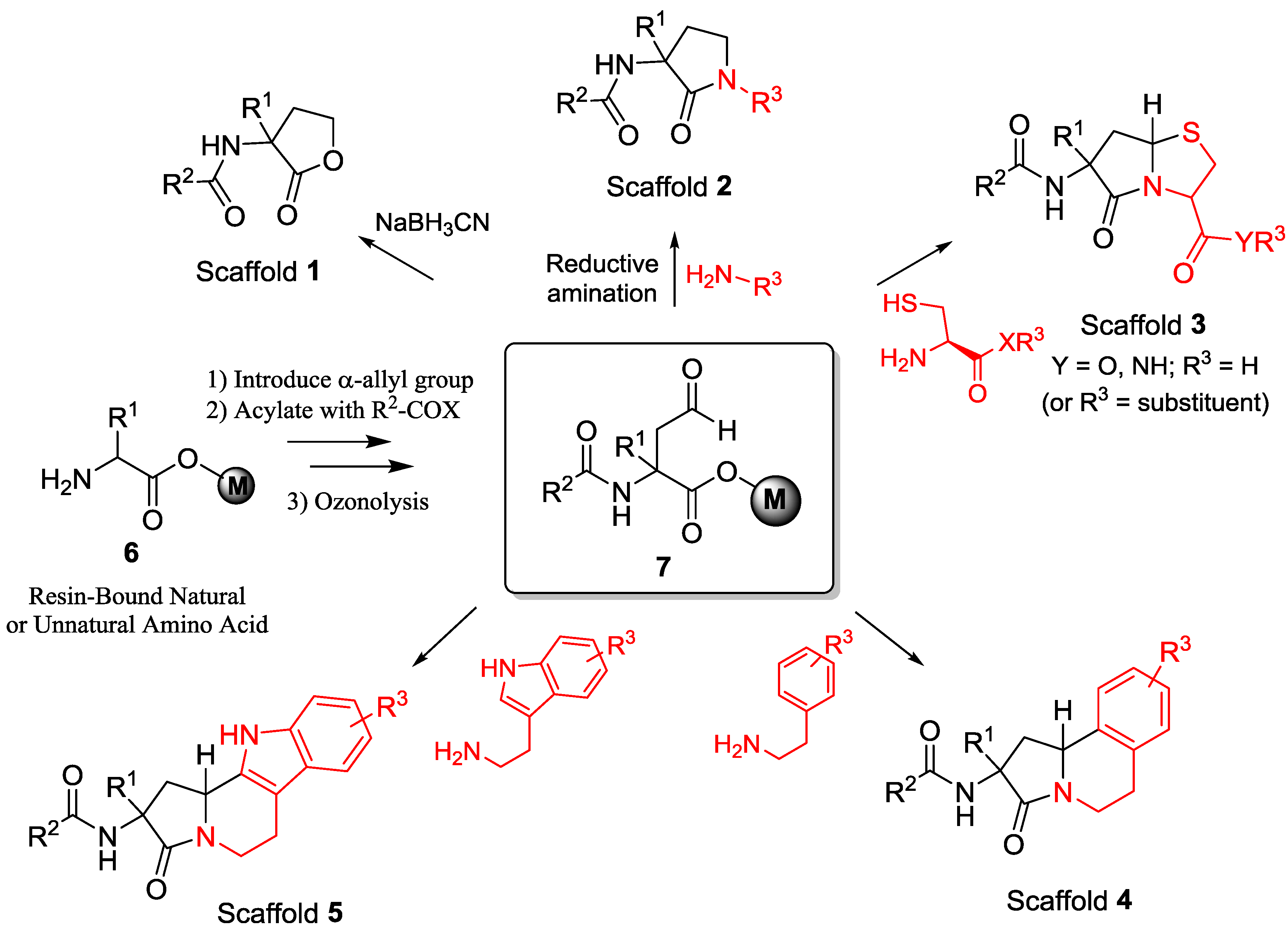
3. Materials and Methods
All of the reagents were purchased from Acros Organics (Geel, Belgium), Advanced Chemtech (Louisville, KY, USA), EMD Millipore (Burlington, MA, USA), or Sigma-Aldrich Chemical (St. Louis, MO, USA), and were used without further purification. Boc-protected amino acids on Merrifield resin were purchased from Polymer Laboratories, Amherst, MA, USA (now Agilent Technologies, Santa Clara, California, USA). Solid-phase peptide synthesis (SPPS) vessels were purchased from Chemglass (Vineland, NJ, USA). 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance III 500 and 400 spectrometers (1H, 500.13 MHz; 13C, 125.76 MHz) (Billerica, MA, USA) using tetramethylsilane as internal standard in CDCl3 or unless otherwise indicated. Chemical shifts are reported in parts per million (ppm, δ units). Coupling constants, J, are reported in Hertz (Hz). Splitting patterns are designated as s: singlet, br s: broad singlet, d: doublet, dd: doublet of doublets, ddd: doublet of doublets of doublets, q: quartet, and m: multiplet. High-resolution mass spectra (HRMS) were obtained using a Thermo Electron Corporation MAT 95XP-Trap (Waltham, MA, USA) or an Agilent 1200 HPLC-6130 MSD Mass Spectrometer (Santa Clara, CA, USA) in the electrospray ionization (ESI) or in the chemical ionization (CI) modes at the Mass Spectrometry Facility of Indiana University, Bloomington, IN. High performance liquid chromatography-mass spectrometry (HPLC/MS) was performed on an Agilent 1100 Series LC/MSD (Santa Clara, CA, USA) in the electrospray ionization positive mode using a 4.6 × 150 mm Agilent Eclipse XDB five-micron C18 reverse-phase column. Nuclear Overhauser enhancement (nOe) 1-D difference spectroscopy was conducted using the Bruker pulse sequence SELNOGP (Billerica, MA, USA) with the following acquisition parameter changes: Spectral width, SW = 10.0 ppm, Transmitter frequency offset, O1P = 4.0 ppm, Duration delay, D[8] = 0.6 s.
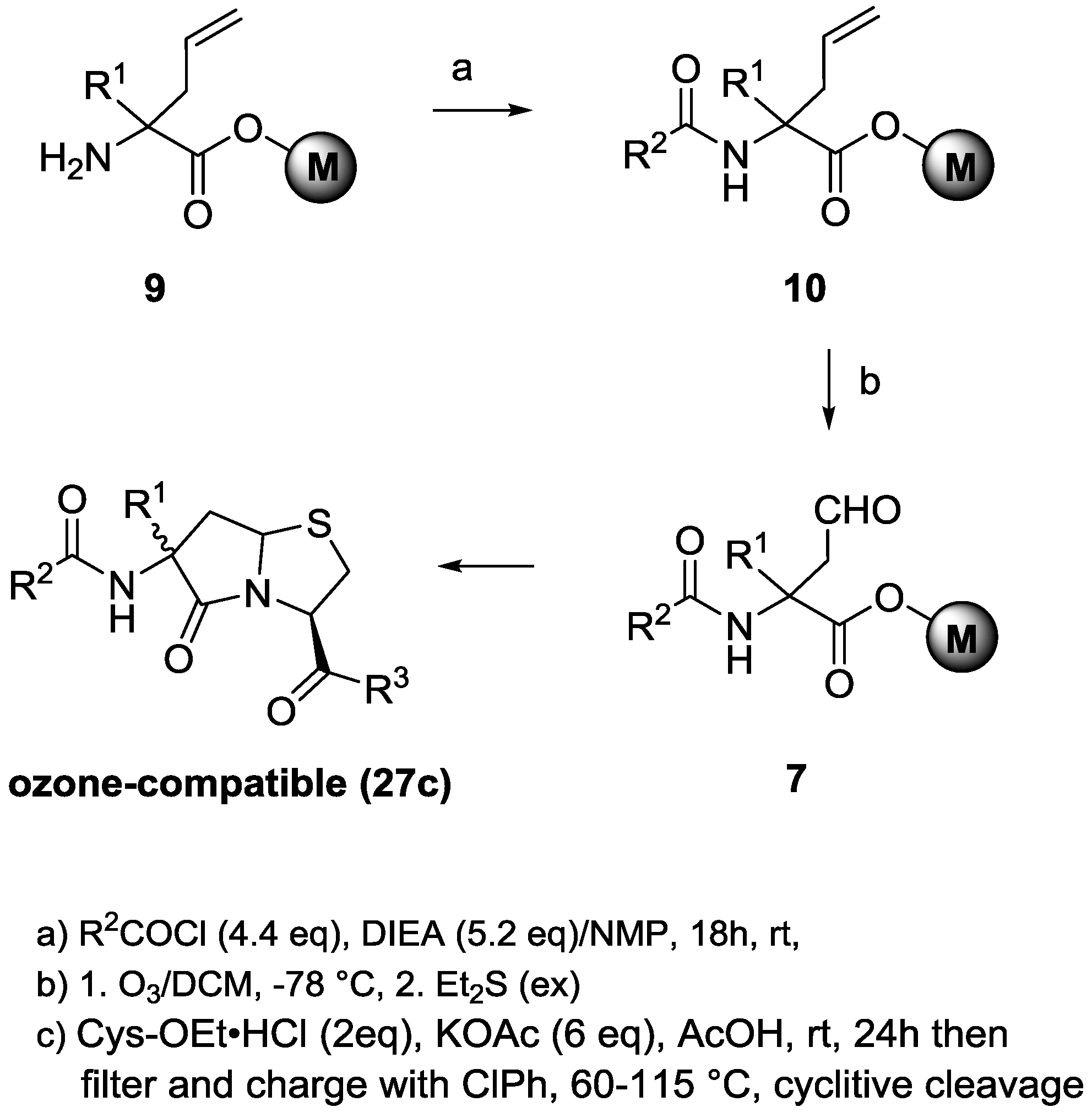
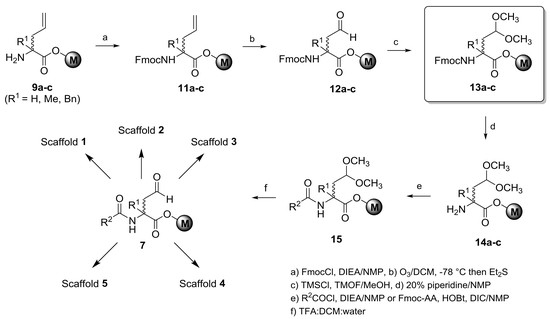
α-Allyl-α-R1-N-(fluorenylmethyloxycarbonyl)glycine on Merrifield resins (11a–c, R1 = H, Me, Bn). The amine resin
9a–
c (R
1 = H, Me, Bn, 7.60–8.50 mmol) [
3], contained in a 500-mL SPPS vessel, was washed with 4 × 10 mL of
N-methyl pyrrolidinone (NMP). To the resin was then added 40 mL of NMP, followed by 3.8 equivalents of diisopropylethylamine (DIEA). The vessel was swirled gently to mix the contents, and 3.0 equivalents of Fmoc chloride in 60–70 mL of NMP was added in one portion. The vessel was rocked on an orbital shaker and after 24 h the vessel was drained, and the resin was washed 3 × 45 mL each with NMP, 1:1 THF:EtOH, THF, and dichloromethane (DCM) to give resins
11a–
c (R
1 = H, Me, Bn). The resin was then dried under a slow stream of dry nitrogen gas overnight.
α-(2-Oxoethyl)-α-R1-N-(fluorenylmethyloxycarbonyl)glycine on Merrifield resins (12a–c, R1 = H, Me, Bn). A 250-mL, three-neck, round-bottomed flask was charged with 1.80–3.20 mmol of resin 11a–c (R1 = H, Me, Bn), a trace of Sudan III red dye, and 40 mL of DCM under dry argon gas. The contents were cooled in a dry ice/acetone bath, and the argon flow was replaced with a subsurface flow of oxygen gas at a rate of 0.6–0.8 L/min. Ozonolysis using an ozone generator was performed at a current of 1.0 ampere until the red dye was rendered colorless (2–3 h). The current was then reduced to zero while oxygen bubbling continued for 10 min. Diethyl sulfide (1.0 mL, 9.3 mmol) was added, and the solution was allowed to gradually warm to room temperature and stir overnight. The resin was collected by filtration into a 50-mL SPPS vessel rinsing over with DCM and THF. The resin was dried under a slow stream of dry argon gas and then under high vacuum to give 12a–c (R1 = H, Me, Bn).
