- Elshafie, H.S.; Mancini, E.; Sakr, S.; de Martino, L.; Mattia, C.A.; de Feo, V.; Camele, I. Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J. Med. Food 2015, 18, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Ghaderi, L.; Moghimi, R.; Aliahmadi, A.; McClements, D.J.; Rafati, H. Development of antimicrobial nanoemulsion-based delivery systems against selected pathogenic bacteria using a thymol-rich Thymus daenensis essential oil. J. Appl. Microbiol. 2017, 123, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; de Feo, V.; Camele, I. Cytotoxic activity of Origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liang, Q.; Yu, F.; Xu, J.; Zhao, Q.; Sun, B. Synthesis and characterization of novel amphiphilic copolymer stearic acid-coupled F127 nanoparticles for nano-technology based drug delivery system. Colloids Surf. B Biointerfaces 2011, 88, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Lee, K.C.; Lee, S.-J.; Kim, D.W.; Lee, W.J. HR-1 Mice: A new inflammatory acne mouse model. Ann. Dermatol. 2015, 27, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Goh, C.C.; Keeble, J.L.; Qin, J.S.; Roediger, B.; Jain, R.; Wang, Y.; Chew, W.K.; Weninger, W.; Ng, L.G. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 2012, 7, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- AVMA—American Veterinary Medical Association. AVMA Guidelines on Euthanasia [Internet]; AVMA: Schaumburg, IL, USA, 2007. [Google Scholar]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- CLSI. CLSI supplement M100. In Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. JoVE 2011, 30, 2437. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.; Weeks, L.; Aldridge, K. Comparison of kill-kinetic studies with agar and broth microdilution methods for determination of antimicrobial activity of selected agents against members of the Bacteroides fragilis group. J. Clin. Microbiol. 1987, 25, 645–649. [Google Scholar] [PubMed]
- Farag, M.A.; Wessjohann, L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012, 77, C1179–C1184. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lv, X.-D.; Wang, G.-E.; Li, Y.-F.; Kurihara, H.; He, R.-R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. Nutr. 2014, 65, 594–601. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the used oregano EO are available from the authors. |
| Essential Oils | MIC | |
|---|---|---|
| S. epidermidis (mg/mL) | P. acnes (mg/mL) | |
| Oregano | 0.67 | 0.34 |
| Thyme | 1.30 | 0.65 |
| Lemongrass | 1.22 | 1.22 |
| Tea tree | 1.27 | 1.28 |
| Lavender | 2.52 | 2.52 |
| Mentha | 5.28 | 2.60 |
| Chamomile | 6.22 | 3.18 |
| Essential Oils | MBC | |
|---|---|---|
| S. epidermidis (mg/mL) | P. acnes (mg/mL) | |
| Oregano | 1.34 | 0.672 |
| Thyme | 2.60 | 1.30 |
| Lemongrass | 2.44 | 2.44 |
| Tea tree | 5.10 | 2.55 |
| Essential Oils | MBIC |
|---|---|
| mg/mL | |
| Oregano | 1.344 |
| Thyme | 2.60 |
| Lemongrass | 2.44 |
| Tea tree | 2.55 |
| Compound | Oregano EO Content % | Thyme EO Content % | Retention Time | Retention Index |
|---|---|---|---|---|
| Thymol a | 99.44 | 72.08 | 12.81 | 1280 |
| p-cymene a | 0.2 | 25.18 | 8.79 | 1005 |
| γ-terpinene | 0.03 | 2.52 | 9.31 | 1038 |
| α-Thujene | 0.01 | 0.01 | 7.15 | 911 |
| Cineole a | 0.06 | − | 8.96 | 1012 |
| Thymol isomer | 0.07 | 0.00 | 12.68 | 1274 |
| Isocaryophyllene a | 0.04 | − | 14.34 | 1428 |
| Carvacrol | − | 0.07 | 12.67 | 1272 |
| Acne-Causing Bacteria | MIC (mg/mL) | MBC (mg/mL) | MBIC (mg/mL) |
|---|---|---|---|
| S. epidermidis | 0.7 | 2.8 | 2.8 |
| P. acnes | 0.7 | 1.4 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study. Molecules 2018, 23, 2164. https://doi.org/10.3390/molecules23092164
Taleb MH, Abdeltawab NF, Shamma RN, Abdelgayed SS, Mohamed SS, Farag MA, Ramadan MA. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study. Molecules. 2018; 23(9):2164. https://doi.org/10.3390/molecules23092164
Chicago/Turabian StyleTaleb, Mohammed H., Nourtan F. Abdeltawab, Rehab N. Shamma, Sherein S. Abdelgayed, Sarah S. Mohamed, Mohamed A. Farag, and Mohammed A. Ramadan. 2018. "Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study" Molecules 23, no. 9: 2164. https://doi.org/10.3390/molecules23092164
APA StyleTaleb, M. H., Abdeltawab, N. F., Shamma, R. N., Abdelgayed, S. S., Mohamed, S. S., Farag, M. A., & Ramadan, M. A. (2018). Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study. Molecules, 23(9), 2164. https://doi.org/10.3390/molecules23092164








