Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization
Abstract
1. Introduction
2. Results
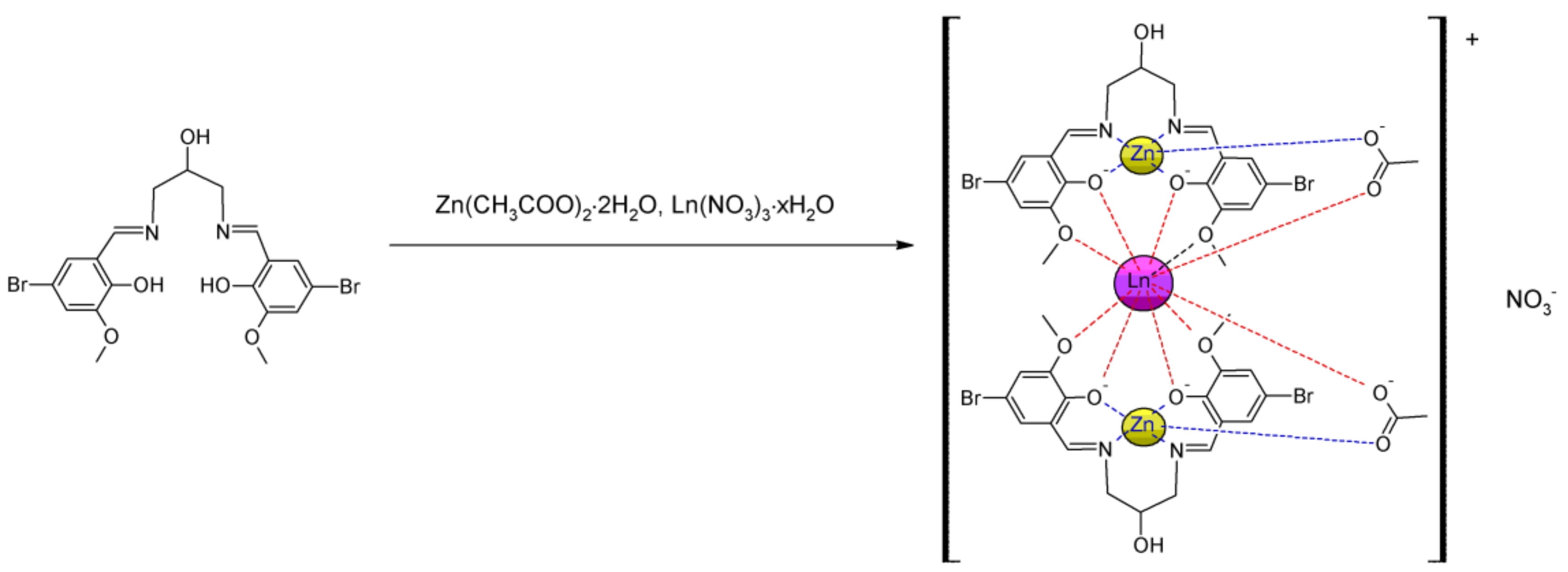
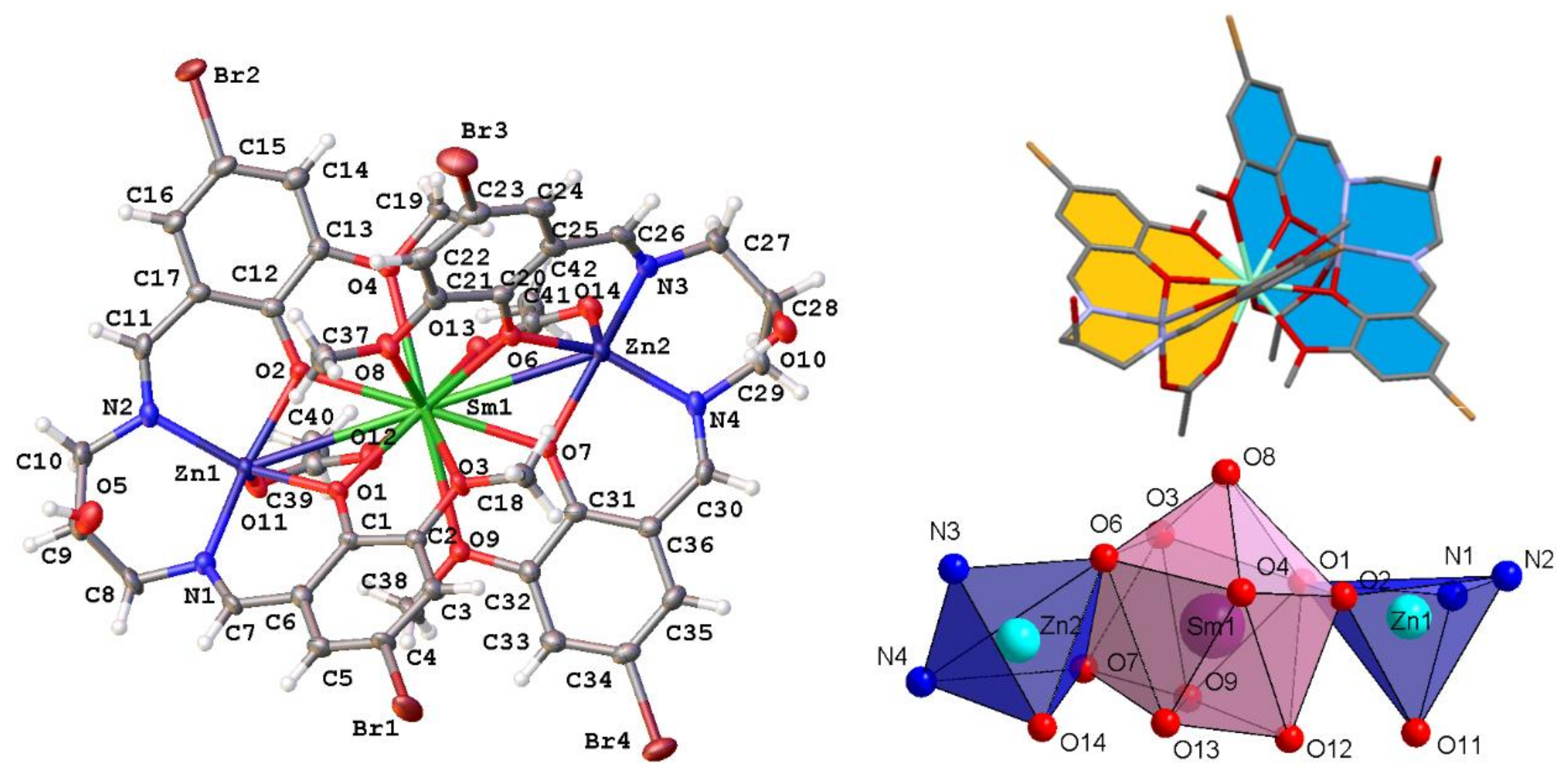
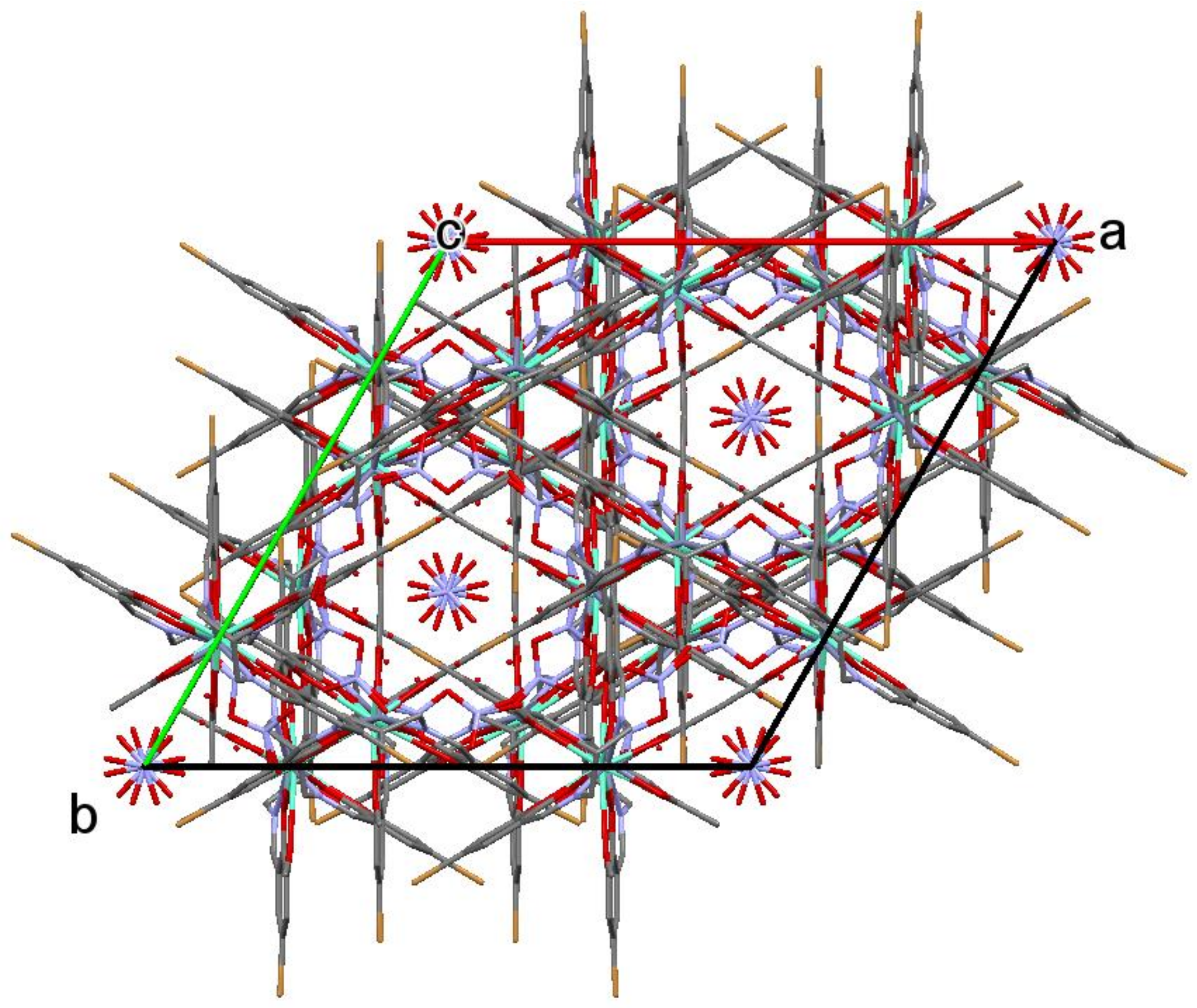
2.1. Crystal Structure
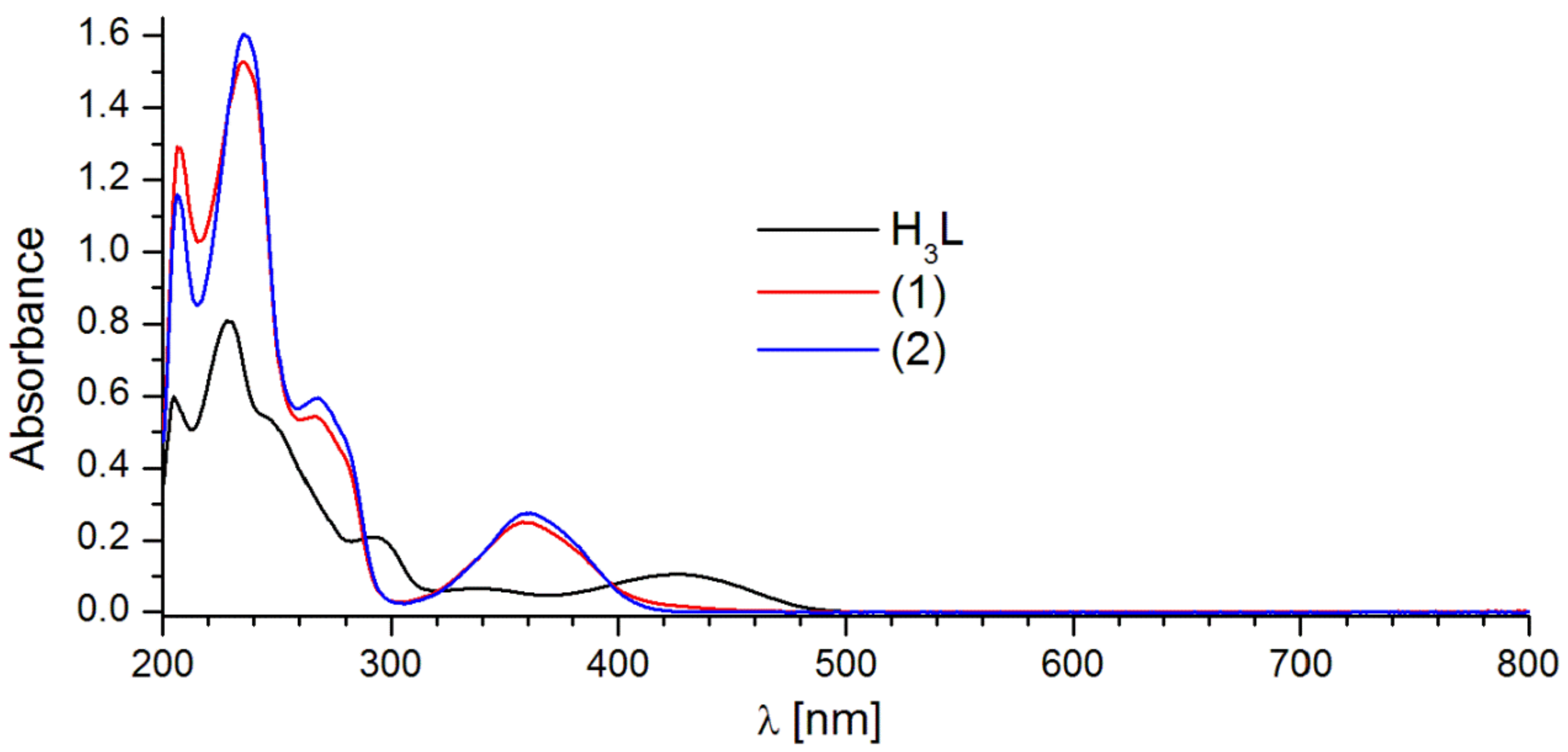
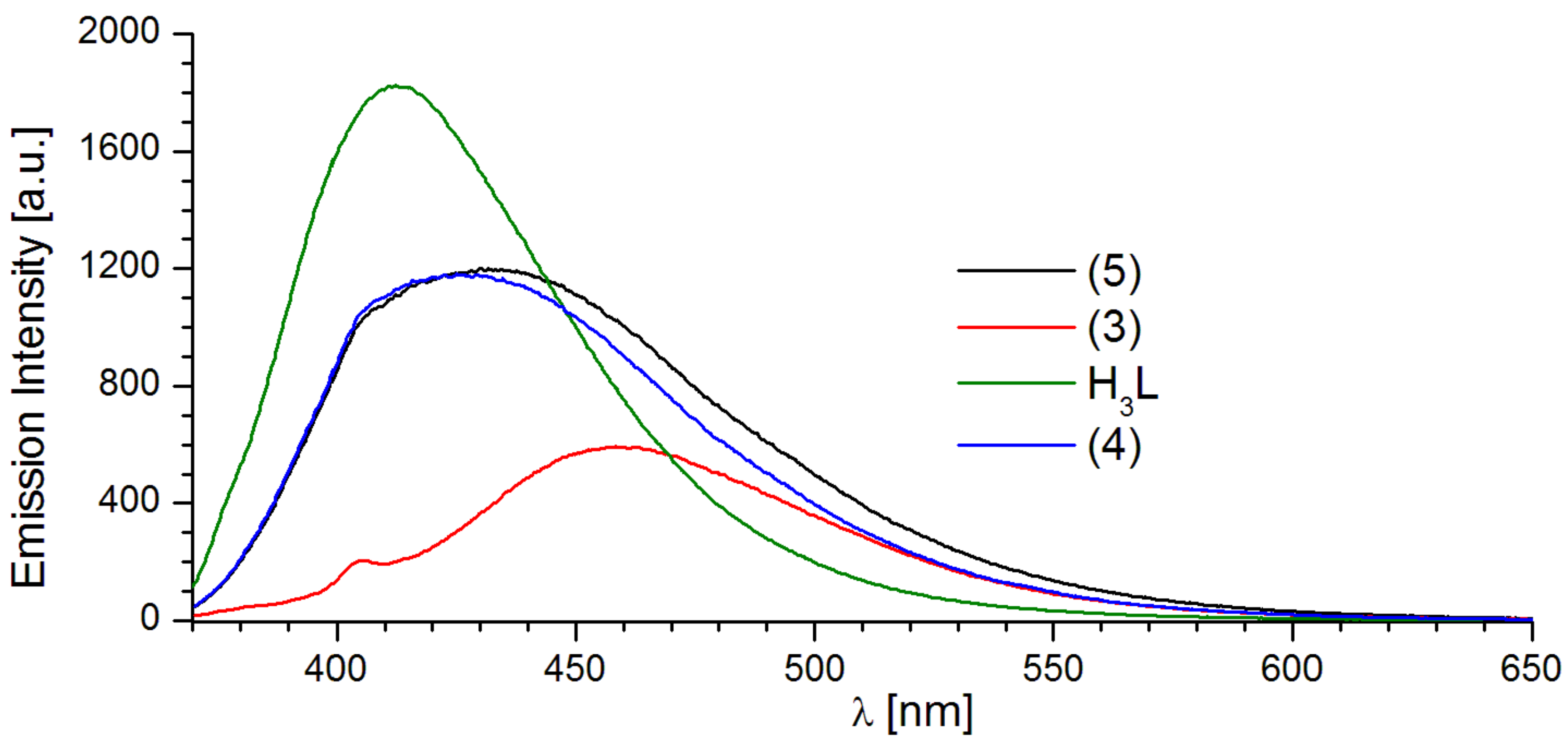
2.2. Luminescent Properties
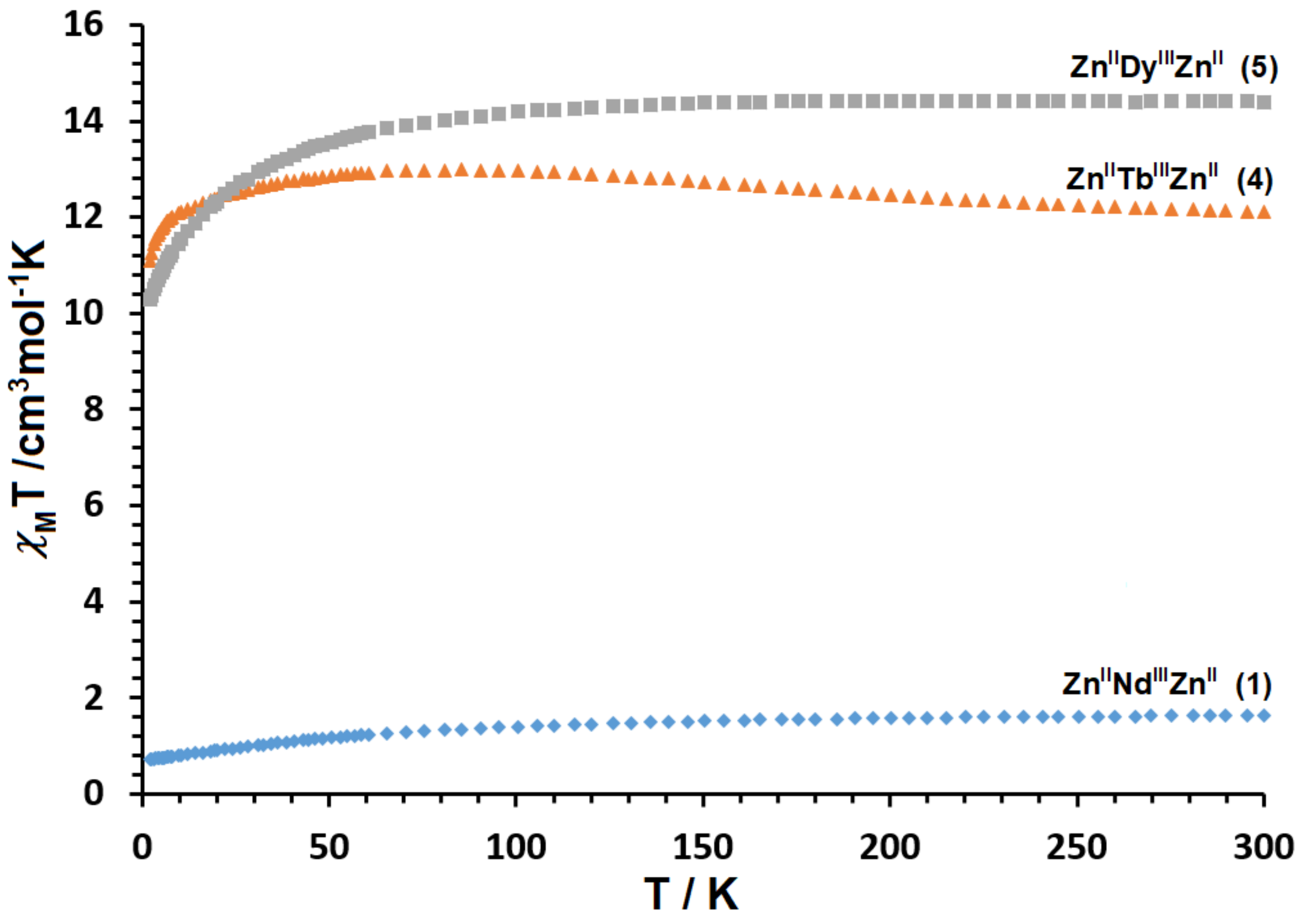
2.3. Magnetic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the H3L
3.3. Synthesis of the ZnII–LnIII–ZnII Complexes
3.3.1. [Zn2Nd(ac)2(HL)2]NO3·3H2O (1)
3.3.2. [Zn2Sm(ac)2(HL)2]NO3·3CH3OH·0.3H2O (2)
3.3.3. [Zn2Eu(ac)2(HL)2]NO3·5.33H2O (3)
3.3.4. [Zn2Tb(ac)2(HL)2]NO3·5.33H2O (4)
3.3.5. [Zn2Dy(ac)2(HL)2]NO3·5.33H2O (5)
3.4. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schmitz, S.; van Leusen, J.; Izarova, N.V.; Lan, Y.; Wernsdorfer, W.; Kögerler, P.; Monakhov, K.Y. Supramolecular 3d-4f Single-Molecule Magnet Architectures. Dalton Trans. 2016, 45, 16148–16152. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Tsipis, A.C.; Kumar, P.; Townrow, O.P.E.; Abdul-Sada, A.; Akien, G.R.; Baldansuren, A.; Spivey, A.C.; Kostakis, G.E. 3d/4f Coordination Clusters as Cooperative Catalysts for Highly Diastereoselective Michael Addition Reactions. Inorg. Chem. 2017, 56, 9563–9573. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.-Z.; Chen, Q.-S.; Zhang, C.-J.; Li, P.-X.; Wang, M.-S.; Guo, G.-C. Photochromism and Photomagnetism of a 3d-4f Hexacyanoferrate at Room Temperature. J. Am. Chem. Soc. 2015, 137, 10882–10885. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Li, H.-F.; Chen, P.; Gao, T.; Sun, W.-B.; Li, G.-M.; Yan, P.-F. Synthesis, Structure, and Tunable White Light Emission of Heteronuclear Zn2Ln2 Arrays Using a Zinc Complex as Ligand. CrystEngComm 2016, 18, 917–923. [Google Scholar] [CrossRef]
- Wong, W.-K.; Liang, H.; Wong, W.-Y.; Cai, Z.; Li, K.-F.; Cheah, K.-W. Synthesis and near-Infrared Luminescence of 3d-4f Bi-Metallic Schiff Base Complexes. New J. Chem. 2002, 26, 275–278. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, W.; Huang, X.; Cheng, Y.; Shen, P. Selective Fluorescent Probe Based on Schiff Base Derived from Hydroxymethyl Coumarin and Aminated Sudan I Dye for Mg2+ Detection. Arab. J. Chem. 2017, 10, S2729–S2735. [Google Scholar] [CrossRef]
