Homogeneous Synthesis of Cationic Chitosan via New Avenue
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure of Quaternized Chitosan (QC)
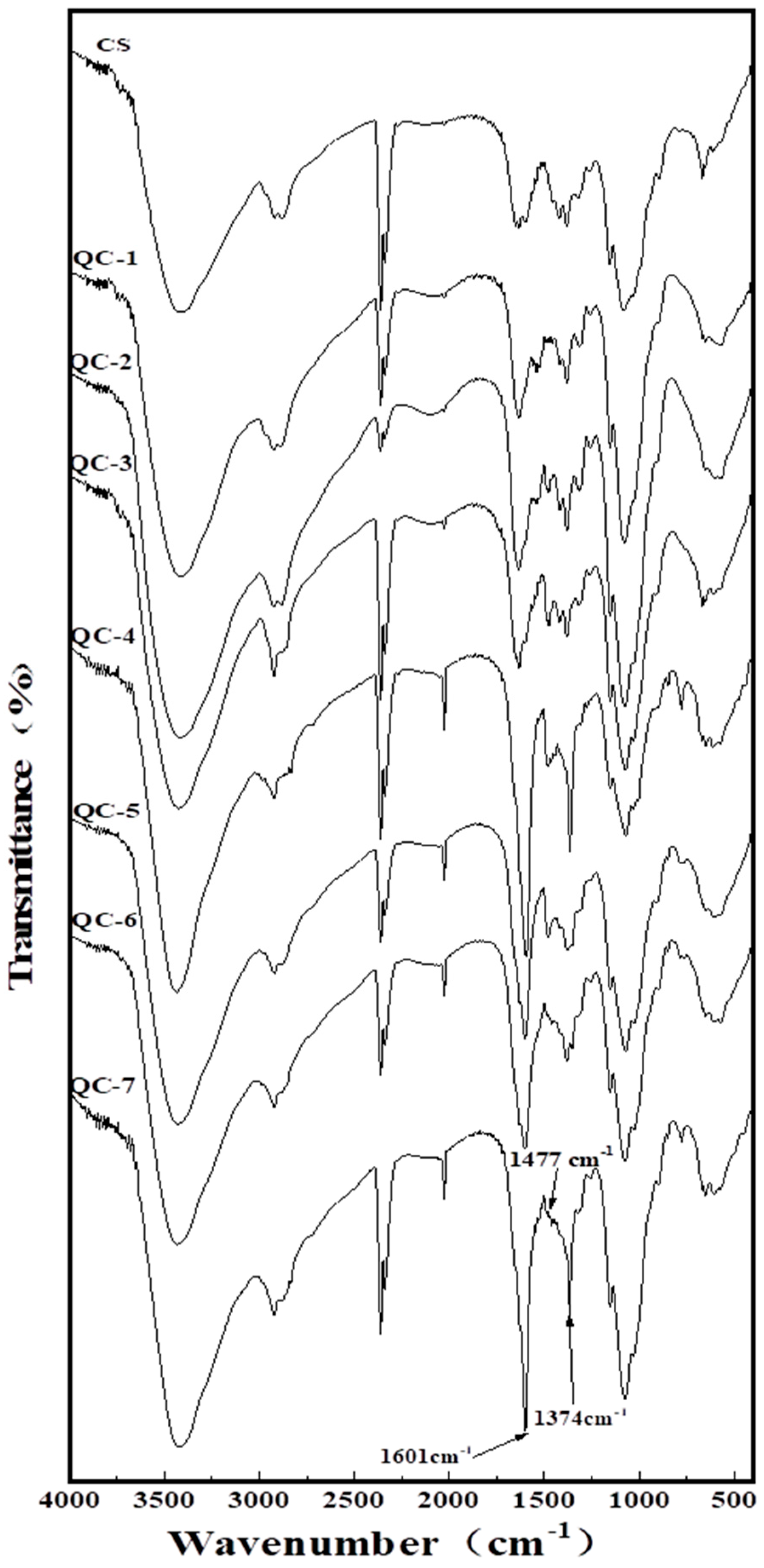
2.2. Fourier-Transform Infrared (FTIR) Spectrometry Analysis
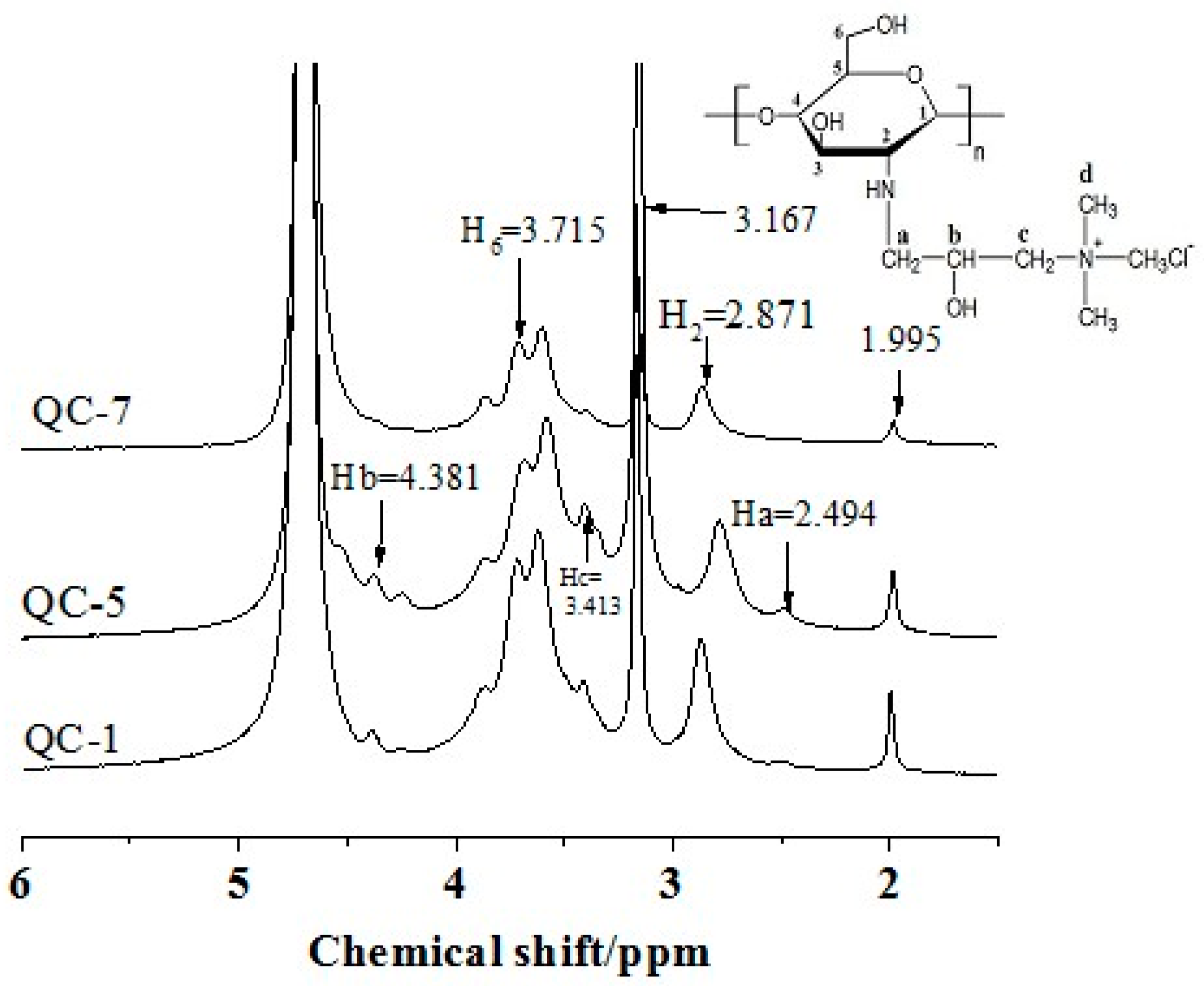
2.3. 1H Nuclear Magnetic Resonance (NMR) Characterization of QCs
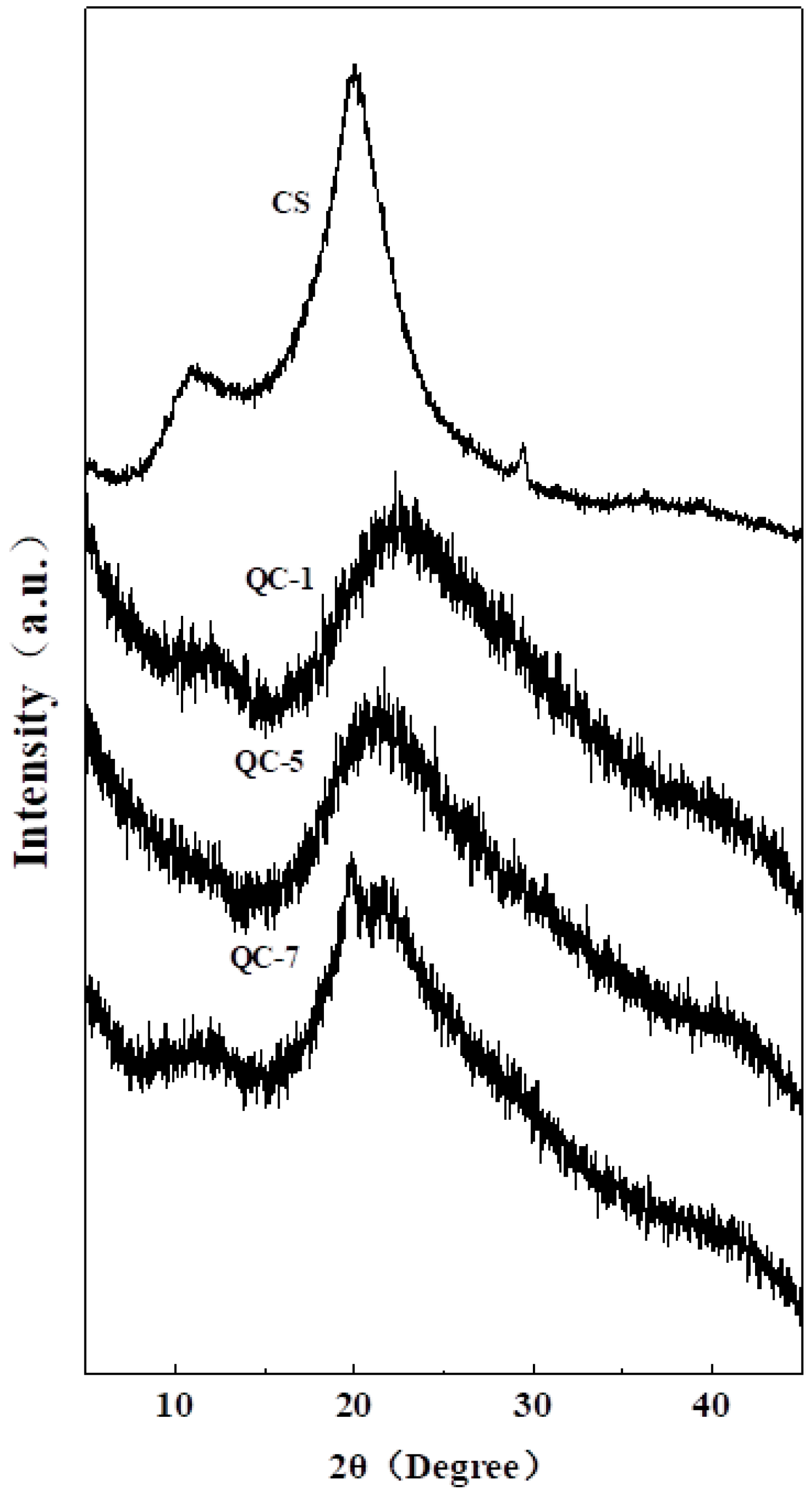
2.4. X-ray Diffraction (XRD) Analysis of QCs
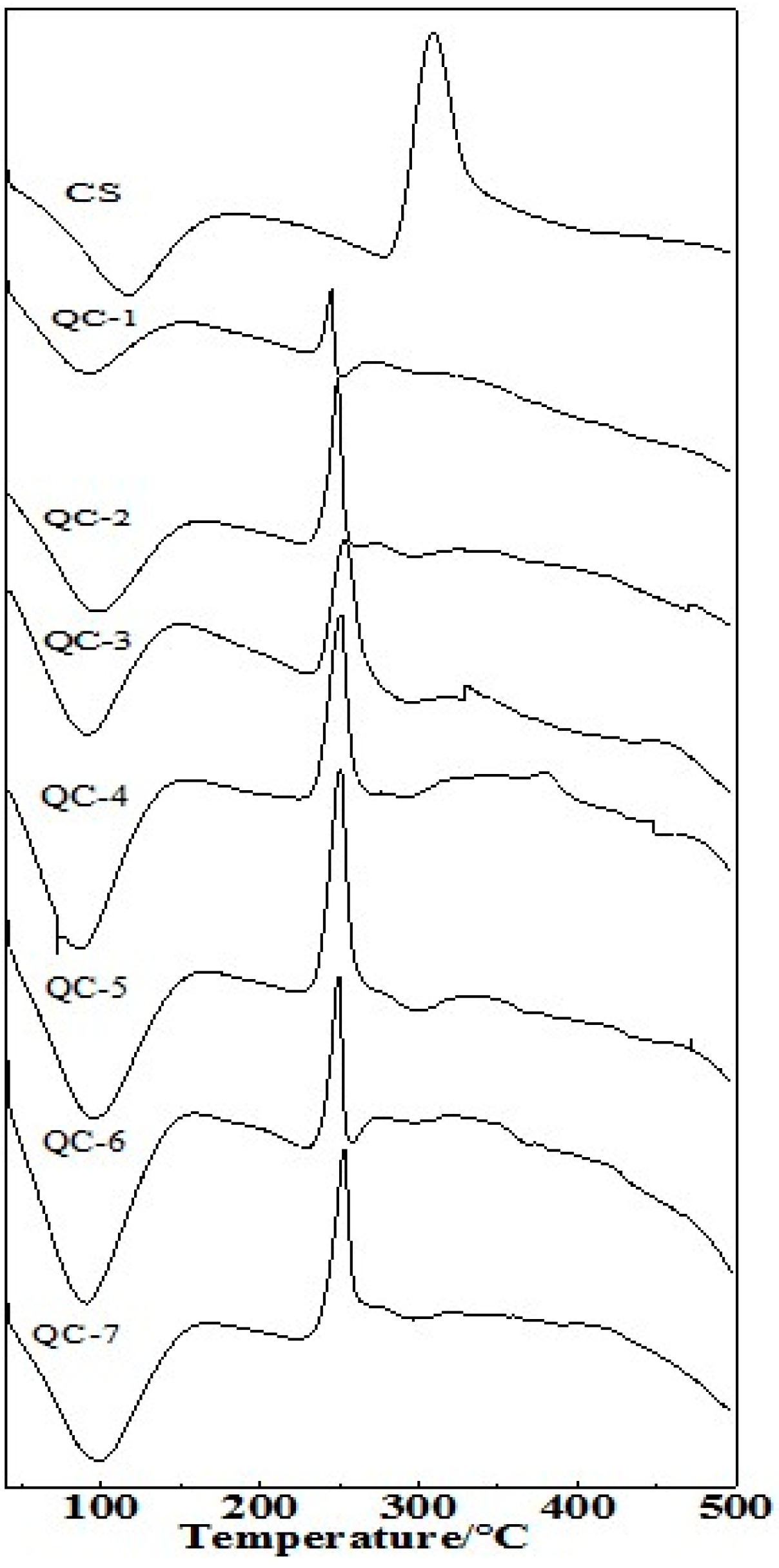
2.5. Differential Scanning Calorimetry (DSC) Analysis of QCs
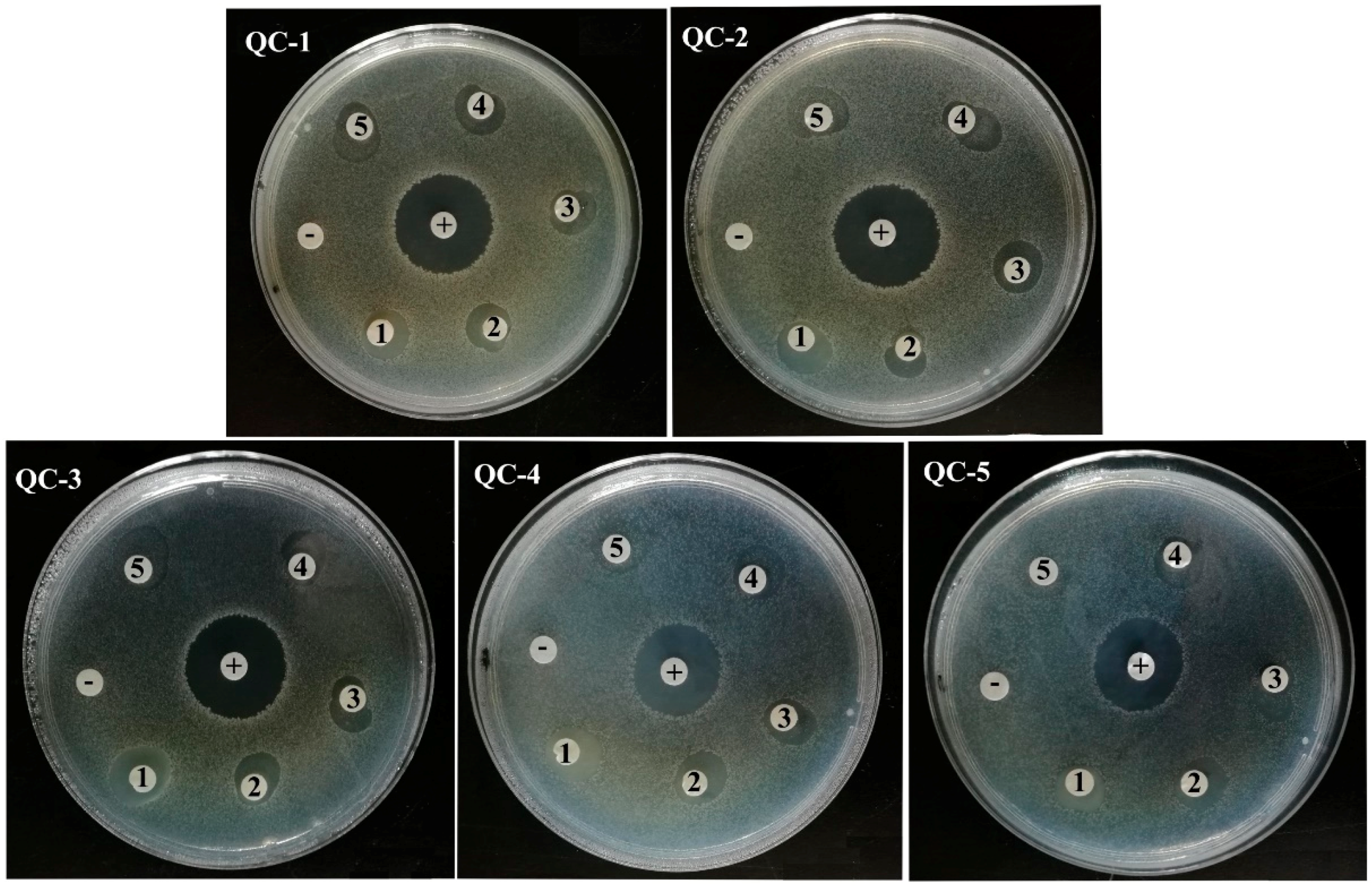
2.6. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Homogeneous Synthesis of QC
3.3. Estimation of Water Solubility
3.4. Characterization of QC
3.5. Degree of Substitution (DS) of QCs
3.6. Zeta Potentials of QC
3.7. Nuclear Magnetic Resonance (NMR) Characterization
3.8. X-ray Diffraction (XRD)
3.9. Differential Scanning Calorimetry (DSC)
3.10. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Dilution Gradient | Diameter of Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|
| QC-1 | QC-2 | QC-3 | QC-4 | QC-5 | |
| 1 | 11.64 | 13.32 | 15.96 | 12.44 | 11.66 |
| 2 | 10.50 | 12.30 | 12.28 | 13.46 | 12.46 |
| 3 | 10.76 | 12.54 | 12.30 | 12.22 | 13.06 |
| 4 | 12.02 | 12.94 | 12.14 | 12.46 | 12.02 |
| 5 | 11.98 | 13.36 | 13.86 | 13.26 | 12.22 |
| positive control | 23.80 | 24.90 | 23.84 | 21.76 | 22.34 |
| negative control | 11.64 | 13.32 | 15.96 | 12.44 | 11.66 |
References