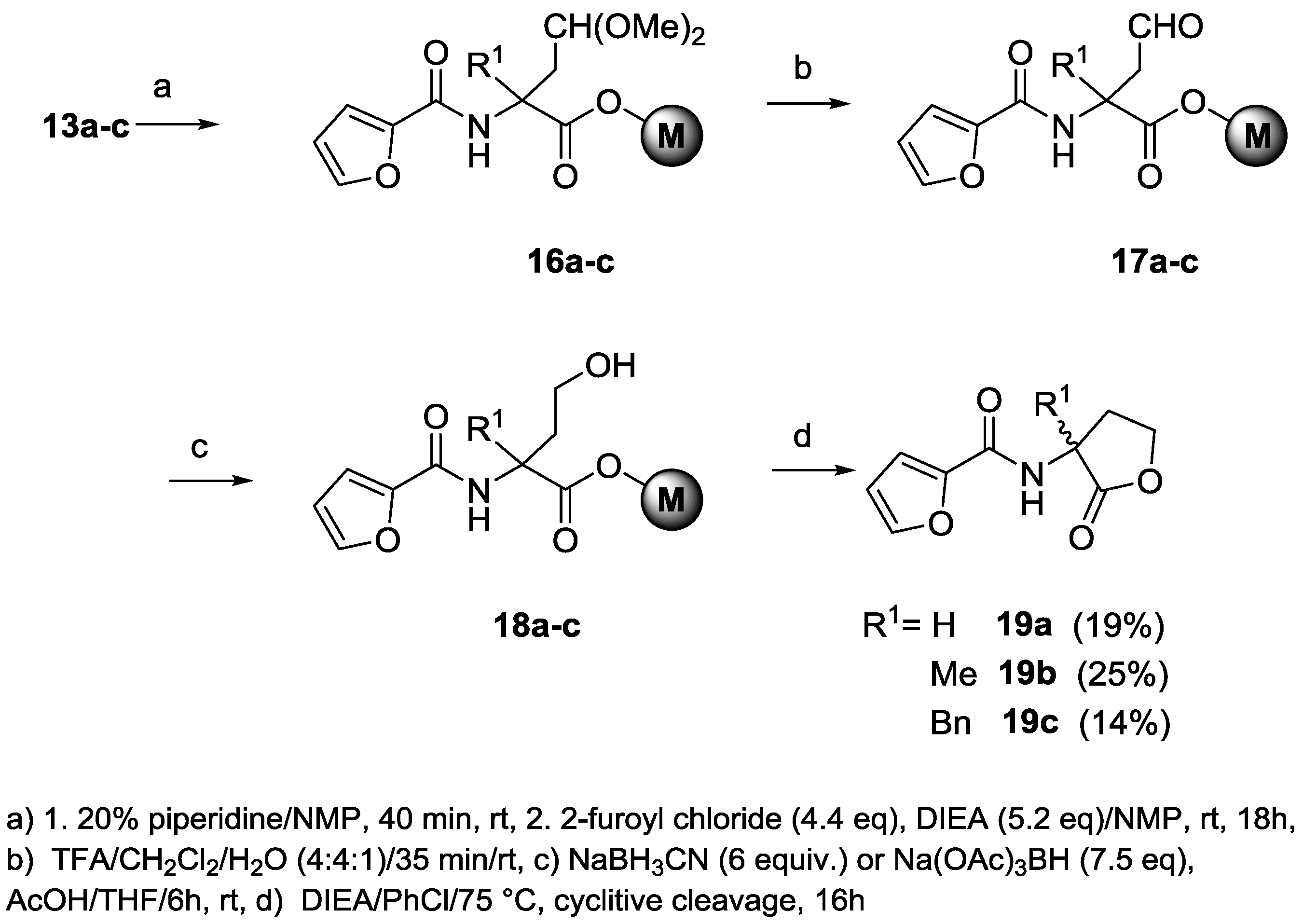
α-(2,2-Dimethoxyethyl)-α-R1-N-(fluorenylmethyloxycarbonyl)glycine on Merrifield resins (13a–c, R1 = H, Me, Bn). To 1.80–3.20 mmol of resin 12a–c (R1 = H, Me, Bn) contained in a 50-mL SPPS vessel, 12–28 mL of absolute methanol, 4.4 equivalents of 1.0 M trimethylsilyl chloride in THF, and 25 equivalent of trimethylorthoformate were added under dry argon. The vessel was rocked overnight, and was then drained to leave a small volume of liquid over the resin. Diisopropylethylamine in absolute methanol (30 mL of a 3% v/v solution) was added. After shaking for 15 min, the vessel was drained, and the resin was washed with 3 × 20 mL of 3% DIEA in MeOH, 3 × 15 mL of NMP, and 8 × 15 mL of DCM and was dried overnight under a slow stream of dry nitrogen gas and then under high vacuum to give resin 13a–c (R1 = H, Me, Bn).
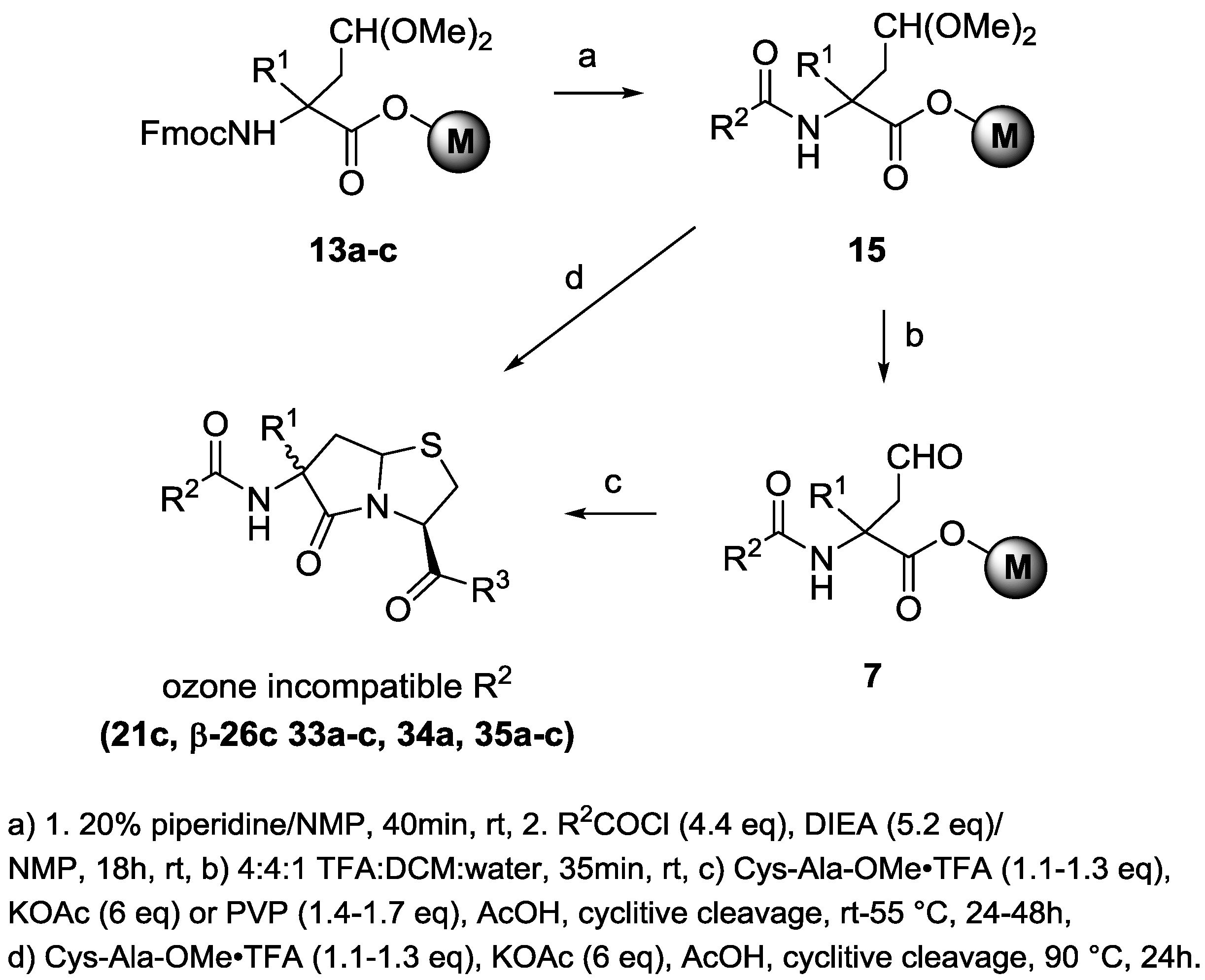
2-(Furan-2-carboxamido)-4,4-dimethoxy-2-R1-butanoic acid on Merrifield resins (16a–c, R1 = H, Me, Bn). Fmoc acetal resin 13a–c (R1 = H, Me, Bn, 100–157 μmol) contained in a 3.5-mL or 5-mL SPPS vessel was treated with 20% piperidine in NMP, and the vessel was gently agitated for 40–50 minutes at room temperature. The vessel was drained, and the resin was washed with 5 × 2–3 mL of NMP. To the deprotected resin was then added 5.2 eq of a 0.50-M solution of diisopropylethylamine (DIEA) in NMP, followed by 4.4 eq of a 0.50 M solution of 2-furoyl chloride in NMP. After 16–18 h, the vessel was drained, and the resin was washed twice each with NMP, 1:1 THF/MeOH (EtOH), and THF, and four times with DCM to give resin 16a–c (R1 = H, Me, Bn).
2-(Furan-2-carboxamido)-2-R1-4-oxobutanoic acid on Merrifield resins (17a–c, R1 = H, Me, Bn). To 100–157 μmol of DCM-swelled resin 16a–c (R1 = H, Me, Bn), 3 mL of TFA:DCM:water (4:4:1) was added. The mixture was gently agitated for 35 min at room temperature. The SPPS vessel was drained, and the resin was washed with 3 × 2 mL each with DCM and then THF to give aldehyde resin 17a–c (R1 = H, Me, Bn).
2-(Furan-2-carboxamido)-4-hydroxy-2-R1-butanoic acid on Merrifield resins (18a–c, R1 = H, Me, Bn). To 100–157 μmol of resin 17a–c (R1 = H, Me, Bn), 6 eq of a 0.90-M solution of sodium cyanoborohydride in 0.50 M of acetic acid in THF (for R1 = H, Me) or 7.5 eq of a suspension of sodium triacetoxyborohydride in 0.50 M of acetic acid in THF (for R1 = Bn) was added. The mixture was gently agitated for 6 h at room temperature. The vessel was drained, and the resin was washed with 3 × 2 mL each with THF, 30% aqueous THF, and THF to give the hydroxyl resin (18a–c, R1 = H, Me, Bn).
N-(3-R1-2-oxotetrahydrofuran-3-yl)furan-2-carboxamide (19a–c, R1 = H, Me, Bn). Resin 18a–c (100–157 μmol, R1 = H, Me, Bn) was washed with 2 × 2 mL of chlorobenzene, and was then treated with 2 mL of chlorobenzene followed by 8 eq of DIEA. The mixture was heated at 75 °C for 16 h. After cooling, the vessel was drained, and the resin was washed with 2 × 2 mL of DCM. The combined filtrates were evaporated to dryness to give a residue that was purified by silica gel chromatography:
N-(2-oxotetrahydrofuran-3-yl)furan-2-carboxamide (19a, R1 = H), dichloromethane and dichloromethane/ethyl acetate mobile phases (94/6, 9/1); 5.7 mg (19% over nine steps) of
19a as a white solid;
1H-NMR δ 2.28 (dq,
J = 11.5 and 8.9 Hz, 1H), 2.92 (dddd,
J = 12.6, 8.6, 5.8, and 1.0 Hz, 1H), 4.34 (ddd,
J = 11.2, 9.2, and 5.8 Hz, 1H), 4.52 (dt,
J = 9.7 and 0.8 Hz, 1H), 4.74 (ddd,
J = 11.7, 8.6, and 6.3 Hz, 1H), 6.52 (dd,
J = 3.5 and 1.8 Hz, 1H), 6.89 (br d,
J = 3.9 Hz, 1H), 7.16 (dd,
J = 3.5 and 0.5 Hz, 1H), 7.47 (dd,
J = 1.6 and 0.6 Hz, 1H);
13C-NMR δ 30.6, 48.1, 66.1, 112.3, 115.3, 144.6, 146.9, 158.6, 175.1; HRMS (TOF ES
+)
m/
z calculated for C
9H
9NO
4 (M + Na) 218.0429; found 218.0429 (As shown in the
Supplementary Materials).