- Jiang, X.-J.; Li, M.; Lu, H.-L.; Xu, L.-H.; Xu, H.; Zang, S.-Q.; Tang, M.-S.; Hou, H.-W.; Mak, T.C.W. A Highly Sensitive C3-Symmetric Schiff-Base Fluorescent Probe for Cd2+. Inorg. Chem. 2014, 53, 12665–12667. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Yang, Z.; Hao, L.; Wang, Z.; Luo, T.; Obst, M.; Liu, P.; Shen, Y.; Zhang, S.; Li, J. A Novel Approach to Study the Structure-Property Relationships and Applications in Living Systems of Modular Cu2+ Fluorescent Probes. Sci. Rep. 2016, 6, 28972. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Majeed, A.; Al-Sammarrae, K.; Salih, N.; Salimon, J.; Abdullah, B. Metal Complexes of Schiff Base: Preparation, Characterization and Antibacterial Activity. Arab. J. Chem. 2017, 10, S1639–S1644. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, D.-Y.; Suo, J.-J.; Gu, W.; Tian, J.-L.; Liu, X.; Yan, S.-P. Synthesis, Magnetism and Spectral Studies of Six Defective Dicubane Tetranuclear {M4O6} (M = NiII, CoII, ZnII) and Three Trinuclear CdII Complexes with Polydentate Schiff Base Ligands. Dalton Trans. 2016, 45, 10233–10248. [Google Scholar] [CrossRef] [PubMed]
- Sivchik, V.V.; Solomatina, A.I.; Chen, Y.-T.; Karttunen, A.J.; Tunik, S.P.; Chou, P.-T.; Koshevoy, I.O. Halogen Bonding to Amplify Luminescence: A Case Study Using a Platinum Cyclometalated Complex. Angew. Chem. Int. Ed. 2015, 54, 14057–14060. [Google Scholar] [CrossRef] [PubMed]
- Sanetra, J.; Armatys, P.; Chrzaszcz, R.; Pielichowski, J.; Barta, P.; Niziol, S.; Saliraoui, B. Synthesis and Luminescent Properties of Br-Substituted Poiy(n-Vinylcarbazoles). Synth. Met. 1999, 101, 82–83. [Google Scholar] [CrossRef]
- Van Deun, R.; Fias, P.; Driesen, K.; Binnemans, K.; Görller-Walrand, C. Halogen Substitution as an Efficient Tool to Increase the near-Infrared Photoluminescence Intensity of Erbium(III) Quinolinates in Non-Deuterated DMSO. Phys. Chem. Chem. Phys. 2003, 5, 2754–2757. [Google Scholar] [CrossRef]
- Albrecht, M.; Osetska, O.; Klankermayer, J.; Fröhlich, R.; Gumy, F.; Bünzli, J.-C.G. Enhancement of near-IR Emission by Bromine Substitution in Lanthanide Complexes with 2-Carboxamide-8-Hydroxyquinoline. Chem. Commun. 2007, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi-Anarjan, P.; Bikas, R.; Hosseini-Monfared, H.; Aleshkevych, P.; Mayer, P. Synthesis, Characterization, EPR Spectroscopy and Catalytic Activity of a New oxidovanadium(IV) Complex with N2O2-Donor Ligand. J. Mol. Struct. 2017, 1131, 258–265. [Google Scholar] [CrossRef]