- Chang, S.S.; Kang, D.H. Alicyclobacillus spp. in the fruit juice industry: History, characteristics, and current isolation/detection procedures. Crit. Rev. Microbiol. 2004, 30, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Walls, I.; Chuyate, R. Spoilage of fruit juices by Alicyclobacillus acidoterrestris. Food Aust. 2000, 52, 286–288. [Google Scholar]
- Steyn, C.E.; Cameron, M.; Witthuhn, R.C. Occurrence of alicyclobacillus in the fruit processing environment—A review. Int. J. Food Microbiol. 2011, 147, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Pascoli, I.C.; dos Anjos, M.M.; da Silva, A.A.; Lorenzetti, F.B.; Garcia Cortez, D.A.; Graton Mikcha, J.M.; Nakamura, T.U.; Nakamura, C.V.; de Abreu Filho, B.A. Piperaceae extracts for controlling Alicyclobacillus acidoterrestris growth in commercial orange juice. Ind. Crops Prod. 2018, 116, 224–230. [Google Scholar] [CrossRef]
- Walker, M.; Phillips, C.A. The effect of intermittent shaking, headspace and temperature on the growth of Alicyclobacillus acidoterrestris in stored apple juice. Int. J. Food Sci. Technol. 2005, 40, 557–562. [Google Scholar] [CrossRef]
- Yamazaki, K.; Teduka, H.; Shinano, H. Isolation and identification of Alicyclobacillus acidoterrestris from acidic beverages. Biosci. Biotechnol. Biochem. 1996, 60, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.; Whitfield, F.B. Role of Alicyclobacillus acidoterrestris in the development of a disinfectant taint in shelf-stable fruit juice. Lett. Appl. Microbiol. 2003, 36, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hassarajani, S.A.; Mamdapur, V.R. A three step synthesis of 11-cycloheptylundecanoic acid, a component of the thermoacidophile alicyclobacillus cycloheptanicus. Molecules 1998, 3, 41–43. [Google Scholar] [CrossRef]