N-(3-methyl-2-oxotetrahydrofuran-3-yl)furan-2-carboxamide (19b, R1 = Me), dichloromethane and dichloromethane/ethyl acetate mobile phases (9/1, 8/2) as mobile phases; 5.2 mg (25% over nine steps) of 19b as a white solid; 1H-NMR δ 1.63 (s, 3H), 2.54 (ddd, J = 13.0, 7.0, and 2.3 Hz, 1H), 2.83 (dt, J = 12.9 and 9.7 Hz, 1H), 4.32 (dt, J = 9.6 and 7.0 Hz, 1H), 4.56 (dt, J = 9.3 and 2.3 Hz, 1H), 6.51 (dd, J = 3.5 and 1.7 Hz, 1H), 6.75 (br s, 1H), 7.13 (d, J = 3.5 Hz, 1H), 7.46 (d, J = 1.6 Hz, 1H); 13C-NMR δ 22.5, 35.1, 55.7, 65.6, 112.3, 115.1, 144.3, 147.2, 157.7, 177.3; HRMS (TOF ES+) m/z calculated for C10H12NO4 (M + H) 210.0761; found 210.0762.
N-(3-benzyl-2-oxotetrahydrofuran-3-yl)furan-2-carboxamide (19c, R1 = Bn), dichloromethane and dichloromethane/ethyl acetate (96/4, 9/1) as mobile phases; 4.3 mg (14% over nine steps) of 19c as a white film; 1H-NMR δ 2.74–2.79 (m, 2H), 3.23 (d, J = 13.2 Hz, 1H), 3.29 (d, J = 13.2 Hz, 1H), 3.49 (dt, J = 9.2 and 7.6 Hz, 1H), 4.31 (dt, J = 8.9 and 3.2 Hz, 1H), 6.51 (dd, J = 3.5 and 1.7 Hz, 1H), 6.83 (br s, 1H), 7.14 (dd, J = 3.5 and 0.7 Hz, 1H), 7.26–7.28 (m, 2H), 7.32–7.35 (m, 3H), 7.45 (dd, J = 1.6 and 0.7 Hz, 1H), 13C-NMR δ 33.0, 42.0, 59.8, 65.9, 112.3, 115.2, 127.9, 128.9, 130.0, 133.6, 144.4, 147.2, 157.7, 179.8; HRMS (TOF ES+) m/z calculated for C16H15NO4Na (M + Na) 308.0899; found 308.0907.
2-Benzyl-4,4-dimethoxy-2-(3,4,5-trimethoxybenzamido)butanoic acid on Merrifield resin (20c). The Fmoc acetal resin 13c (320 mg, 0.298 mmol) was placed in a 5-mL SPPS vessel, washed with DMF (2 × 3 mL), and then slowly washed with 20% piperidine/DMF (6 × 4 mL × 5 min), then with DMF (4 × 4 mL), with CH2Cl2 (3 × 4mL), and again with DMF (3 × 4 mL). The vessel was drained with a stream of argon, and the resin was treated with a freshly prepared solution of 3,4,5-trimethoxybenzoic acid (191 mg, 0.9 mmol, 3 eq) and HOBt-H2O (122 mg, 0.9 mmol, 3 eq) in 3 mL of a 1:1 CH2Cl2-DMF mixture. This was followed by the addition of diisopropylcarbodiimide (DIC, 140 μL, 114 mg, 0.9 mmol) and DIEA (78 μL). The vessel was capped and rotated at room temperature (RT) for 24 h. The solution was then drained from the vessel, and the resin was washed with DMF (3 × 4 mL), and CH2Cl2 (4 × 4 mL), and then dried with a stream of argon for 5 min to afford resin 20c.
Ethyl(3R,6R,S,7aS)-6-benzyl-5-oxo-6-(3,4,5-trimethoxybenzamido)hexahydropyrrolo[2,1-b]-thiazole-3-carboxylate (21c). To 0.298 mmol of resin 20c, a solution of KOAc (147.5 mg, 1.5 mmol, 5 eq) and L-Cys-OEt-HCl (111.4 mg, 0.6 mmol, 2 eq) in glacial AcOH (3 mL) was added. The vessel was capped, rotated for 15 min, and then transferred to an oven preheated to 90 °C. After 24 h, the mixture was cooled to RT, the solution was drained from the resin, and the resin was washed with THF (2 × 3 mL) and then with CH2Cl2 (3 × 3 mL). The combined filtrates were transferred to a separatory funnel containing brine (50 mL), water (50 mL), and CH2Cl2 (60 mL). After extraction, the phases were separated, and the organic phase was extracted with 10% KHCO3/H2O (80 mL). The organic layer was dried over Na2SO4, filtered, and concentrated in vacuo to afford a yellow oil (206 mg). This sample was purified by flash chromatography on silica gel (4.5 g; 45% EtOAc/hexanes). This gave the product diastereomeric thiazolidines 21c as a colorless solidifying oil (41 mg; 27%). A portion (12.5 mg) was chromatographed on a 500-mg HyperSep SI column of silica gel to give 4.6 mg of 21c as a 2:1 mixture of β:α; 1H-NMR (CDCl3) δ 1.31 and 1.32 (2t, 3H, J = 7.2 Hz and 7.2 Hz,), 2.64, (dd, 0.65H, J = 13.3 Hz and 7.3 Hz), 2.71 (dd, 0.37H, J = 14.6 Hz and 2.2 Hz), 2.93 (dd, 0.39H, J = 14.6 Hz and 7.7 Hz ), 3.21–3.49 (m, 5.14H), 3.86–3.87 (s, 3H), 3.86–3.87 (s, 2 × 3H), 4.20–4.31 (m, 2H), 4.78 (t, 0.68H, J = 6.9 Hz), 5.10 (dd, 0.38H, J = 8.1 Hz and 3.7 Hz 1H), 5.14 (dd, 0.64H, J = 7.2 Hz and 3.7 Hz), 5.41 (dd, 0.36H, J = 7.7 Hz and 2.2 Hz), 6.44 (bs, 0.39H), 6.60 (bs, 0.60H), 6.90 (s, 0.73H), 6.92 (s, 1.26H), 7.23–7.39 (m, 5.05H); 13C-NMR (CDCl3) δ 14.1, 14.2, 33.7, 35.4, 36.5, 41.7, 42.7, 42.9, 56.3, 56.4, 58.2, 58.4, 60.9, 62.06, 62.09, 63.0, 63.1, 65.4, 77.2, 104.47, 104.53, 127.5, 127.8, 128.5, 128.9, 129.3, 130.4, 130.6, 134.6, 135.0, 141.4, 153.20, 153.24, 166.1, 166.4, 168.8, 169.3, 173.0, 174.6. The diastereomers were separated on a 2.1 × 100 mm Agilent Eclipse XDB-C18 column using the mobile phase 65% MeOH, 35% 0.1% formic acid, and 5% acetonitrile at a flow rate of 0.3 µL/min, and their accurate masses were determined: 5.19 min, calculated for C26H31N2O7S (M + H) 515.1846; found 515.1848; 6.07 min, calculated for C26H31N2O7S (M + H) 515.1846; found 515.1848.
2-((S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)propanamido)-2-benzyl-4,4-dimethoxybutanoic acid on Merrifield resin (22c). Resin 13c (87.1 μmol) was treated with 2.5 mL of 20% piperidine in NMP for 30 min with gentle agitation. The vessel was drained, and the deprotected resin was washed with 5 × 3 mL of NMP. To this resin 262 mg (5.00 eq) of Fmoc-Ala anhydride in NMP in 3 mL of NMP was added. After 48 h, the vessel was drained, and the resin was washed with 4 × 3 mL each of NMP, THF, and NMP to give resin 22c.
2-Benzyl-2-((S)-2-(4-chlorobenzamido)propanamido)-4,4-dimethoxybutanoic acid on Merrifield resin (23c). Resin 22c (87.1 μmol) was treated with 2.5 mL of 20% piperidine in NMP for 30 min with gentle agitation. The vessel was drained, and the deprotected resin was washed with 6 × 3 mL of NMP. The resin was then treated with 4.4 eq of 1.0 M solutions of 4-chlorobenzoyl chloride followed by 5.2 eq of DIEA. After 18 h, the vessels were drained, and the resin was washed with 4 × 3 mL each of NMP, 1:1 THF:MeOH, THF, and DCM to give resin 23c.
N-((2S)-1-((3-Benzyl-2-oxotetrahydrofuran-3-yl)amino)-1-oxopropan-2-yl)-4-chlorobenz-amide (24c). Resin 23c (87.1 μmol) was treated with 3 mL of 4:4:1 TFA:DCM:water for 35 min with gentle agitation. The vessel was drained, and the resin was washed with 5 × 3 mL of DCM and 3 × 3 mL of THF to afford the aldehyde resin. The resin was then treated with 3 mL of 0.50 M acetic acid in THF for 10–15 min. The vessel was drained, and the resin was treated with 1 mL of 0.50 M acetic acid in THF followed by 1.04 mL (6.3 eq) of a 0.53-M solution of sodium cyanoborohydride in 0.50 M of acetic acid in THF. The vessel was gently agitated for 6 h at room temperature; then, it was drained, and the resin was washed with 3 × 3 mL each of THF, 30% aqueous THF, and 4 × 3 mL of THF. The resin was dried under a stream of nitrogen gas, and was then washed with 3 mL of chlorobenzene. Chlorobenzene (3 mL) was then added, followed by 7.9 eq (690 μmol) of DIEA. The resin was then heated at 75 °C for 16 h. After cooling, the vessel was drained, and the filtrate was evaporated to dryness to yield 4.6 mg of crude 24c. Separation of the diastereomers by reverse-phase HPLC chromatography gave 1.4 mg of the stereoisomer with the earlier retention time: 1H-NMR (methanol-d4) δ 1.49 (d, 3H, J = 7.2 Hz), 2.51 (ddd, 1H, J = 13.3 Hz, 8.2 Hz, and 3.0 Hz), 2.65–2.73 (m, 2H), 3.10 (d, 1H, J = 13.0 Hz), 3.15–3.21 (m, 1H), 3.20 (d, 1H, J = 13.0 Hz), 4.18 (ddd, 1H, J = 10.4 Hz, 8.9 Hz, and 3.0 Hz), 4.57 (q, 1H, J = 7.2 Hz), 7.30–7.35 (br m, 5H), 7.49 (d, 2H, J = 8.7 Hz), 7.87 (d, 2H, J = 8.8 Hz); 13C-NMR (methanol-d4) δ 16.2, 31.0, 41.5, 49.3, 60.0, 65.1, 127.4, 128.28, 128.33, 128.9, 130.0, 132.4, 133.6, 137.6, 167.6, 173.2, 177.7; HRMS (ES+) m/z calculated for C21H21ClN3O4S (M + Na) 423.1082; found 423.1085, and 1.5 mg of the stereoisomer with the later retention time: 1H-NMR (CDCl3) δ 1.46 (d, 3H, J = 7.0 Hz), 2.59 (ddd, 1H, J = 13.2 Hz, 7.6 Hz, and 4.9 Hz), 2.64–2.72 (m, 1H), 3.07 (d, 1H, J = 13.3 Hz), 3.16 (d, 1H, J = 13.2 Hz), 3.51 (m, 1H), 4.30 (dt, 1H, J = 9.5 Hz and 2.8 Hz), 4.67 (quintet, 1H, J = 7.1 Hz), 6.78 (br d, 1H, J = 7.3 Hz), 6.92 (br s, 1H), 7.21–7.24 (m, 2H), 7.28–7.31 (m, 3H), 7.42 (d, 2H, J = 8.6 Hz), 7.75 (d, 2H, J = 8.6 Hz); 13C-NMR (CDCl3) 17.6, 32.3, 41.9, 49.0, 59.8, 65.5, 128.0, 128.6, 128.90, 128.95, 130.1, 132.0, 133.4, 138.3, 166.6, 171.7, 176.7; HRMS (ES+) m/z calculated for C21H21ClN3O4S (M + Na) 423.1082; found 423.1085.