- Datta, A.; Das, K.; Massera, C.; Clegg, J.K.; Sinha, C.; Huang, J.-H.; Garribba, E. A Mixed Valent Heterometallic CuII/NaI Coordination Polymer with Sodium–phenyl Bonds. Dalton Trans. 2014, 43, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Liu, X.; Tian, J.; Yan, S. Three Series of Heterometallic NiII–LnIII Schiff Base Complexes: Synthesis, Crystal Structures and Magnetic Characterization. Dalton Trans. 2017, 46, 12558–12573. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Mayans, J.; Shipman, M.A.; Tizzard, G.J.; Coles, S.J.; Blight, B.A.; Escuer, A.; Kostakis, G.E. Four New Families of Polynuclear Zn-Ln Coordination Clusters. Synthetic, Topological, Magnetic, and Luminescent Aspects. Cryst. Growth Des. 2017, 17, 1524–1538. [Google Scholar] [CrossRef]
- Tsuchimoto, M.; Ishii, T.; Imaoka, T.; Yamamoto, K.; Yoshioka, N.; Sunatsuki, Y. Synthesis and Structures of Vanadium–Cerium Trinuclear Complexes with Schiff-Base Ligands. Bull. Chem. Soc. Jpn. 2006, 79, 1393–1397. [Google Scholar] [CrossRef]
- Tsuchimoto, M.; Ishii, T.; Imaoka, T.; Yamamoto, K. Synthesis and Electrochemical Properties of Oxovanadium Complexes with a Pentadentate Schiff Base Ligand. Bull. Chem. Soc. Jpn. 2004, 77, 1849–1854. [Google Scholar] [CrossRef]
- Lan, Y.; Novitchi, G.; Clérac, R.; Tang, J.-K.; Madhu, N.T.; Hewitt, I.J.; Anson, C.E.; Brooker, S.; Powell, A.K. Di-, Tetra- and Hexanuclear iron(III), manganese(II/III) and copper(II) Complexes of Schiff-Base Ligands Derived from 6-Substituted-2-Formylphenols. Dalton Trans. 2009, 10, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Chakrabarty, P.P.; Saha, S.; Jana, A.D.; Schollmeyer, D.; García-Granda, S. Ligand Mediated Structural Diversity and Role of Different Weak Interactions in Molecular Self-Assembly of a Series of copper(II)–sodium(I) Schiff-Base Heterometallic Complexes. Inorg. Chim. Acta 2013, 408, 172–180. [Google Scholar] [CrossRef]
- Elmali, A.; Zeyrek, C.T.; Elerman, Y. Crystal Structure, Magnetic Properties and Molecular Orbital Calculations of a Binuclear copper(II) Complex Bridged by an Alkoxo-Oxygen Atom and an Acetate Ion. J. Mol. Struct. 2004, 693, 225–234. [Google Scholar] [CrossRef]