- Song, Z.; Yuan, Y.; Niu, C.; Dai, L.; Wei, J.; Yue, T. Iron oxide nanoparticles functionalized with nisin for rapid inhibition and separation of Alicyclobacillus spp. RSC Adv. 2017, 7, 6712–6719. [Google Scholar] [CrossRef]
- Anjos, M.M.; Endo, E.H.; Leimann, F.V.; Goncalves, O.H.; Dias-Filho, B.P.; de Abreu Filho, B.A. Preservation of the antibacterial activity of enzymes against Alicyclobacillus spp. Through microencapsulation. LWT Food Sci. Technol. 2018, 88, 18–25. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Ciuffreda, E.; Sinigaglia, M.; Corbo, M.R. Effects of lysozyme on Alicyclobacillus acidoterrestris under laboratory conditions. Int. J. Food Sci. Technol. 2014, 49, 224–229. [Google Scholar] [CrossRef]
- Pei, J.; Yue, T.; Yuan, Y.; Dai, L. Activity of paracin c from lactic acid bacteria against alicyclobacillus in apple juice: Application of a novelty bacteriocin. J. Food Saf. 2017, 37, e12350. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef] [PubMed]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Franca, E.F.; Freitas, L.C.G.; Lins, R.D. Chitosan molecular structure as a function of N-acetylation. Biopolymers 2011, 95, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Dos Santos, B.R.; Bacalhau, F.B.; dos Santos Pereira, T.; Souza, C.F.; Faez, R. Chitosan-montmorillonite microspheres: A sustainable fertilizer delivery system. Carbohydr. Polym. 2015, 127, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Dananjaya, S.H.S.; Godahewa, G.I.; Jayasooriya, R.G.P.T.; Lee, J.; De Zoysa, M. Antimicrobial effects of chitosan silver nano composites (CAgNCs) on fish pathogenic Aliivibrio (Vibrio) salmonicida. Aquaculture 2016, 450, 422–430. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.-Z.; Chen, W.; Bai, Z.-W. Synthesis and characterization of chitosan alkyl urea. Carbohydr. Polym. 2016, 145, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Baruch, L.; Machluf, M. Alginate–chitosan complex coacervation for cell encapsulation: Effect on mechanical properties and on long-term viability. Biopolymers 2006, 82, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Dai-Hung, N.; Thanh-Sang, V.; Dai-Nghiep, N.; Kang, K.-H.; Je, J.-Y.; Hoang Nguyen-Duc, P.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar]
- Dai, L.; Jin, S.; Fan, M.; Zhou, P. Preparation of quaternized cellulose/chitosan microspheres for selective enrichment of phosphopeptides. Anal. Bioanal. Chem. 2017, 409, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of double quaternized chitosan derivatives. Molecules 2017, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Gao, J.; Wang, K.; Zhao, Y.; Li, H.; Ma, Y. Quaternized chitosan-intercalated montmorillonite composite for cyanobacterial bloom inhibition. Desalination Water Treat. 2016, 57, 19665–19676. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Soininen, P.; Masson, M.; Jarvinen, T. Synthesis of novel quaternary chitosan derivatives via n-chloroacyl-6-o-triphenylmethylchitosans. Biomacromolecules 2006, 7, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Preparation and physicochemical evaluation of chitosan/poly(vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohydr. Polym. 