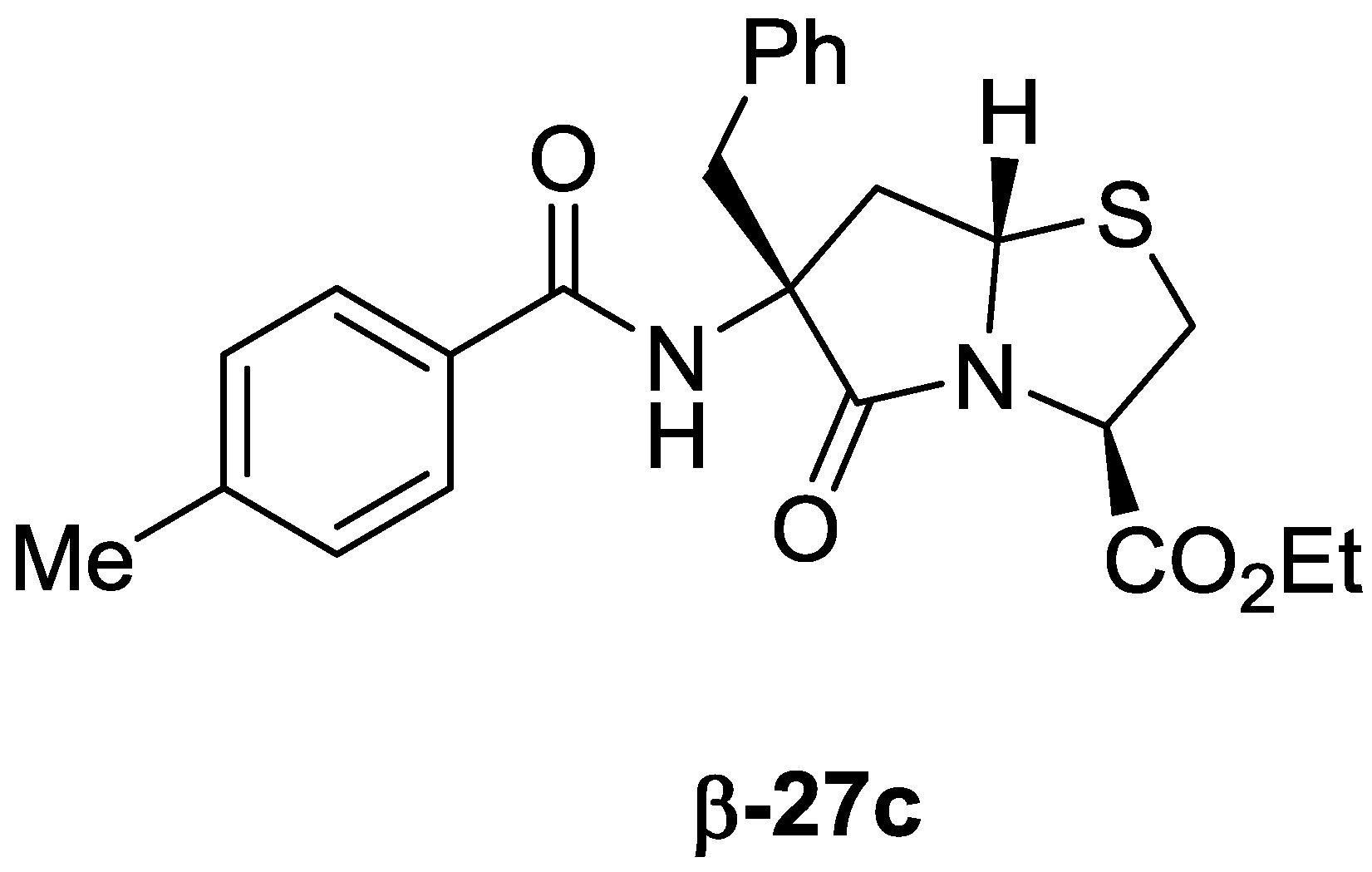
2-Benzyl-4,4-dimethoxy-2-(4-methylbenzamido)butanoic acid on Merrifield resin (25c): Resin 13c (791 μmol) in a 50-mL SPPS vessel was washed with 3 × 20 mL of NMP, followed by 4 × 25 mL × five minutes of 20% piperidine in NMP and then with 3 × 20 mL of NMP. To the deprotected resin was added 20 mL of NMP, 722 μL (5.24 eq) of DIEA, and then 542 mg (4.4 eq) of p-toluoyl chloride. The vessel was placed on an orbital shaker, and after 40 h was drained, and the resin was washed with 3 × 20 mL of NMP, 2 × 20 mL of 1:1 THF:MeOH, 2 × 20 mL of THF, and 3 × 20 mL of DCM to give resin 25c, which was dried under vacuum to afford a mass of 1.19 g.
2-((3R)-6-Benzyl-6-(4-methylbenzamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxamido)-acetic acid (β-26c): Resin 25c (118 μmol), 56 mg (570 μmol, 4.8 eq) of potassium acetate and 42.9 mg (241 μmol, 2.0 eq) of Cys-Gly-OH in 1.5 mL of acetic acid contained in a 3.5-mL reaction vessel was heated at 90 °C for 24 h. After cooling, the vessel was drained, and the resin was washed with 3 × 2 mL of dichloromethane. The combined filtrates were evaporated, and the residue was partitioned between 25 mL of dichloromethane and 25 mL of 1.0 N HCl. After separation, the aqueous phase was extracted with 10 mL of dichloromethane, and the combined organics were washed with 20 mL of 1.0 N HCl and were dried (Na2SO4). Concentration gave 9.7 mg, which was triturated under 250 μL of dichloromethane to afford 3.7 mg (7% over 10 steps) of β-26c as a white solid; 1H-NMR (CD3OD) δ 2.64 (dd, J = 13.2 Hz and 6.6 Hz, 1H), δ 2.90 (dd, J = 13.3 Hz and 7.3 Hz, 1H), 3.15 (m, 2H), 3.28 (dd, J = 10.8 Hz and 3.4 Hz, 1H), 3.32 (10.8 Hz and 6.5 Hz, 1H), 3.76 (m, 2H), 4.10 (t, J = 7.0 Hz, 1H), 4.91 (dd, J = 6.5 Hz and 3.4 Hz, 1H), 6.55 (br t, J = 5.8 Hz, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.21–7.30 (m, 5H), 7.63 (d, J = 8.2 Hz, 2H), 8.37 (br s, 1H); 13C-NMR (CD3OD) δ 21.5, 34.6, 41.5, 41.8, 43.0, 61.7, 62.1, 67.4, 128.6, 128.8, 129.8, 130.2, 131.6, 132.3, 135.9, 143.8, 169.7, 170.9, 172.3, 176.2; HRMS (TOF ES+) m/z calculated for C24H26N3O5S (M + H) 468.1593; found 468.1609. Recrystallization from ethanol gave a sample for x-ray analysis.
2-((S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)propanamido)-4,4-dimethoxy-2-methylbutanoic acid on Merrifield resin (28b). A 25-mL SPPS vessel was charged with 773 mg (363 μmol) of resin 13b, and was swelled in NMP for 30 minutes. The vessel was drained, and the resin was treated with 5 mL of 20% piperidine in NMP for 5 min. The vessel was drained, the resin was treated with 10 mL of 20% piperidine, and the vessel was rocked on an orbital shaker for 40 min. Then, it was drained, and the resin was washed with 5 × 10 mL of NMP. The deprotected resin was then treated with 859 mg (1.11 mmol, 3.05 eq) of Fmoc-Ala anhydride in 4.5 mL of NMP. The vessel was rocked for 42 h, drained, and the resin was washed with 6 × 15 mL of NMP to give resin 28b.
4,4-Dimethoxy-2-methyl-2-((S)-2-(4-nitrobenzamido)propanamido)butanoic acid on Merrifield resin (29b). To 363 μmol of resin 28b in a 25-mL SPPS vessel, 5 mL of 20% piperidine in NMP was added. After five min, the vessel was drained, and the resin was treated with 15 mL of 20% piperidine. The vessel was rocked for 40 min, drained, and the resin was washed with 6 × 15 mL of NMP. To the deprotected resin, 3.80 mL (1.90 mmol, 5.2 eq) of a 0.50-M solution of DIEA in NMP was added, followed by 3.21 mL (1.60 mmol, 4.4 eq) of a 0.50-M solution of 4-nitrobenzoyl chloride in NMP. The vessel was rocked for 18 h, drained, and the resin was washed with 2 × 15 mL of NMP, 3 × 15 mL of 1:1 THF:MeOH, 3 × 15 mL of THF, and 5 × 15 mL of DCM to give resin 29b.
Fmoc-Ala Anhydride, (Fmoc-Ala)2O. A variation of the method of Izdebski and Pawlak [
48] is described here. A 50-mL, three-neck, round-bottomed flask under argon was charged with 1.25 g (4.00 mmol) of Fmoc-Ala-OH. The flask was fitted with a thermometer and two rubber septa, and 11 mL of DCM was added via syringe. The mixture was treated with 1 mL of anhydrous DMF, and then cooled to 3 °C. To the mixture, 1.69 mL (252 mg, 2.00 mmol) of 1.0 M diisopropylcarbodiimide (DIC) in DCM was added dropwise via syringe over a two-minute period. After stirring at 3 °C for 30 minutes, the mixture was allowed to warm to room temperature, and was stirred an additional 10 minutes. The contents were filtered to afford 469 mg of crude anhydride (70 mol%) containing 30 mol% diisopropylurea (DIU),
1H-NMR 1.14 (d, 12H,
J = 6.5 Hz, DIU), 1.49 (d, 6H,
J = 7.0 Hz), 3.83 (octet, 2H,
J = 6.6 Hz, DIU), 3.99 (br s, 2H, DIU), 4.21 (t, 2H,
J = 7.0 Hz), 4.38–4.49 (2m, 6H), 5.26 (m, 2H), 7.31 (t, 4H,
J =7.2 Hz), 7.40 (t, 4H,
J =7.5 Hz), 7.58 (br t, 4H,
J =6.1 Hz), 7.76 (d, 4H,
J =7.6 Hz). Further drying gave 288 mg. The mother liquor was concentrated to a small volume and afforded an additional 463 mg (81 mol% anhydride) after drying. The combined yield of
(Fmoc-Ala)2O was 56%.