- Mitra, M.; Maji, A.K.; Ghosh, B.K.; Raghavaiah, P.; Ribas, J.; Ghosh, R. Catecholase Activity of a Structurally Characterized Dinuclear iron(III) Complex [FeIII2(L)2] [H3L = N,N′-bis(3-Methoxysalicylaldimine)-1,3-Diaminopropan-2-Ol]. Polyhedron 2014, 67, 19–26. [Google Scholar] [CrossRef]
- Smith, K.I.; Borer, L.L.; Olmstead, M.M. Vanadium(IV) and Vanadium(V) Complexes of Salicyladimine Ligands. Inorg. Chem. 2003, 42, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- Dolai, M.; Ali, M.; Titiš, J.; Boča, R. Cu(II)–Dy(III) and Co(III)–Dy(III) Based Single Molecule Magnets with Multiple Slow Magnetic Relaxation Processes in the Cu(II)–Dy(III) Complex. Dalton Trans. 2015, 44, 13242–13249. [Google Scholar] [CrossRef] [PubMed]
- Chiboub Fellah, F.Z.; Boulefred, S.; Chiboub Fellah, A.; El Rez, B.; Duhayon, C.; Sutter, J.-P. Binuclear CuLn Complexes (LnIII = Gd, Tb, Dy) of Alcohol-Functionalized Bicompartmental Schiff-Base Ligand. Hydrogen Bonding and Magnetic Behaviors. Inorg. Chim. Acta 2016, 439, 24–29. [Google Scholar] [CrossRef]
- Datta, A.; Clegg, J.K.; Huang, J.-H.; Pevec, A.; Garribba, E.; Fondo, M. Hydroxo-Bridged 1-D Coordination Polymer of Cu(II) Incorporating with Salicyladimine Precursor: Spectral and Temperature Dependent Magneto Structural Correlation. Inorg. Chem. Commun. 2012, 24, 216–220. [Google Scholar] [CrossRef]
- Liao, S.; Yang, X.; Jones, R.A. Self-Assembly of Luminescent Hexanuclear Lanthanide Salen Complexes. Cryst. Growth Des. 2012, 12, 970–974. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Dey, A.; Das, S.; Rouzières, M.; Clérac, R. Syntheses, Structures, and Magnetic Properties of a Family of Heterometallic Heptanuclear [Cu5Ln2] (Ln = Y(III), Lu(III), Dy(III), Ho(III), Er(III), and Yb(III)) Complexes: Observation of SMM Behavior for the Dy(III) and Ho(III) Analogues. Inorg. Chem. 2013, 52, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Maeda, M.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. SMM Behavior Observed in Ce(III)Zn(II)2 Linear Trinuclear Complex. Chem. Lett. 2013, 42, 1276–1278. [Google Scholar] [CrossRef]
