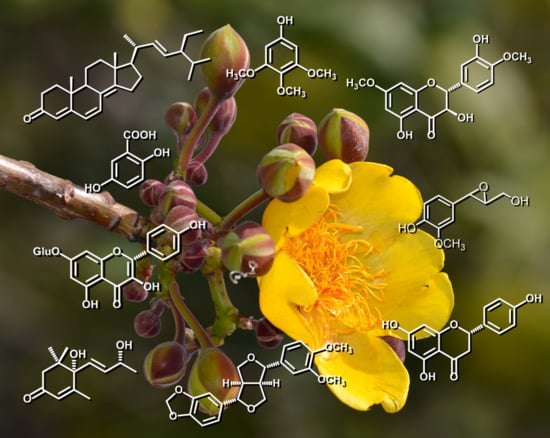
Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity
Abstract
1. Introduction
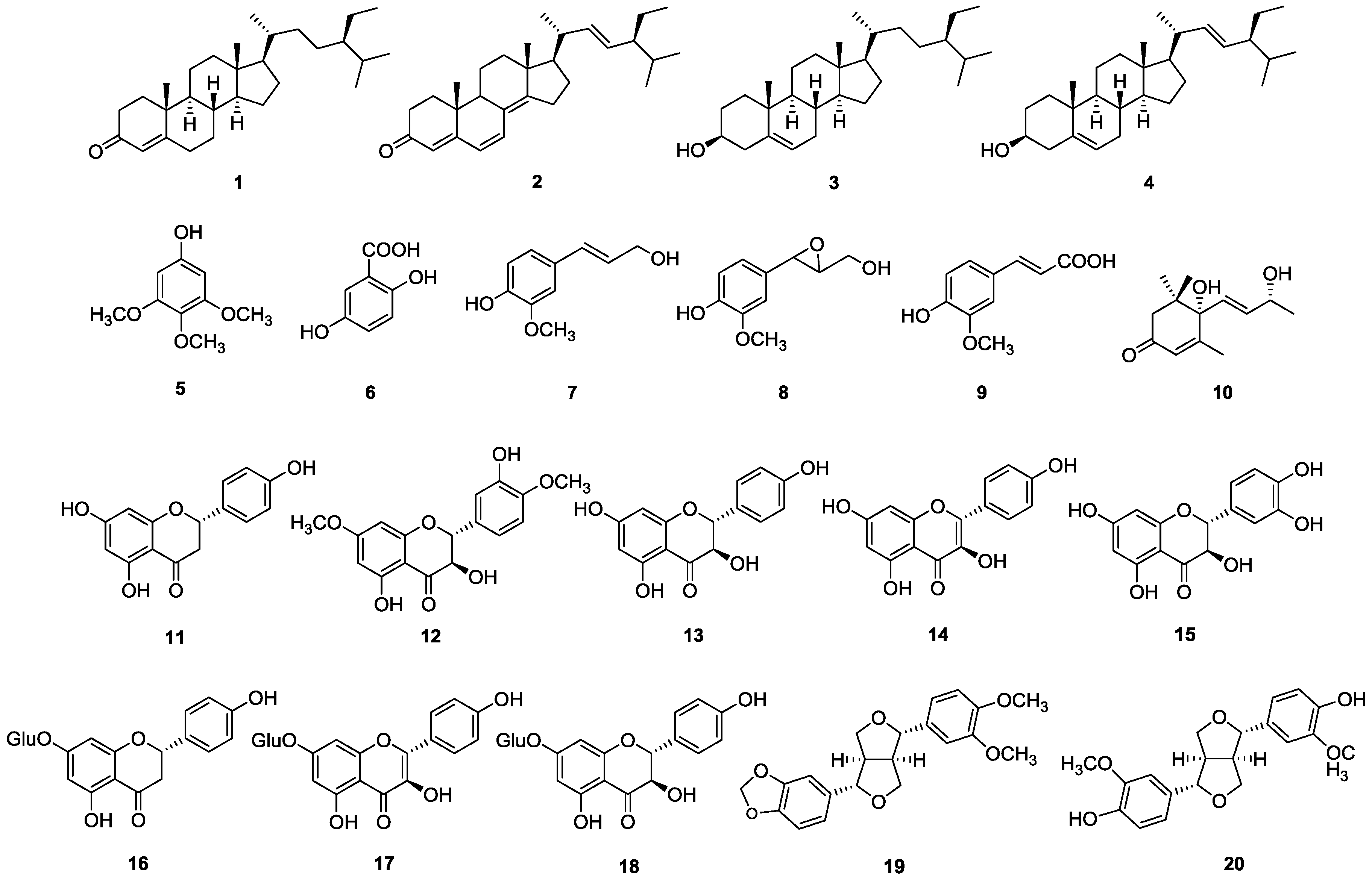
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamien-Meda, A.; Kiendrebeogo, M.; Compaoré, M.; Meda, R.N.T.; Bacher, M.; Koenig, K.; Pacher, T.; Fuehrer, H.-P.; Noedl, H.; Willcox, M.; et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry 2015, 119, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.F.; Knox, J.R. Flavonoids from Cochlospermum gillivraei. Phytochemistry 1975, 14, 2510–2511. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Suresh, K.I.; Rama Rao, B.; Vijaya Saradhi, U.V.R.; Prabhakar Rao, T. Morphological, physico-chemical and structural characterization of gum kondagogu (Cochlospermum gossypium): A tree gum from India. Food Hydrocoll. 2008, 22, 899–915. [Google Scholar] [CrossRef]
- Hongsing, P.; Palanuvej, C.; Ruangrungsi, N. Chemical compositions and biological activities of selected exudate gums. J. Chem. Pharm. Res. 2012, 4, 4174–4180. [Google Scholar]
- Bate-Smith, E.C. Chemotaxonomie der Pflanzen. Bull. Soc. Bot. Mem. 1964, 435. [Google Scholar] [CrossRef]
- Anaga, A.O.; Oparah, N. Investigation of the methanol root extract of Cochlospermum planchonii for pharmacological activities in vitro and in vivo. Pharm. Biol. 2009, 47, 1027–1034. [Google Scholar] [CrossRef]
- Solon, S.; Carollo, C.A.; Brandão, L.F.G.; Macedo, C.; Klein, A.; Dias-Junior, C.A.; Siqueira, J.M. Phenolic derivatives and other chemical compounds from Cochlospermum regium. Quim. Nova 2012, 35, 1169–1172. [Google Scholar] [CrossRef]
- Diallo, B.; Vanhaelen, M.; Vanhaelen-Fastré, R.; Konoshima, T.; Kozuka, M.; Tokuda, H. Studies on inhibitors of skin-tumor promotion. Inhibitory effects of triterpenes from Cochlospermum tinctorium on Epstein-Barr Virus Activation. J. Nat. Prod. 1989, 52, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Diallo, B.; Vanhaelen-Fastré, R.; Vanhaelen, M. Triacylbenzenes and long-chain volatile ketones from Cochlospermum tinctorium rhizome. Phytochemistry 1991, 30, 4153–4156. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Traore, M.; Tinto, H.; Sittie, A.; Mølgaard, P.; Olsen, C.E.; Kharazmi, A.; Christensen, S.B. Antiplasmodial compounds from Cochlospermum tinctorium. J. Nat. Prod. 2002, 65, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Xenofonte de Almeida, S.C.; Gomes de Lemos, L.T.; Rocha Silveira, E.; Loiola Pessoa, O.D. Constituintes químicos voláteis e não-voláteis de Cochlospermum vitifolium (Willdenow) Sprengel. Quim. Nov. 2005, 28, 57–60. [Google Scholar] [CrossRef]
- Achenbach, H.; Blümm, E.; Waibel, R. Vitixanthin and dihydrovitixanthin - new unusual 7′-apocarotenoic acids from. Tetrahedron Lett. 1989, 30, 3059–3060. [Google Scholar] [CrossRef]
- Dixit, B.S.; Srivastava, S.N. Flavonoids and carotenoids of Cochlospermum vitifolium flowers. Fitoterapia 1992, 63, 270. [Google Scholar]