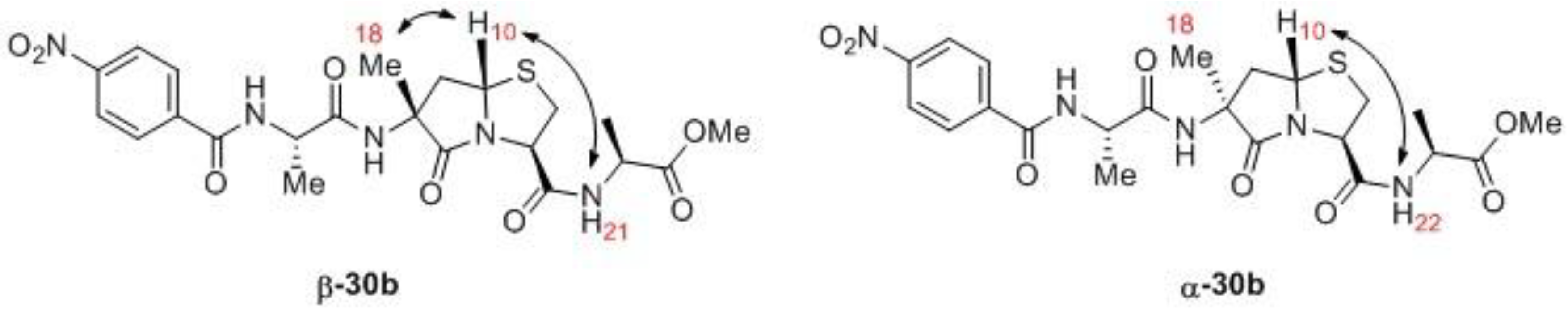
Methyl((3R,7aS)-6-methyl-6-((S)-2-(4-nitrobenzamido)propanamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carbonyl)-L-alaninate (30b).Hydrolysis of dimethyl acetal functional group: Resin 29b (363 μmol) was treated with 13 mL of TFA:DCM:water (4:4:1) for 35 min at room temperature. The SPPS vessel was drained, and the resin was washed with 6 × 15 mL of DCM to give the aldehyde resin. Preparation of Cys-Ala-OMe·TFA: Boc-Cys(Trt)-Ala-OMe (262 mg, 478 μmol, 1.3 eq) was treated with 5 mL of trifluoroacetic acid (TFA): triethylsilane (TES) (97.5:2.5) for two hours at room temperature. The volatiles were removed in vacuo, and the residue was treated with 5 mL of 1:1 diethyl ether:hexanes, and then decanted. This was repeated again with 5 mL of 1:1 diethyl ether: hexanes. The residue was treated with 5 mL of diethyl ether to induce solidification, and then the ether was evaporated to give the TFA salt of Cys-Ala-OMe. This material was dissolved in 3 mL of acetic acid, and was added to the pre-formed aldehyde resin described above followed by 294 mg (3.00 mmol, 8.3 eq) of potassium acetate. The vessel was rocked overnight at room temperature and then drained. The resin was then washed with 2 × 5 mL of acetic acid. Acetic acid (5 mL) was added, and the vessel was heated at 55–60 °C for 48 h. After cooling, the vessel was drained, and the resin was washed with 2 × 5 mL of acetic acid. The three filtrates were combined and evaporated to dryness to give 65.6 mg of crude 30b. The crude material was chromatographed on 2.0 g of normal phase silica gel 60 slurried in DCM. Elution with DCM, 98/2 DCM/MeOH, and 95/5 DCM/MeOH afforded 43.4 mg, which was then separated into its two major diastereomers by reverse-phase chromatography on a 5-micron, 21.4 × 250 mm, C18 column using 50/50 1:1 MeOH/MeCN (5 mM NH4OAc)/water (5 mM NH4OAc) to give 12.5 mg (7% over 13 steps) of α-30b: 1H-NMR 1.45 (d, J = 6.8 Hz, 3H), 1.45 (d, J = 7.2 Hz, 3H), 1.51 (s, 3H), 2.28 (dd, J = 14.3 Hz and 4.3 Hz, 1H), 2.80 (dd, J = 14.3 and 7.8 Hz, 1H), 3.56–3.64 (m, 2H), 3.63 (s, 3H), 4.66 (quintet, J = 7.6 Hz, 1H), 4.79–4.82 (m, 2H), 5.26 (dd, J = 7.8 Hz and 4.3 Hz, 1H), 7.25 (br s, 1H), 7.57 (br d, J = 7.8 Hz, 1H), 7.67 (br d, J = 8.2 Hz, 1H), 8.01 (d, J = 8.8 Hz, 2H), 8.26 (d, J = 8.8 Hz, 2H); 13C-NMR δ 17.0, 18.4, 25.6, 36.7, 38.7, 47.9, 49.0, 52.4, 57.5, 60.8, 62.9, 123.7, 128.6, 139.4, 149.8, 166.2, 168.3, 172.2, 172.6, 173.5; HRMS (TOF ES+) m/z calculated for C22H27N5O8SNa (M + Na) 544.1478; found 544.1493 and 16.2 mg (9% over 13 steps) of α-30b; 1H-NMR δ 1.38 (d, J = 7.2 Hz, 3H), 1.52 (d, J = 6.9 Hz, 3H), 1.60 (s, 3H), 2.65 (dd, J = 12.9 Hz and 7.3 Hz, 1H), 2.82 (dd, J = 12.9 Hz and 6.6 Hz, 1H), 3.48 (dd, J = 11.7 Hz and 7.4 Hz, 1H), 3.67 (dd, J = 11.8 Hz and 5.8 Hz, 1H), 3.76 (s, 3H), 4.50 (quintet, J = 7.1 Hz, 1H), 4.83 (quintet, J = 7.0 Hz, 1H), 4.95 (t, J = 6.6 Hz, 1H), 5.15 (t, J = 7.0 Hz, 1H), 7.06 (br s, 1H), 7.35 (br d, J = 8.6 Hz, 1H), 7.37 (br d, J = 8.0 Hz, 1H), 7.97 (d, J = 8.6 Hz, 2H), 8.27 (d, J = 8.6 Hz, 2H); 13C-NMR δ 18.0, 19.1, 23.6, 35.4, 41.7, 48.4, 49.3, 52.6, 58.8, 61.9, 62.6, 123.8, 128.4, 139.1, 149.8, 165.0, 168.0, 171.7, 172.9, 174.4; HRMS (TOF ES+) m/z calculated for C22H27N5O8SNa (M + Na) 544.1478; found 544.1478.
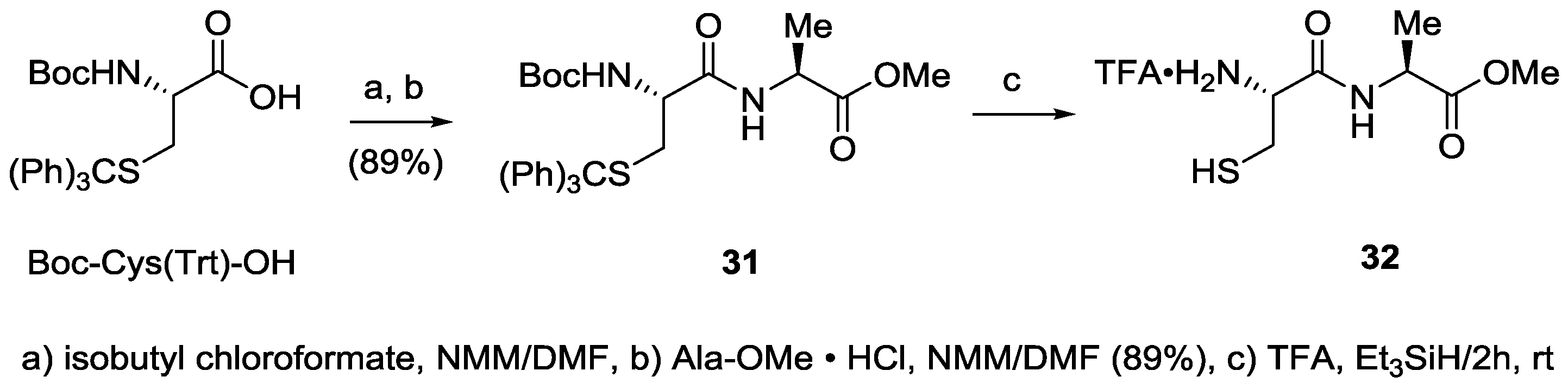
Boc-Cys(Trt)-Ala-OMe (31). A solution of 1.28 g (2.76 mmol) of Boc-Cys(Trt)-OH and 303 μL (2.76 mmol) of N-methylmorpholine in 7 mL of anhydrous DMF was prepared in a 20-mL scintillation vial fitted with a rubber septum and under dry argon gas. This solution was transferred via syringe to a 50-mL, three-neck, round-bottomed flask also under argon. The solution was then cooled in an ice/acetone bath (−9 °C) and treated via syringe with 358 μL (2.76 mmol) of isobutyl chloroformate over a 30 s interval. After three minutes, a solution of 385 mg (2.76 mmol) alanine methyl ester hydrochloride and 303 μL (2.76 mmol) of N-methylmorpholine in 7 mL of anhydrous DMF cooled in ice/acetone was added via syringe. The mixture was stirred at −9 °C for one hour, and then at room temperature for one hour. The reaction mixture was transferred to a 250-mL beaker and was evaporated with a stream of nitrogen overnight. The residue was partitioned between 75 mL of ethyl acetate and 30 mL of pH 2 buffer. The layers were separated, and the organic phase was washed with pH 2 buffer, saturated sodium bicarbonate, twice with water (to pH 7), and then dried over sodium sulfate. Concentration gave 1.38 g (89%) of 31. LC/MS analysis (4.6 × 75 mm, 3.5-micron, Agilent Zorbax SB-C18 column, 70–100% solvent B over 20 min at 0.5 mL/min (solvent B: 1/1 MeCN/MeOH w 5 mM NH4OAc―solvent A: 5 mM NH4OAc) showed that no epimerization had occurred (Rt = 17.0 min, M + 59 = 607, 100%). A portion (518 mg) was recrystallized from 7 mL of methanol to afford 346 mg of 31. 1H-NMR δ 1.35 (d, 3H, J = 7.1 Hz), 1.42 (s, 9H), 2.53 (br dd, 1H, J = 13.0 Hz and 5.0 Hz), 2.74 (br m, 1H), 3.70 (s, 3H), 3.82 (br s, 1H), 4.49 (quintet, 1H, J = 7.2 Hz), 4.77 (br s, 1H), 6.52 (br d, 1H, J = 5.6 Hz), 7.22 (t, 3H, J = 7.2 Hz), 7.29 (t, 6H, J = 7.5 Hz), 7.42 (d, 6H, J = 7.3 Hz); 13C-NMR δ 18.4, 28.3, 33.7, 48.1, 52.4, 53.5, 67.2, 80.3, 126.9, 128.1, 129.6, 144.4, 155.3,170.0, 172.8; HRMS (ESI) m/z calculated for C31H36N2O5SNa (M + Na) 571.2237; found 571.2239.
Cys-Ala-OMe, trifluoroacetic acid salt (32). Boc-Cys(Trt)-Ala-OMe (411 mg, 750 μmol) was treated with 6 mL of trifluoroacetic acid/triethylsilane (97.5/2.5) solution. The mixture was stirred at room temperature for two hours, and was then concentrated to a residue that was treated with 8 mL of 1:1 hexane:diethyl ether, and then decanted from the insoluble oil. This decantation was performed two additional times using 4 mL of 1:1 hexane:diethyl ether. The oil was then triturated under diethyl ether to induce solidification. The mixture was then evaporated to give 32 as a white solid, which was immediately used in the cyclitive cleavage.
Compounds 33a–
c, 34a, and 35a–
c were prepared according to the methods outlined in
Scheme 11 and
Scheme 12:
(3R)-Ethyl 6-(4-chlorobenzamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxylate (α-33a and β-33a): A mixture of 242 μmol of 15 (R1 = H, R2 = 4-ClPh) in 10 mL of dichloromethane contained in a 25-mL glass reaction vessel for 15 minutes was drained, and the resin was then treated with 10 mL of 4:4:1 TFA:CH2Cl2:water. The vessel was rocked for 35 minutes, drained, and the resin was washed with 5 × 3 mL of dichloromethane, and then with 2 × 3 mL of acetic acid. The now-formed aldehyde resin 7 (R1 = H, R2 = 4-ClPh) was then converted to 33a using Method C by treatment with 340 μmol (1.40 equiv.) of polyvinylpyridine, 193 μmol of cysteine ethyl ester hydrochloride, and 5 mL of acetic acid. The vessel was rocked overnight at room temperature. LC/MS analysis indicated product formation. The contents were then heated/rocked at 55 °C for 24 h. The vessel was drained, and the filtrate was evaporated to dryness affording 41.5 mg, which was chromatographed on 3.0 g of silica gel eluting with toluene and 80/20 toluene/ethyl acetate to afford 5.9 mg (8% over 11 steps) of α-33a as an oil; 1H-NMR δ 1.32 (t, J = 7.2 Hz, 3H), 2.49 (dt, J = 14.9 Hz and 7.4 Hz, 1H), 2.86 (ddd, J = 14.2 Hz, 9,3 Hz, and 1.2 Hz, 1H), 3.40 (dd, J = 11.5 Hz and 4.6 Hz, 1H), 3.54 (dd, J = 11.5 Hz and 8.6 Hz, 1H), 4.26 (q, J = 7.1 Hz, 2H), 4.77 (ddd, J = 9.2 Hz, 8.3 Hz, and 6.3 Hz, 1H), (dd, J = 8.7 Hz and 4.6 Hz, 1H), 5.24 (dd, J = 7.1 Hz and 1.3 Hz, 1H), 6.92 (br d, J = 6.2 Hz, 1H), 7.40 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.6 Hz, 2H); 13C-NMR δ 14.1, 30.1, 37.2, 51.6, 58.6, 62.3, 64.4, 128.6, 128.9, 131.6, 138.3, 166.6, 169.7, 175.1; HRMS (TOF ES+) m/z calculated for C16H18ClN2O4S (M + H) 369.0670; found 369.0668. Compound β-33a was eluted afterwards with 80/20 toluene/ethyl acetate to afford 5.6 mg (8% over 11 steps) as an oil; 1H-NMR δ 1.31 (t, J = 7.2 Hz, 3H), 2.11 (ddd, J = 12.7 Hz, 10.6 Hz, and 7.6 Hz, 1H), 3.35 (ddd, J = 12.8 Hz, 8.1 Hz, and 6.2 Hz, 1H), 3.42 (d, J = 5.7 Hz, 2H), 4.25 (m, 2H), 5.00 (dd, J = 10.5 Hz, 8.2 Hz, and 5.7 Hz, 1H), 5.17 (t, J = 5.4 Hz, 1H), 5.21 (dd, J = 7.4 Hz and 6.4 Hz, 1H), 6.95 (br d, J = 5.5 Hz, 1H), 7.38 (d, J = 8.6 Hz, 2H), 7.72 (d, J = 8.6 Hz, 2H); 13C-NMR δ 14.1, 35.3, 38.6, 54.0, 57.8, 62.2, 62.3, 128.6, 128.9, 131.6, 138.3, 166.4, 168.9, 171.5; HRMS (TOF ES+) m/z calculated for C16H18ClN2O4S (M + H) 369.0670; found 369.0672.