- Maeda, M.; Hino, S.; Yamashita, K.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. Correlation between Slow Magnetic Relaxation and the Coordination Structures of a Family of Linear Trinuclear Zn(II)–Ln(III)–Zn(II) Complexes (Ln = Tb, Dy, Ho, Er, Tm and Yb). Dalton Trans. 2012, 41, 13640–13648. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Morita, Y.; Utsuno, F.; Nabeshima, T. Multiple Folding Structures Mediated by Metal Coordination of Acyclic Multidentate Ligand. Inorg. Chem. 2009, 48, 10670–10678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, W.; Liu, H.; Zhang, Z.; Lü, X.; Song, J.; Fan, D.; Wong, W.-K.; Jones, R.A. Photo-Luminescent Hetero-Trinuclear Zn2Ln (Ln = Nd, Yb, Er or Gd) Complexes Based on the Binuclear Zn2L Precursor. Inorg. Chem. Commun. 2012, 24, 148–152. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Ma, J.-C.; Zhu, L.-C.; Dong, W.-K.; Zhang, Y. Four 3d-4f Heteromultinuclear zinc(II)–lanthanide(III) Complexes Constructed from a Distinct Hexadentate N2O2-Type Ligand: Syntheses, Structures and Luminescence Properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Tian, Y.-M.; Li, H.-F.; Han, B.-L.; Zhang, Q.; Sun, W.-B. A Salen-Type Trinuclear Zn2Gd Complex. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, m1500–m1501. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.; Yang, X.; Stanley, J.M.; Jones, R.A.; Holliday, B.J. Synthesis and Crystal Structure of a New Heterotrinuclear Schiff-Base Zn–Gd Complex. J. Chem. Crystallogr. 2010, 40, 1060–1064. [Google Scholar] [CrossRef]
- Yang, X.; Jones, R.A.; Lynch, V.; Oye, M.M.; Holmes, A.L. Synthesis and near Infrared Luminescence of a Tetrametallic Zn2Yb2 Architecture from a Trinuclear Zn3L2 Schiff Base Complex. Dalton Trans. 2005, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Schipper, D.; Liao, A.; Stanley, J.M.; Jones, R.A.; Holliday, B.J. Anion Dependent Self-Assembly of Luminescent Zn–Ln (Eu and Tb) Salen Complexes. Polyhedron 2013, 52, 165–169. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Miroslaw, B. Synthesis, Crystal Structures and Magnetic Behavior of NiII–4f–NiII Compounds. Polyhedron 2012, 43, 47–54. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Pełka, R.; Miroslaw, B.; Hnatejko, Z. Heterometallic Trinuclear 3d-4f-3d Compounds Based on a Hexadentate Schiff Base Ligand. Polyhedron 2014, 68, 180–190. [Google Scholar] [CrossRef]