2010, 79, 711–716. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, B.R.; Rhee, Y.H. Imparting durable antimicrobial properties to cotton fabrics using alginate-quaternary ammonium complex nanoparticles. Carbohydr. Polym. 2010, 79, 1057–1062. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdottir, B.E.; Gudjonsson, T.; Baldursson, O.; Masson, M. N-alkylation of highly quaternized chitosan derivatives affects the paracellular permeation enhancement in bronchial epithelia in vitro. Eur. J. Pharm. Biopharm. 2014, 86, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. The influence of the cationic of quaternized chitosan on antifungal activity. Int. J. Food Microbiol. 2007, 118, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, Y.; Zhang, X.; Zhou, J.; Zhang, L. Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules 2008, 9, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Vallapa, N.; Wiarachai, O.; Thongchul, N.; Pan, J.; Tangpasuthadol, V.; Kiatkamjornwong, S.; Hoven, V.P. Enhancing antibacterial activity of chitosan surface by heterogeneous quaternization. Carbohydr. Polym. 2011, 83, 868–875. [Google Scholar] [CrossRef]
- Liu, P.; Meng, W.; Wang, S.; Sun, Y.; Ashraf, M.A. Quaternary ammonium salt of chitosan: Preparation and antimicrobial property for paper. Open Med. 2015, 10, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Katsamenis, O.L.; Bouropoulos, N.; Nazar, H.; Thurner, P.J.; van der Merwe, S.M.; Fatouros, D.G. Preparation and characterization of bioadhesive microparticles comprised of low degree of quaternization trimethylated chitosan for nasal administration: Effect of concentration and molecular weight. Langmuir 2014, 30, 12337–12344. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Peppley, B.; Creber, K.A.M.; Bui, V.T. Anion-exchange membranes composed of quaternized-chitosan derivatives for alkaline fuel cells. J. Power Sources 2010, 195, 3785–3793. [Google Scholar] [CrossRef]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Li, P.-W.; Yang, Z.-M.; Peng, Z.; Quan, W.-Y.; Yang, X.-H.; Yang, L.; Dong, J.-J. Synthesis and characterization of chitosan quaternary ammonium salt and its application as drug carrier for ribavirin. Drug Deliv. 2014, 21, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Kittur, F.S.; Prashanth, K.V.H.; Sankar, K.U.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chelminiak, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. J. Therm. Anal. Calorim. 2015, 119, 499–506. [Google Scholar] [CrossRef]
- Hu, S.; Song, L.; Pan, H.; Hu, Y. Effect of a novel chitosan-based flame retardant on thermal and flammability properties of polyvinyl alcohol. J. Therm. Anal. Calorim. 2013, 112, 859–864. [Google Scholar] [CrossRef]
- Chen, C.; Gu, X.; Jin, X.; Sun, J.; Zhang, S. The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohydr. Polym. 2017, 157, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Felix, F.; Castillo-Ortega, M.M.; Yepiz-Gomez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Julieta Bof, M.; Carina Bordagaray, V.; Elisa Locaso, D.; Alejandra Garcia, M. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocoll. 2015, 51, 281–294. [Google Scholar]