(3R)-Ethyl6-(4-chlorobenzamido)-6-methyl-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxylate (β-33b and α-33b): A mixture of 133.5 μmol of 15 (R1 = Me, R2 = 4-ClPh) and 2.4 mL of 4:4:1 TFA:CH2Cl2:water contained in a 5-mL glass reaction vessel was rotated for 35 minutes, drained, and the resin was washed with 6 × 1.5 mL of dichloromethane. The resin was then dried under a stream of nitrogen, and then under vacuum. The now-formed aldehyde resin 7 (R1 = Me, R2 = 4-ClPh) was then converted to 33b using Method B by treatment with 270 μmol (2.0 equiv) of cysteine ethyl ester hydrochloride in 2 mL of NMP, followed by 927 μmol (6.9 equiv.) of potassium acetate in 0.6 mL of acetic acid. The vessel was rotated for 24 h at room temperature, drained, and the resin was washed with 2 × 3 mL each of NMP, 5% DIEA in NMP, 5% DIEA in dichloromethane, and 3 × 3 mL of dichloromethane. The resin was dried under a stream of argon, and then treated with 2 mL of chlorobenzene. The contents were heated at 60 °C for 67 h, cooled, the vessel was drained, and the resin was washed with 3 × 4 mL of dichloromethane. The combined filtrates were evaporated to afford 1.7 mg. The resin was then heated at 75 °C in 3 mL of chlorobenzene for 50 h. The vessel was drained, and the resin was washed with 3 × 4 mL of dichloromethane. The combined filtrates were evaporated to afford 4.1 mg. This process was repeated at 75 °C for 40 h to afford 2.2 mg. The combined materials (1.7 mg, 4.1 mg, and 2.2 mg) were chromatographed on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using a step gradient beginning with 6/4 1:1 MeCN/MeOH with 5.0 mM ammonium acetate/water with 5.0 mM ammonium acetate to afford 2.5 mg (5% over 11 steps) of β-33b as an oil; 1H-NMR δ 1.31 (t, J = 7.2 Hz, 3H), 1.69 (s, 3H), 2.67 (dd, J = 13.1 Hz and 7.4 Hz, 1H), 3.03 (dd, J = 13.0 Hz and 6.5 Hz, 1H), 3.40 (dd, J = 11.1 Hz and 3.0 Hz, 1H), 3.50 (dd, J = 11.2 Hz and 7.3 Hz, 1H), 4.24 (m, 2H), 5.19 (dd, J = 7.2 Hz and 3.0 Hz, 1H), 5.21 (t, J = 7.0 Hz, 1H), 6.63 (br s, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.72 (d, J = 8.6 Hz, 2H); 13C-NMR δ 14.1, 23.1, 35.3, 43.7, 57.9, 61.6, 61.7, 62.1, 128.5, 128.8, 132.1, 138.2, 165.5, 169.0, 173.9; HRMS (TOF ES+) m/z calculated for C17H19ClN2O4SNa (M + Na) 405.0652; found 405.0642. The resin was subjected to methoxide cleavage conditions by treating with 1.75 mL of anhydrous THF followed by 700 μL (660 mg, 3.00 mmol, 22 equiv.) of 25% sodium methoxide in methanol for 3 h. The vessel was drained under positive argon pressure while the resin was washed with 4 mL of absolute methanol. The combined filtrates were added to a rapidly stirred, cold mixture of 20 mL of dichloromethane and 20 mL of 1 N HCl. The layers were separated, the aqueous phase was extracted once with 10 mL of dichloromethane, and the combined organics were dried (Na2SO4). Concentration gave 13.9 mg of a mixture consisting primarily of the carboxylic acid of 33b, which was esterified with ethyl iodide (300 mg) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (648 μmol) overnight at room temperature. The mixture was concentrated to a residue that was partitioned between ethyl acetate/5% citric acid/brine. The organic phase was washed with 5% citric acid and dried (MgSO4). Concentration gave a wet residue that was diluted with dichloromethane and then dried with sodium sulfate. Concentration gave 8.7 mg, which was chromatographed on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using a step gradient beginning with 1/1 1:1 MeCN/MeOH with 5.0 mM ammonium acetate/water with 5.0 mM ammonium acetate to afford 2.1 mg (4% over 13 steps) of α-33b as an oil; 1H-NMR δ 1.33 and 1.34 (2t, J = 7.2 Hz and 7.2 Hz, 3H), 1.65 (s, 3H), 2.51 (d, J = 14.5 Hz, 1H), 3.10 (dd, J = 14.4 Hz and 7.8 Hz, 1H), 3.41 (dd, J = 11.4 Hz and 4.3 Hz, 1H), 3.48 (dd, J = 11.3 Hz and 8.4 Hz, 1H), 4.26 (m, 2H), 5.07 (dd, J = 8.2 Hz and 4.2 Hz, 1H), 5.34 (d, J = 8.7 Hz, 1H), 6.40 (br s, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.71 (d, J = 8.6 Hz, 2H); 13C-NMR δ 14.1, 25.8, 36.2, 36.5, 58.3, 59.6, 62.1, 63.3, 128.5, 128.9, 132.0, 138.2, 165.8, 169.4, 176.0; HRMS (TOF ES+) m/z calculated for C17H19ClN2O4SNa (M + Na) 405.0652; found 405.0667.
(3R)-Ethyl6-benzyl-6-(4-chlorobenzamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxylate (β-33c and α-33c): A mixture of 260 μmol of 15 (R1 = benzyl, R2 = 4-ClPh) and 2.4 mL of 4:4:1 TFA:CH2Cl2:water contained in a 5-mL glass reaction vessel was rotated for 35 minutes, drained, and the resin was washed with 6 × 1.5 mL of dichloromethane. The resin was then dried under a stream of nitrogen, and then under vacuum. The now-formed aldehyde resin 7 (R1 = benzyl, R2 = 4-ClPh) was then converted to 33c using Method F by treatment with 1.56 mL (1.56 mmol, 6.00 equivalent) of 1.0 M potassium acetate in acetic acid followed by 1.04 mL (0.520 mmol, 2.00 equivalent) of cysteine ethyl ester hydrochloride in acetic acid. After rotation at room temperature for 18 h, the vessel was drained, and the resin was washed once with 3 mL of THF, and then with 4 × 2.5 mL of 5% DIEA in dichloromethane, and then with 4 × 2 mL of dichloromethane. The resin was dried in vacuo, and then treated with 3 mL of chlorobenzene followed by 0.270 mL (200 mg, 1.55 mmol, 6.0 equiv.) of DIEA. The mixture was heated at 55 °C for 24 h, the vessel was drained, and the resin washed with 2 × 3 mL of dichloromethane. Evaporation of the combined filtrates gave only 5.0 mg. The resin was then treated with 3 mL of acetic acid, and the vessel was heated at 75 °C for 40 h. After cooling, the vessel was drained, and the resin was washed with 2 × 2 mL of acetic acid. The combined filtrates were evaporated to give 46.6 mg, which was triturated under 500 μL of warm acetonitrile to afford 15.6 mg (13% over 11 steps) of β-33c as a white solid; 1H-NMR δ 1.31 (t, J = 7.2 Hz, 3H), 2.61 (dd, J = 13.4 Hz and 7.4 Hz, 1H), 3.23 (d, J = 13.3 Hz, 1H), 3.32 (dd, J = 13.4 Hz and 6.4 Hz, 1H), 3.37 (dd, J = 11.3 Hz and 3.6 Hz, 1H), 3.44 (dd, J = 11.3 Hz and 7.5 Hz, 1H), 3.45 (d, J = 13.4 Hz, 1H), 4.25 (m, 2H), 4.75 (t, J = 6.8 Hz, 1H), 5.14 (dd, J = 7.1 Hz and 3.6 Hz, 1H), 6.66 (br s, 1H), 7.21 (m, 2H), 7.28–7.31 (m, 3H), 7.39 (d, J = 8.6 Hz, 2H), 7.67 (d, J = 8.5 Hz, 2H); 13C-NMR δ 14.2, 35.3, 41.5, 42.9, 58.1, 62.06, 62.07, 65.5, 127.5, 128.4, 128.5, 128.9, 130.2, 132.2, 134.4, 138.2, 165.6, 168.8, 172.9; HRMS (TOF ES+) m/z calculated for C23H24ClN2O4S (M + H) 459.1140; found 459.1147. The filtrate was evaporated to a solid that was taken up in 650 μL of 1:1 MeCN/MeOH, decanted with syringe, and the filtrate was diluted with 350 μL of water. The mixture was filtered through a 0.45 micron filter, and was injected onto a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) and chromatographed using a step gradient beginning with 65/35 1:1 MeCN/MeOH with 5.0 mM ammonium acetate/water with 5.0 mM ammonium acetate to afford 1.3 mg (1% over 11 steps) of α-33c as a film; 1H-NMR δ 1.31 (t, J = 7.1 Hz, 3H), 2.70 (dd, J = 14.6 Hz and 2.3 Hz, 1H), 2.93 (dd, J = 14.6 Hz and 7.7 Hz, 1H), 3.19 (d, J = 11.5 Hz, 1H), 3.21 (d, J = 11.2 Hz, 1H), 3.31 (d, J = 11.5 Hz, 1H), 3.33 (dd, J = 11.0 Hz and 3.6 Hz, 1H), 4.25 (m, 2H), 5.08 (dd, J = 8.1 Hz and 3.5 Hz, 1H), 5.39 (dd, J = 7.8 Hz and 2.3 Hz, 1H), 6.47 (br s, 1H), 7.27–7.34 (m, 5H), 7.39 (d, J = 8.5 Hz, 2H), 7.63 (d, J= 8.6 Hz, 2H); 13C-NMR δ 14.1, 33.6, 36.4, 42.8, 58.2, 62.1, 63.0, 63.2, 127.8, 128.4, 128.9, 129.0, 130.6, 132.0, 134.7, 138.2, 165.5, 169.3, 174.3; HRMS (TOF ES+) m/z calculated for C23H24ClN2O4S (M + H) 459.1140; found 459.1146.
(2S)-Methyl2-((3R)-6-(4-fluorobenzamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxamido)-4-methylpentanoate (β-34a and α-34a): Prepared using Method C and
Scheme 12 Preparation of Cys-Leu-OMe. Fmoc-Leu-Wang resin (559 μmol) contained in an SPPS vessel was washed four times with NMP, and was then treated with 6 × 8 mL × 5 min 20% (
v/
v) piperidine in NMP. To the deprotected resin was then added a mixture of Boc-Cys(Trt)-OH (3.0 equivalent), DIEA (6.0 equivalent),
N,
N,
N′,
N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate, and O-(benzotriazol-1-yl)-
N,
N,
N′,
N′-tetramethyluronium hexafluorophosphate (HBTU, 3 equivalent) in 6 mL of 85/15 DCM/DMF. The vessel was rocked for 20 h, drained, and the resin was washed three times each with DMF and 1:1 THF:MeOH, and then four times with methanol to give Boc-Cys(Trt)-Leu on Wang resin. The resin was then treated with 12 mL 30% (
v/
v) triethylamine in methanol, and the vessel was placed in an oven at 55–60 °C for 48 h. The contents were allowed to cool, the vessel was drained, and the resin was washed with 2 × 20 mL of methanol. The combined filtrates were then concentrated to give 287 mg of crude Boc-Cys(Trt)-OMe;
1H-NMR δ 0.89 (d, 3H,
J = 6.2 Hz), 0.90 (d, 3H,
J = 6.1 Hz), 1.42 (s, 9H), 1.51 (m, 1H), 1.57–1.63 (m, 2H), 2.50 (dd, 1H,
J = 13.0 Hz and 5.0 Hz), 2.75 (br m, 1H), 3.68 (s, 3H), 3.82 (br m), 4.54 (br m), 4.74 (br s), 6.41 (br s), 7.22 (t, 3H,
J = 7.2 Hz), 7.30 (t, 6H,
J = 7.7 Hz), 7.42 (d, 6H,
J = 7.7 Hz). Chromatography on 2.0 g of silica gel afforded 227.5 mg (57%) of purified Boc-Cys(Trt)-OMe. To 7 mL of trifluoroacetic acid (TFA):triethylsilane (TES) (97.5:2.5) under argon was added 175 mg (297 μmol) of purified Boc-Cys(Trt)-OMe. The mixture was stirred at 35 °C for one hour, and was then concentrated to a residue that was triturated twice with 15 mL of 1:1 hexanes:diethyl ether and decanted. The residue was then dried under vacuum to give a white powder that was dissolved in 2.7 mL of acetic acid and used immediately in the cyclitive cleavage, which is described as follows.