- Lo, W.K.; Wong, W.K.; Wong, W.Y.; Guo, J.; Yeung, K.T.; Cheng, Y.K.; Yang, X.; Jones, R.A. Heterobimetallic Zn(II)-Ln(III) Phenylene-Bridged Schiff Base Complexes, Computational Studies, and Evidence for Singlet Energy Transfer as the Main Pathway in the Sensitization of near-Infrared Nd3+ Luminescence. Inorg. Chem. 2006, 45, 9315–9325. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Thakurta, S.; Sadhukhan, D.; Pilet, G.; Rosair, G.M.; Nonat, A.; Charbonnière, L.J.; Mitra, S. Thermally Stable Luminescent zinc–Schiff Base Complexes: A Thiocyanato Bridged 1D Coordination Polymer and a Supramolecular 1D Polymer. Polyhedron 2013, 65, 6–15. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, B.; Li, Y. Synthesis and Luminescence Properties of Polymer-Rare Earth Complexes Containing Salicylaldehyde-Type Bidentate Schiff Base Ligand. Luminescence 2017, 32, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Panja, S.K.; Verma, A.; Takaya, T.; Iwata, K.; Sunkari, S.S.; Saha, S. NIR Luminescent Heterodinuclear [ZnIILnIII] Complexes: Synthesis, Crystal Structures and Photophysical Properties. J. Lumin. 2017, 192, 156–165. [Google Scholar] [CrossRef]
- Feng, X.; Feng, Y.-Q.; Chen, J.J.; Ng, S.-W.; Wang, L.-Y.; Guo, J.-Z. Reticular Three-Dimensional 3d-4f Frameworks Constructed through Substituted Imidazole-Dicarboxylate: Syntheses, Luminescence and Magnetic Properties Study. Dalton Trans. 2015, 44, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-S.; Dong, W.-K.; Zhang, Y.; Chen, L.; Ding, Y.-J. Four Salamo-Type 3d-4f Hetero-Bimetallic [ZnIILnIII] Complexes: Syntheses, Crystal Structures, and Luminescent and Magnetic Properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Pasatoiu, T.D.; Madalan, A.M.; Kumke, M.U.; Tiseanu, C.; Andruh, M. Temperature Switch of LMCT Role: From Quenching to Sensitization of Europium Emission in a ZnII−EuIII Binuclear Complex. Inorg. Chem. 2010, 49, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Görrler-Warland, C.; Binnemans, K.B. Spectral Intensities of F-F Transitions. In Handbook on the Physics and Chemistry of Rare Earths; Geschneidner, K.A., Eyring, L., Lander, G.H., Eds.; Elsevier: New York, NY, USA, 1998; pp. 101–264. [Google Scholar]
- Tanner, P.A. Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Hänninen, P., Härmä, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Tao, C.-H.; Ma, J.-C.; Zhu, L.-C.; Zhang, Y.; Dong, W.-K. Heterobimetallic 3d-4f Zn(II)–Ln(III) (Ln = Sm, Eu, Tb and Dy) Complexes with a N2O4 Bisoxime Chelate Ligand and a Simple Auxiliary Ligand Py: Syntheses, Structures and Luminescence Properties. Polyhedron 2017, 128, 38–45. [Google Scholar] [CrossRef]