- Gonil, P.; Sajomsang, W.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Novel quaternized chitosan containing β-cyclodextrin moiety synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2011, 83, 905–913. [Google Scholar] [CrossRef]
- Stompor, M.; Zarowska, B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Starbova, K.; Markova, N.; Manolova, N.; Rashkov, I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol). Carbohydr. Res. 2006, 341, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Du, Y.M.; Wu, X.J.; Zhan, H.Y. Effect of molecular weight and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids Surf. A 2004, 242, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, S.; Zhang, J.; Wu, W. Antibacterial activity composition of the fermentation broth of Streptomyces djakartensis NW35. Molecules 2013, 18, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, T.; Ramasamy, P.; Wahid, M.E.A.; Segaran, T.C.; Vairappan, C.S. Biological activity of carbazole alkaloids and essential oil of Murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules 2011, 16, 9651–9664. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds QC1–QC7 are available from the authors. |
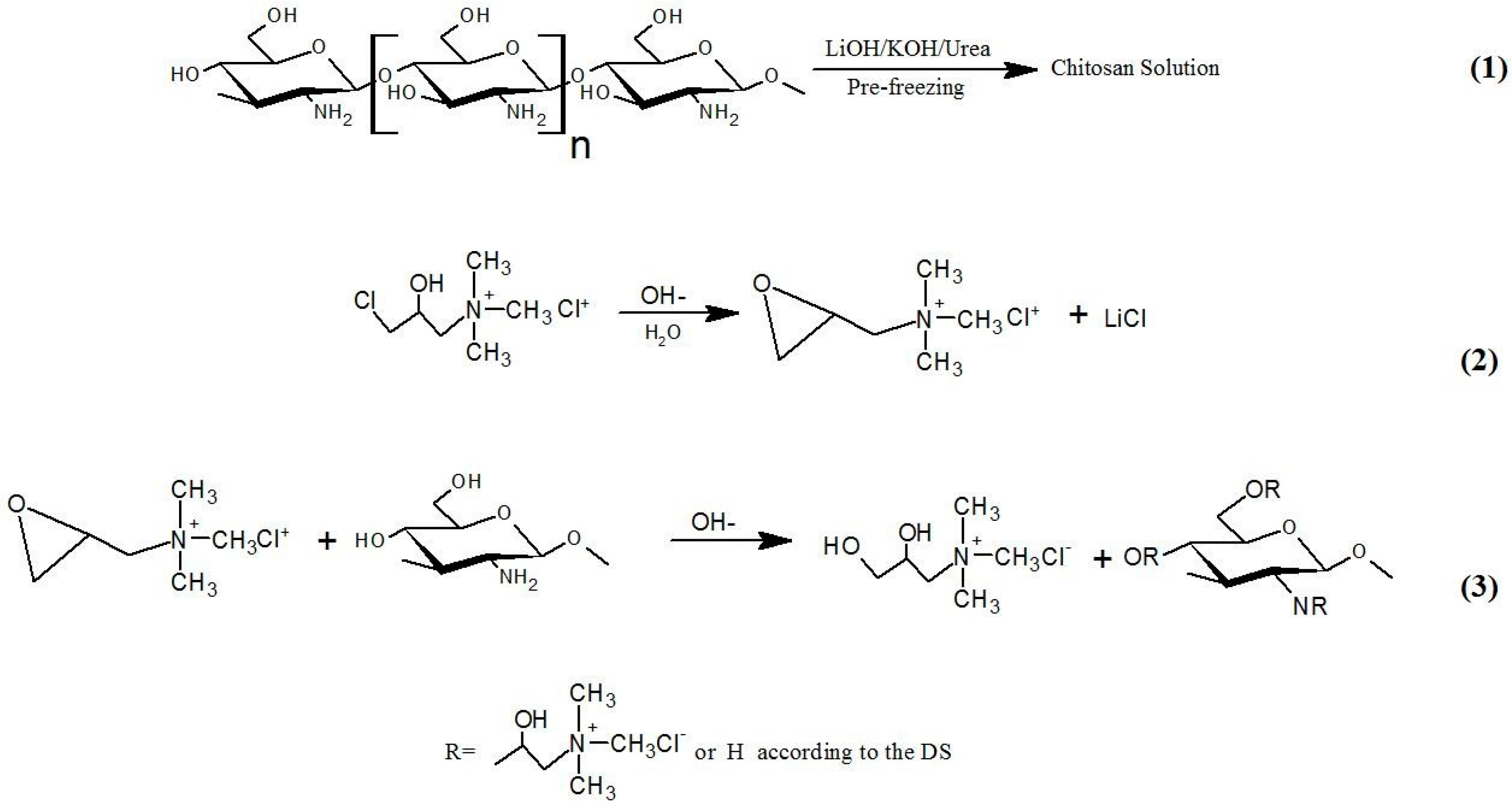
| Sample | Volume of CHPTAC | React Time (h) | React Temperature (°C) | Yield (%) | Degree of Substitution (%) | Solubility | Zeta Potentials (mv) |
|---|---|---|---|---|---|---|---|
| QC1 | 6 | 15 | 25 | 68.9 | 32.9 | ++ | 45.8 ± 1.1 |
| QC2 | 12 | 15 | 25 | 78.6 | 36.1 | ++ | 48.1 ± 1.5 |
| QC3 | 24 | 15 | 25 | 85.2 | 44.5 | ++ | 49.6 ± 0.5 |