- López, J.A. Flavonoids in Cochlospermum vitifolium Willd (Cochlospermaceae). Ing. Cienc. Quim. 1981, 5, 101–102. [Google Scholar]
- Esposito-avella, M.; Brown, P.; Tejeira, I.; Buitrago, R.; Barrios, L.; Sanchez, C.; Gupta, M.P.; Cedeño, J. Pharmacological screening of Panamanian medicinal plants. Part 1. Int. J. Crude Drug Res. 1985, 23, 17–25. [Google Scholar] [CrossRef]
- Zamora-Martinez, M.C.; de Pascual Pola, C.N. Medicinal plants used in some rural populations of Oaxaca, Puebla and Veracruz, Mexico. J. Ethnopharmacol. 1992, 35, 229–257. [Google Scholar] [CrossRef]
- Monroy-Ortiz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos, 2nd ed.; Universidad Autónoma del Estado de Morelos: Cuernavaca, México, 2007; ISBN 968-878-277-7. [Google Scholar]
- Banos, G.; Perez-Torres, I.; El Hafidi, M. Medicinal agents in the metabolic syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salgado, J.C.; Ortiz-Andrade, R.R.; Aguirre-Crespo, F.; Vergara-Galicia, J.; León-Rivera, I.; Montes, S.; Villalobos-Molina, R.; Estrada-Soto, S. Hypoglycemic, vasorelaxant and hepatoprotective effects of Cochlospermum vitifolium (Willd.) Sprengel: A potential agent for the treatment of metabolic syndrome. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salgado, J.C.; Castillo-España, P.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Estrada-Soto, S. Cochlospermum vitifolium induces vasorelaxant and antihypertensive effects mainly by activation of NO/cGMP signaling pathway. J. Ethnopharmacol. 2010, 130, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Caballero-George, C.; Vanderheyden, P.M.L.; Solis, P.N.; Pieters, L.; Shahat, A.A.; Gupta, M.P.; Vauquelin, G.; Vlietinck, A.J. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine 2001, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Andrade, R.; Torres-Piedra, M.; Sánchez-Salgado, J.C.; García-Jiménez, S.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Gallardo-Ortíz, I.; Estrada-Soto, S. Acute and sub-chronic effects of Cochlospermum vitifolium in blood glucose levels in normoglycemic and STZ-nicotinamide-induced diabetic rats. Rev. Latinoamer. Quím. 2009, 37, 122–132. [Google Scholar]
- Deharo, E.; Baelmans, R.; Gimenez, A.; Quenevo, C.; Bourdy, G. In vitro immunomodulatory activity of plants used by the Tacana ethnic group in Bolivia. Phytomedicine 2004, 11, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Recillas, A.; Mantecón-Reyes, P.; Castillo-España, P.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Estrada-Soto, S. Tracheal relaxation of five medicinal plants used in Mexico for the treatment of several diseases. Asian Pac. J. Trop. Med. 2014, 7, 179–183. [Google Scholar] [CrossRef]
- HEPAMAP A Roadmap for Hepatology Research in Europe: An Overview for Policy Makers. Available online: www.easl.eu/medias/EASLimg/News/3f9dd90221ef292_file.pdf (accessed on 10 July 2018).