- Cristóvão, B.; Hnatejko, Z. Lanthanide(III) Compounds with the N2O4-Donor Schiff Base—Synthesis, Spectral, Thermal, Magnetic and Luminescence Properties. J. Mol. Struct. 2015, 1088, 50–55. [Google Scholar] [CrossRef]
- Craze, A.R.; Huang, X.-D.; Etchells, I.; Zheng, L.-M.; Bhadbhade, M.M.; Marjo, C.E.; Clegg, J.K.; Moore, E.G.; Avdeev, M.; Lindoy, L.F.; et al. Synthesis and Characterisation of New Tripodal Lanthanide Complexes and Investigation of Their Optical and Magnetic Properties. Dalton Trans. 2017, 46, 12177–12184. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.P.; Titos-Padilla, S.; Oyarzabal, I.; Gupta, T.; Duhayon, C.; Rajaraman, G.; Colacio, E. Analysis of the Role of Peripheral Ligands Coordinated to ZnII in Enhancing the Energy Barrier in Luminescent Linear Trinuclear Zn-Dy-Zn Single-Molecule Magnets. Chemistry 2015, 21, 15785–15796. [Google Scholar] [CrossRef] [PubMed]
- Dolai, M.; Mistri, T.; Panja, A.; Ali, M. Diversity in Supramolecular Self-Assembly through Hydrogen-Bonding Interactions of Non-Coordinated Aliphatic –OH Group in a Series of Heterodinuclear CuIIM (M = NaI, ZnII, HgII, SmIII, BiIII, PbII and CdII). Inorg. Chim. Acta 2013, 399, 95–104. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. Crysalis-Pro Software System ver. 1.171.38.46; Rigaku Corporation: Oxford, UK, 2016. [Google Scholar]
- Clark, R.C.; Reid, J.S. The Analytical Calculation of Absorption in Multifaceted Crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |


| Parameter. | 1 | 2 | Parameter | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Ln1–Zn1 | 3.513(1) | 3.5268(7) | Ln1–Zn1 | 3.458(1) | 3.4242(9) | 3.414(1) |
| Ln1–Zn2 | 3.556(1) | 3.4915(7) | Ln1–O1 | 2.354(5) | 2.330(5) | 2.367(4) |
| Ln1–O1 | 2.472(5) | 2.414(3) | Ln1–O2 | 2.409(5) | 2.386(5) | 2.303(4) |
| Ln1–O2 | 2.442(5) | 2.414(3) | Ln1–O3 | 2.637(6) | 2.649(5) | 2.879(4) |
| Ln1–O3 | 2.825(6) | 2.650(4) | Ln1–O4 | 2.856(5) | 2.905(5) | 2.612(5) |
| Ln1–O4 | 2.729(6) | 2.704(3) | Ln1–O7 | 2.375(5) | 2.345(5) | 2.324(4) |
| Ln1–O6 | 2.442(5) | 2.412(3) | Zn1–O1 | 2.056(6) | 2.064(5) | 2.094(4) |
| Ln1–O7 | 2.457(5) | 2.440(3) | Zn1–O2 | 2.082(5) | 2.089(5) | 2.054(5) |
| Ln1–O8 | 2.671(5) | 2.689(4) | Zn1–O6 | 1.997(6) | 2.001(5) | 1.988(5) |