A 3.5-mL reaction vessel was charged with 106 μmol of resin 7 (R1 = H, R2 = 4-FPh), 19 mg (181 μmol, 1.7 equivalent) of polyvinylpyridine, 0.8 mL (85 μmol, 0.80 equivalent) of a 0.106 M solution of Cys-Leu-OMe in acetic acid, and 0.8 mL of acetic acid, and heated at 55 °C for 20 h. The vessel was drained, the resin was washed with 2 × 2 mL of acetic acid, and the combined filtrates were evaporated to give 23 mg. Further exposure (72 h) to 55 °C gave an additional 4.1 mg. Both quantities were combined and chromatographed on 1.0 g of silica gel. The higher Rf material, α-34a, was eluted with 1/1 hexanes/ethyl acetate to afford 5.7 mg, while the lower Rf material, β-34a, was eluted with 1/2 hexanes/ethyl acetate to afford 6.4 mg. Each of these materials was separately further purified by reverse-phase HPLC on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using step gradients of 1:1 MeCN/MeOH with 5.0 mM ammonium acetate/water with 5.0 mM ammonium acetate to afford 4.6 mg (12% over seven steps) of α-34a as a film; 1H-NMR δ 0.92 and 0.93 (2d, J = 6.3 Hz and 6.3 Hz, 6H), 1.54–1.64 (m, 2H), 1.65–1.69 (m, 1H), 2.14 (ddd, J = 12.8 Hz, 10.8 Hz, and 7.6 Hz, 1H), 3.35 (ddd, 12.7 Hz, 8.4 Hz, and 6.3 Hz, 1H), 3.36 (dd, J = 12.0 Hz and 6.3 Hz, 1H), 3.75 (s, 3H), 3.79 (dd, J = 12.0 Hz and 6.4 Hz, 1H), 4.55–4.60 (m, 1H), 4.88 (t, J = 6.8 Hz, 1H), 5.09 (dd, J = 8.4 Hz and 6.1 Hz, 1H), 5.11 (dd, J = 7.3 Hz and 6.5 Hz, 1H), 6.84 (br d, J = 5.9 Hz, 1H), 6.95 (br d, J = 7.9 Hz, 1H), 7.12 (t, J = 8.6 Hz, 2H), 7.82 (dd, J = 8.7 Hz and 5.2 Hz, 2H); 13C-NMR δ 22.0, 22.7, 25.0, 34.8, 37.7, 41.3, 51.3, 52.5, 54.1, 59.3, 63.1, 115.7 (d, 2JCF = 22.0 Hz), 129.3 (d, 4JCF = 3.1 Hz), 129.5 (d, 3JCF = 9.1 Hz), 165.0 (d, 1JCF = 253 Hz), 166.4, 167.6, 172.8, 173.1; HRMS (TOF ES+) m/z calculated for C21H27FN3O5S (M + H) 452.1650; found 452.1654. β-34a: 4.7 mg (12% over 7 steps); 1H-NMR δ 0.97 and 0.98 (2d, J = 6.7 Hz and 6.6 Hz, 6H), 1.72–1.79 (m, 3H), 2.56 (ddd, J = 14.3 Hz, 7.5 Hz, and 4.7 Hz, 1H), 2.65 (ddd, J = 14.5 Hz, 10.5 Hz, and 4.2 Hz, 1H), 3.60 (dd, J = 11.6 Hz and 8.6 Hz, 1H), 3.69 (dd, J = 11.7 Hz and 4.9 Hz, 1H), 3.76 (s, 3H), 4.36 (ddd, J = 11.1 Hz, 6.7 Hz, and 4.9 Hz, 1H), 4.53–4.57 (m, 1H), 4.93 (dd, J = 8.5 Hz and 4.8 Hz, 1H), 5.31 (dd, J = 7.6 Hz and 4.2 Hz, 1H), 6.98 (t, J = 8.6 Hz, 2H), 7.47 (br d, J = 7.6 Hz, 1H), 7.63 (dd, J = 8.7 Hz and 5.2 Hz, 2H), 8.16 (br d, J = 6.7 Hz, 1H); 13C-NMR δ 21.8, 22.8, 25.0, 32.1, 36.5, 40.6, 51.4, 52.3, 54.8, 57.8, 64.7, 115.5 (d, 2JCF = 22.0 Hz), 128.3 (d, 4JCF = 3.0 Hz), 129.5 (d, 3JCF = 9.2 Hz), 165.0 (d, 1JCF = 253 Hz), 166.4, 168.8, 172.6, 172.9; HRMS (TOF ES+) m/z calculated for C21H27FN3O5S (M + H) 452.1650; found 452.1656.
(2S)-Methyl2-((3R)-6-(4-chlorobenzamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxamido)propanoate (α-35a and β-35a): Separated by reverse-phase HPLC on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using a step gradient beginning with 60/40 of 1:1 MeCN/MeOH with 5.0 mM of ammonium acetate/water with 5.0 mM ammonium acetate to afford 6.3 mg (6% over 11 steps) of α-35a; 1H-NMR δ 1.53 (d, J = 7.3 Hz, 3H), 2.56 (ddd, J = 14.3 Hz, 7.6 Hz, and 4.9 Hz, 1H), 2.65 (ddd, J = 14.5 Hz, 10.5 Hz, and 4.1 Hz, 1H), 3.60 (dd, J = 11.6 Hz and 8.6 Hz, 1H), 3.69 (dd, J = 11.6 Hz and 4.7 Hz, 1H), 3.80 (s, 3H), 4.37 (ddd, J = 10.8 Hz, 6.7 Hz, and 4.9 Hz, 1H), 4.56 (quintet, J = 7.2 Hz, 1H), 4.95 (dd, J = 8.7 Hz and 4.8 Hz, 1H), 5.32 (dd, J = 7.6 Hz and 4.1 Hz, 1H), 7.26 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 7.58 (br d, J = 7.1 Hz, 1H), 8.42 (br d, J = 6.8 Hz, 1H); 13C-NMR δ 17.5, 31.8, 36.4, 48.6, 52.5, 54.7, 57.8, 64.6, 128.5, 128.7, 130.3, 138.2, 166.5, 168.5, 172.8, 172.9; HRMS (TOF ES+) m/z calculated for C18H20ClN3O5SNa (M + Na) 448.0710; found 448.0725 and 4.6 mg (4% over 11 steps) of β-35a; 1H-NMR 1.42 (d, J = 7.2 Hz, 3H), 2.14 (ddd, J = 12.9 Hz, 10.7 Hz, and 7.5 Hz, 1H), 3.35 (dd, J = 8.3 Hz and 6.3 Hz, 1H), 3.38 (dd, J = 12.0 Hz and 7.2 Hz, 1H), 3.77 (s, 3H), 3.78 (dd, J = 12.1 Hz and 6.4 Hz, 1H), 4.54 (quintet, J = 7.1 Hz, 1H), 4.86 (t, J = 6.8 Hz, 1H), 5.08 (ddd, J = 10.6 Hz, 8.5 Hz and 5.9 Hz, 1H), 5.13 (t, J = 6.8 Hz, 1H), 6.74 (br d, J = 5.6 Hz, 1H), 7.10 (br d, J = 6.9 Hz, 1H), 7.42 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H); 13C-NMR δ 18.2, 34.9, 37.6, 48.6, 52.7, 54.2, 59.3, 63.1, 128.6, 129.0, 131.5, 138.4, 166.4, 167.5, 172.8, 173.0; HRMS (TOF ES+) m/z calculated for C18H20ClN3O5SNa (M + Na) 448.0710; found 448.0710.
(2S)-Methyl2-((3R)-6-(4-chlorobenzamido)-6-methyl-5-oxohexahydro-pyrrolo[2,1-b]thiazole-3carboxamido)propanoate (β-35b and α-35b): Separated on 2.0 g of silica gel 60 using hexanes/ethyl acetate eluents (40/60 and 1/2) to afford 10.3 mg (9% over 11 steps) of β-35b; 1H-NMR δ 1.41 (d, J = 7.1 Hz, 3H), 1.69 (s, 3H), 2.73 (dd, J = 13.1 Hz and 7.3 Hz, 1H), 2.99 (dd, J = 13.1 Hz and 6.6 Hz, 1H), 3.47 (dd, J = 12.9 Hz and 7.3 Hz, 1H), 3.73 (dd, J = 11.9 Hz and 6.0 Hz, 1H), 3.76 (s, 3H), 4.51 (quintet, J = 7.1 Hz, 1H), 4.89 (dd, J = 6.9 Hz and 6.3 Hz, 1H), 5.13 (t, J = 6.9 Hz, 1H), 6.71 (br s, 1H), 7.22 (br d, J = 6.8 Hz, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.6 Hz, 2H); 13C-NMR δ 19.2, 23.5, 35.0, 42.2, 48.5, 52.6, 59.2, 62.0, 62.7, 128.5, 128.9, 131.9, 138.3, 165.5, 167.8, 172.9, 175.4; HRMS (TOF ES+) m/z calculated for C19H23ClN3O5S (M + H) 440.1041; found 440.1039 and 10.1 mg (9% over 11 steps) of α-35b; 1H-NMR δ 1.51 (d, J = 7.3 Hz, 3H), 1.64 (s, 3H), 2.34 (dd, J = 14.3 Hz and 3.8 Hz, 1H), 2.88 (dd, J = 14.3 and 7.9 Hz, 1H), 3.55 (dd, J = 11.6 Hz and 8.6 Hz, 1H), 3.68 (dd, J = 11.7 Hz and 4.8 Hz, 1H), 3.69 (s, 3H), 4.54 (quintet, J = 7.2 Hz, 1H), 4.93 (dd, J = 8.6 Hz and 4.8 Hz, 1H), 5.26 (dd, J = 7.9 Hz and 3.8 Hz, 1H), 6.59 (br s, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.62 (br d, J = 7.1 Hz, 1H), 7.67 (d, J = 8.6 Hz, 2H); 13C-NMR δ 17.6, 26.2, 36.0, 38.7, 48.5, 52.4, 57.8, 60.5, 62.5, 128.5, 129.0, 131.4, 138.5, 166.4, 168.7, 172.5, 173.1; HRMS (TOF ES+) m/z calculated for C19H23ClN3O5S (M + H) 440.1041; found 440.1044.