- American Liver Foundation The Liver Lowdown–Liver Disease: The Big Picture. Available online: https://liverfoundation.org/for-patients/resources/liver-lowdown/ (accessed on 10 July 2018).
- Wang, F.-S.; Fan, J.-G.; Zhang, Z.; Gao, B.; Wang, H.-Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Yang, H.J.; Lee, J.-Y.; Na, Y.-C.; Kwon, S.-Y.; Kim, Y.-C.; Lee, J.-H.; Jang, H.-J. Effects of β-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis. BMC Complement. Altern. Med. 2014, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-S.; Chen, J.-H.; Leong, P.-K.; Leung, H.-Y.; Chan, W.-M.; Ko, K.-M. β-Sitosterol protects against carbon tetrachloride hepatotoxicity but not Gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules 2014, 19, 17649–17662. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Li, X.-F.; Kang, K.-H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Nafees, S.; Ahmad, S.T.; Arjumand, W.; Rashid, S.; Ali, N.; Sultana, S. Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. J. Pharm. Pharmacol. 2012, 64, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Geng, C.-A.; Ma, Y.-B.; Zhang, X.-M.; Chen, J.-J. Three new secoiridoids, swermacrolactones A–C and anti-hepatitis B virus activity from Swertia macrosperma. Fitoterapia 2013, 89, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Gerin, F.; Erman, H.; Erboga, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pan, Z.; Dong, M.; Yu, C.; Niu, Y. Ferulic acid suppresses activation of hepatic stellate cells through ERK1/2 and Smad signaling pathways in vitro. Biochem. Pharmacol. 2015, 93, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Jin, X.; Hong, Y.-S.; Lee, J.J. An isoaurone and other constituents from trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-κB. J. Nat. Prod. 2010, 73, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-R.; Liu, C.-J.; Yeh, C.-C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Kim, N.H.; Kim, J.-Y.; Park, J.-H.; Shin, S.-Y.; Kwon, Y.-S.; Lee, H.J.; Kim, S.-S.; Chun, W. Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-κB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol. Ther. 2013, 21, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Miyata, S.; Iwase, M.; Shimizu, M.; Inoue, J.; Sato, R. Kaempferol stimulates gene expression of low-density lipoprotein receptor through activation of Sp1 in cultured hepatocytes. Sci. Rep. 2016, 6, 24940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.; Zhu, P.; Fujino, M.; Takahara, T.; Toyama, S.; Tomita, A.; Zhao, L.; Yang, Z.; Hei, M.; et al. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int. Immunopharmacol. 2015, 28, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, C.-A.; Sun, C.-L.; Ma, Y.-B.; Huang, X.-Y.; Cao, T.-W.; He, K.; Wang, H.; Zhang, X.-M.; Chen, J.-J. Polyacetylenes and anti-hepatitis B virus active constituents from Artemisia capillaris. Fitoterapia 2014, 95, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lee, H.Y.; Chang, C.H.; Namba, T.; Masao, H. Evaluation of the liver protective principles from the root of Cudrania cochinchinensis var. gerontogea. Phyther. Res. 1996, 10, 13–17. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.-K.; Choi, J.-H.; Jung, J.-Y.; Oh, W.-Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.-H.; et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 3, 4, 11 and 18 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Guadarrama, A.B.; Rios, M.Y. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules 2018, 23, 1952. https://doi.org/10.3390/molecules23081952
Aguilar-Guadarrama AB, Rios MY. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules. 2018; 23(8):1952. https://doi.org/10.3390/molecules23081952
Chicago/Turabian StyleAguilar-Guadarrama, A. Berenice, and María Yolanda Rios. 2018. "Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity" Molecules 23, no. 8: 1952. https://doi.org/10.3390/molecules23081952
APA StyleAguilar-Guadarrama, A. B., & Rios, M. Y. (2018). Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules, 23(8), 1952. https://doi.org/10.3390/molecules23081952