| Ln1–O9 | 2.730(6) | 2.803(4) | Zn1–N1 | 2.082(7) | 2.083(7) | 2.034(6) |
| Ln1–O12 | 2.437(6) | 2.369(4) | Zn1–N2 | 2.035(7) | 2.049(7) | 2.092(6) |
| Ln1–O13 | 2.384(6) | 2.407(4) | Zn1–O1–Ln1 | 103.0(1) | 102.2(2) | 103.0(2) |
| Zn1–O1 | 2.067(5) | 2.063(3) | Zn1–O2–Ln1 | 100.4(1) | 99.6(2) | 99.7(2) |
| Zn1–O2 | 2.044(5) | 2.077(3) | Δ b (Zn1) | 0.48 | 0.49 | 0.48 |
| Zn1–O11 | 1.996(6) | 2.005(4) | σc (Zn1) | 35.5 | 36.0 | 34.9 |
| Zn1–N1 | 2.022(8) | 2.075(4) | ωd | 62.4(4) | 61.6(3) | 62.2(2) |
| Zn1–N2 | 2.084(7) | 2.069(4) | εe | 62.0(3) | 61.3(4) | 62.0(3) |
| Zn2–O6 | 2.064(5) | 2.059(4) | φf | 173.3(2) | 172.8(1) | 172.9(1) |
| Zn2–O7 | 2.068(5) | 2.077(3) | ||||
| Zn2–O14 | 2.009(6) | 1.993(4) | ||||
| Zn2–N3 | 2.052(8) | 2.079(5) | ||||
| Zn2–N4 | 2.074(6) | 2.050(4) | ||||
| Zn1–O1–Ln1 | 101.1(2) | 103.7(1) | ||||
| Zn1–O2–Ln1 | 102.7(2) | 103.3(1) | ||||
| Zn2–O6–Ln1 | 103.9(2) | 102.4(1) | ||||
| Zn2–O7–Ln1 | 103.3(2) | 100.9(1) | ||||
| Δ b (Zn1; Zn2) | 0.46; 0.50 | 0.51; 0.49 | ||||
| σc (Zn1; Zn2) | 34.7; 34.8 | 34.5; 35.7 | ||||
| ωd | 36.2(4) | 34.6 (3) | ||||
| εe | 42.6(4) | 42.1(3) | ||||
| φf | 178.8(1) | 178.8(3) |
| Compound | λabs a (nm) | λex/λem a (nm) | λex/λem b (nm) |
|---|---|---|---|
| H3L | 229.0; 292.5; 341.5; 425.5 | 337/414.0 | 363/541.0 |
| 1 | 235.5; 267.0; 358.5 | 359/421.0 | 357/457.0 |
| 2 | 235.5; 268.0; 359.5 | 359/458.0; 561.0; 598.0; 644.0 | 357/460.0; 561.0; 598.0; 644.0 |
| 3 | 234.5; 267.5. 359.0 | 359/458.0 | 357/461.0 |
| 4 | 236.0. 267.0; 359.0 | 359/424.0 | 357/507.0 |
| 5 | 234.0; 267.5; 359.5 | 359/430.0 | 357/519.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroslaw, B.; Cristóvão, B.; Hnatejko, Z. Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules 2018, 23, 1761. https://doi.org/10.3390/molecules23071761
Miroslaw B, Cristóvão B, Hnatejko Z. Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules. 2018; 23(7):1761. https://doi.org/10.3390/molecules23071761
Chicago/Turabian StyleMiroslaw, Barbara, Beata Cristóvão, and Zbigniew Hnatejko. 2018. "Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization" Molecules 23, no. 7: 1761. https://doi.org/10.3390/molecules23071761
APA StyleMiroslaw, B., Cristóvão, B., & Hnatejko, Z. (2018). Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules, 23(7), 1761. https://doi.org/10.3390/molecules23071761