| QC4 | 24 | 30 | 25 | 89.7 | 46.6 | ++ | 53.8 ± 1.9 |
| QC5 | 24 | 45 | 25 | 88.5 | 46.8 | ++ | 54.2 ± 0.5 |
| QC6 | 6 | 15 | 40 | 48.7 | 25.3 | + | - |
| QC7 | 6 | 15 | 60 | 32.6 | 16.5 | - | - |
| Sample | Onset/°C | Peak/°C | Terminal/°C | DH (J/g) |
|---|---|---|---|---|
| QC-1 | 51.7 238.4 | 89.6 245.0 | 152.4 252.6 | 284.8 −73.3 |
| QC-2 | 47.4 240.4 | 96.2 249.0 | 160.6 262.5 | 298.1 −71.8 |
| QC-3 | 45.0 238.5 | 90.4 253.7 | 150.4 293.0 | 277.2 −108.6 |
| QC-4 | 41.7 236.7 | 72.0 251.2 | 151.9 274.2 | 302.5 −89.1 |
| QC-5 | 44.5 237.3 | 94.0 250.8 | 148.9 293.9 | 347.5 −121.0 |
| QC-6 | 48.4 240.5 | 93.6 249.8 | 136.5 258.4 | 285.2 −38.9 |
| QC-7 | 51.0 241.6 | 98.5 253.1 | 153.1 296.2 | 280.9 −92.1 |
| CS | 64.7 288.1 | 116.8 308.1 | 179.3 338.4 | 230.1 −196.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Wu, H.; Li, S.; Tian, H.; Li, Y.; Wang, J. Homogeneous Synthesis of Cationic Chitosan via New Avenue. Molecules 2018, 23, 1921. https://doi.org/10.3390/molecules23081921
Song H, Wu H, Li S, Tian H, Li Y, Wang J. Homogeneous Synthesis of Cationic Chitosan via New Avenue. Molecules. 2018; 23(8):1921. https://doi.org/10.3390/molecules23081921
Chicago/Turabian StyleSong, Huanlu, Hao Wu, ShuJing Li, Huafeng Tian, YanRu Li, and JianGuo Wang. 2018. "Homogeneous Synthesis of Cationic Chitosan via New Avenue" Molecules 23, no. 8: 1921. https://doi.org/10.3390/molecules23081921
APA StyleSong, H., Wu, H., Li, S., Tian, H., Li, Y., & Wang, J. (2018). Homogeneous Synthesis of Cationic Chitosan via New Avenue. Molecules, 23(8), 1921. https://doi.org/10.3390/molecules23081921