(2S)-Methyl2-((3R)-6-benzyl-6-(4-chlorobenzamido)-5-oxohexahydro-pyrrolo[2,1-b]thiazole-3carboxamido)propanoate (β-35c and α-35c): Separated on two 2.0 g of silica gel 60 using hexanes/ethyl acetate eluents (70/30, 60/40, 55/45, 1/1) to afford 10.3 mg (8% over 11 steps) of β-35c; 1H-NMR δ 1.43 (d, J = 7.2 Hz, 3H), 2.69 (dd, J = 13.7 Hz and 7.1 Hz, 1H), 3.27 (dd, J = 13.7 Hz and 6.7 Hz, 1H), 3.30 (d, J = 12.9, 1H), 3.34 (dd, J = 8.6 Hz and 7.5 Hz, 1H), 3.35 (d, J = 13.1 Hz, 1H), 3.64 (dd, J = 11.8 Hz and 5.8 Hz, 1H), 3.80 (s, 3H), 4.25 (t, J = 6.8 Hz, 1H), 4.59 (quintet, J = 7.3 Hz, 1H), 4.85 (t, J = 6.4 Hz, 1H), 6.73 (br s, 1H), 6.94 (br d, J = 7.4 Hz, 1H), 7.23–7.25 (m, 2H), 7.29–7.31 (m, 3H), 7.41 (d, J = 8.5 Hz, 2H), 7.71 (d, J = 8.5 Hz, 2H); 13C-NMR δ 18.2, 34.6, 41.5, 42.2, 48.3, 52.6, 60.1, 62.7, 66.0, 127.9, 128.5, 128.7, 128.9, 130.1, 132.0, 134.3, 138.3, 165.5, 167.3, 172.7, 174.9; HRMS (TOF ES+) m/z calculated for C25H26ClN3O5SNa (M + Na) 538.1179; found 538.11 and 5.1 mg (3% over 11 steps) of α-35c (90% by-NMR); 1H-NMR δ 1.50 (d, J = 7.2 Hz, 3H), 2.62 (dd, J = 14.3 and 7.6 Hz, 1H), 2.68 (dd, J = 14.3 and 4.5 Hz, 1H), 3.19 (d, J = 13.6 Hz, 1H), 3.27 (d, J = 13.5 Hz, 1H), 3.35 (dd, J = 11.3 Hz and 8.4 Hz, 1H), 3.63 (dd, J = 11.4 Hz and 4.3 Hz, 1H), 3.71 (s, 3H), 4.53 (quintet, J = 7.2 Hz, 1H), 4.94 (dd, J = 8.4 Hz and 4.3 Hz, 1H), 5.25 (dd, J = 7.6 Hz and 4.5 Hz, 1H), 6.56 (br s, 1H), 7.31–7.33 (m, 2H), 7.37–7.43 (m, 5H), 7.56 (d, J = 8.6 Hz, 2H), 7.68 (br d, J = 7.2 Hz, 1H); 13C-NMR δ 17.5, 35.8, 37.0, 43.5, 48.6, 52.3, 57.9, 62.5, 63.9, 128.2, 128.3, 129.0, 129.3, 130.2, 131.6, 134.0, 138.6, 165.9, 168.5, 171.8, 172.5; HRMS (TOF ES+) m/z calculated for C25H27ClN3O5S (M + H) 516.1354; found 516.1355.
General procedure for the preparation of 36a–
c and 30b,
c (Method D cleavage). According to
Scheme 10, 250 μmol of resin
13a–
c contained in a SPPS vessel was treated with 20% piperidine in NMP at room temperature for 40 minutes with gentle agitation. The vessel was drained, and the resin was washed with 6 × 4 mL of NMP. To the deprotected resin, Fmoc-Ala, HOBt, and DIC (5 eq each) were added in NMP for R
1 = H or (Fmoc-Ala)
2O (3 eq) in NMP for R
1 = Me and Bn. The vessel was agitated for 18–42 h, drained, and the resin was washed with 6 × 6 mL of NMP to give resin
22a–
c. Resin
22a–
c was treated with 20% piperidine in NMP at room temperature for 40 minutes with gentle agitation. The vessel was drained, and the resin was washed with 6 × 4 mL of NMP. To the deprotected resin 4-chlorobenzoyl chloride (4.4 eq) in NMP was added, followed by DIEA (5.2 eq) in NMP. The vessel was rocked for 18 h to 24 h, drained, and the resin was washed with 3 × 6 mL of NMP, 3 × 6 mL of 1:1 THF:MeOH, 3 × 6 mL of THF, and 5 × 6 mL of DCM. The resin was treated with 5 mL of 4:4:1 TFA:CH
2Cl
2:water for 35 min at room temperature. The mixture was filtered, and the resin was washed with 5 × 2 mL of dichloromethane, and then dried under a stream of nitrogen. To the resulting resin
38a–
c, 1.0–1.3 equivalents of a 0.25-M solution of
32 in acetic acid was added, followed by 6.2–8.6 equivalents of a 1.6-M solution of potassium acetate in acetic acid. The vessel was rotated at room temperature overnight (16–22 h) and was then drained, and the resin was washed with acetic acid. The combined filtrates were evaporated to a residue that was taken up in dichloromethane and washed two times with saturated sodium bicarbonate and dried (MgSO
4). Concentration gave a crude sample that was analyzed by LC/MS and quantitative NMR. The resin was then treated with 3 mL of acetic acid, and was heated at 55 °C for 24 h. The vessel was drained, the resin was washed with acetic acid, and the combined filtrates were evaporated to a residue. A further exposure of the resin/acetic acid to 55 °C for 24 h resulted in a minor amount of cyclitive cleavage product. The residues were combined, and the diastereomers were separated by normal phase or reverse-phase chromatography.
(2S)-Methyl2-((3R)-6-((S)-2-(4-chlorobenzamido)propanamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxamido)propanoate (β-36a and α-36a): Separated by chromatography on 2.0 g of silica gel 60 using CH2Cl2 and CH2Cl2/MeOH (98/2, 97/3, and 95/5) to afford 4.1 mg (4% over 13 steps) of β-36a; 1H-NMR δ 1.37 (d, J = 7.2 Hz, 3H), 1.49 (d, J = 7.0 Hz, 3H), 2.07 (ddd, J = 12.8 Hz, 10.8 Hz, and 7.4 Hz, 1H), 3.14 (ddd, J = 12.9 Hz, 8.5 Hz, and 6.3 Hz, 1H), 3.33 (dd, J = 11.8 Hz and 7.2 Hz, 1H), 3.71 (dd, J = 11.8 Hz and 5.8 Hz, 1H), 3.74 (s, 3H), 4.51 (quintet, J = 7.2 Hz, 1H), 4.78 (quintet, J = 7.2 Hz, 1H), 4.84–4.89 (m, 2H), 5.06 (t, J = 6.8 Hz, 1H), 6.95 (br d, J = 7.3 Hz, 1H), 7.14 (br d, J = 6.3 Hz, 1H), 7.20 (br d, J = 7.1 Hz, 1H), 7.41 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 8.5 Hz, 1H); 13C-NMR δ 18.0, 18.3, 34.8, 37.0, 48.5, 49.0, 52.6, 53.7, 59.1, 62.7, 128.6, 128.9, 132.0, 138.3, 166.4, 167.7, 172.5, 172.6, 172.9; HRMS (TOF ES+) m/z calculated for C21H26ClN4O6S (M + H) 497.1256; found 497.1260 and 2.7 mg (3% over 13 steps) of α-36a; 1H-NMR δ 1.44 (d, J = 7.1 Hz, 3H), 1.46 (d, J = 7.3 Hz, 3H), 2.48 (ddd, J = 14.3 Hz, 7.3 Hz, and 4.8 Hz, 1H), 2.56 (ddd, J = 14.4 Hz, 10.2 Hz, and 4.3 Hz, 1H), 3.59 (d, J = 7.1 Hz, 2H), 3.67 (s, 3H), 4.20 (ddd, J = 11.7 Hz, 7.1 Hz, 4.8 Hz, 1H), 4.58 (quintet, J = 7.4 Hz, 1H), 4.72 (quintet, J = 7.2 Hz, 1H), 4.76 (t, J = 7.1 Hz, 1H), 5.29 (dd, J = 7.3 Hz and 4.3 Hz, 1H), 7.27 (br d, J = 7.7 Hz, 1H), 7.41 (d, J = 8.5 Hz, 2H), 7.56 (br d, J = 7.6 Hz, 1H), 7.75 (br d, J = 7.0 Hz, 1H), 7.79 (d, J = 8.6 Hz, 2H); 13C-NMR δ 17.4, 17.8, 31.7, 36.9, 48.2, 49.4, 52.4, 54.3, 58.0, 64.9, 128.78, 128.85, 131.8, 138.3, 166.7, 168.5, 171.9, 173.3, 173.6; HRMS (TOF ES+) m/z calculated for C21H26ClN4O6S (M + H) 497.1256; found 497.1253.
(2S)-Methyl2-((3R)-6-((S)-2-(4-chlorobenzamido)propanamido)-6-methyl-5-oxohexahydropyrrolo-[2,1-b]thiazole-3-carboxamido)propanoate (α-36b and β-36b): Separated by reverse-phase HPLC on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using a step gradient beginning with 60/40 of 1:1 MeCN/MeOH with 5.0 mM ammonium acetate/water with 5.0 mM ammonium acetate to afford 4.2 mg (4% over 13 steps) of α-36b; 1H-NMR δ 1.38 (d, J = 7.1 Hz, 3H), 1.49 (d, J = 7.0 Hz, 3H), 1.59 (s, 3H), 2.63 (dd, J = 13.0 Hz and 7.4 Hz, 1H), 2.79 (dd, J = 13.0 Hz and 6.6 Hz, 1H), 3.47 (dd, J = 11.9 Hz and 7.5 Hz, 1H), 3.69 (dd, J = 11.9 Hz and 5.9 Hz, 1H), 3.75 (s, 3H), 4.50 (quintet, J = 7.1 Hz, 1H), 4.78 (quintet, J = 7.0 Hz, 1H), 4.94 (dd, J = 7.0 and 6.2 Hz, 1H), 5.11 (t, J = 7.0 Hz, 1H), 6.97 (br d, J = 7.2 Hz, 1H), 7.02 (br s, 1H), 7.35 (br d, J = 6.9 Hz, 1H), 7.41 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H); 13C-NMR δ 18.0, 18.9, 23.6, 35.2, 41.7, 48.4, 49.1, 52.6, 58.8, 61.7, 62.5, 128.5, 128.9, 131.9, 138.2, 166.0, 168.1, 171.7, 172.9, 174.5; HRMS (TOF ES+) m/z calculated for C22H28ClN4O6S (M + H) 511.1413; found 511.1416 and 4.1 mg (4% over 13 steps) of β-36b; 1H-NMR δ 1.42 (d, J = 6.9 Hz, 3H), 1.45 (d, J = 7.2 Hz, 3H), 1.50 (s, 3H), 2.26 (dd, J = 14.2 Hz and 4.3 Hz, 1H), 2.78 (dd, J = 14.3 Hz and 7.8 Hz, 1H), 3.55–3.62 (m, 2H), 3.64 (s, 3H), 4.63 (quintet, J = 7.5 Hz, 1H), 4.77 (quintet, J = 7.2 Hz, 1H), 4.81 (dd, J = 8.1 Hz and 5.8 Hz, 1H), ), 5.26 (dd, J = 7.8 Hz and 4.4 Hz, 1H), 7.21 (br s, 1H), 7.26 (br d, J = 6.8 Hz, 1H), 7.38 (d, J = 8.4 Hz, 2H), 7.66 (br d, J = 7.9 Hz, 1H), 7.77 (d, J = 8.5 Hz, 2H); 13C-NMR δ 16.9, 18.2, 25.6, 36.6, 38.8, 47.9, 48.8, 52.3, 57.5, 60.7, 62.9, 128.7, 128.8, 132.1, 138.2, 167.1, 168.4, 172.2, 172.8, 173.3; HRMS (TOF ES+) m/z calculated for C22H28ClN4O6S (M + H) 511.1413; found 511.1411.
(2S)-Methyl2-((3R)-6-benzyl-6-((S)-2-(4-chlorobenzamido)propanamido)-5-oxohexahydropyrrolo-[2,1-b]thiazole-3-carboxamido)propanoate (α-36c and β-36c): Compound α-36c was separated from β-36c by chromatography on 1.5 g of silica gel. It was further purified by reverse-phase HPLC on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using a step gradient beginning with 70/30 of 1:1 MeCN/MeOH with 5.0 mM of ammonium acetate/water with 5.0 mM of ammonium acetate to afford 2.1 mg (1% over 13 steps) of α-36c; 1H-NMR δ 1.37 (d, J = 6.9 Hz, 3H), 1.44 (d, J = 7.2 Hz, 3H), 2.54 (d, J = 6.2 Hz, 2H), 3.02 (d, J = 13.6 Hz, 1H), 3.10 (d, J = 13.6 Hz, 1H), 3.42 (dd, J = 11.6 Hz and 8.6 Hz, 1H), 3.50 (dd, J = 11.6 Hz and 5.2 Hz, 1H), 3.67 (s, 3H), 4.63 (quintet, J = 7.6 Hz, 1H), 4.72 (quintet, J = 7.4 Hz, 1H), 4.78 (dd, J = 8.5 Hz and 5.2 Hz, 1H), 5.23 (t, J = 6.2 Hz, 1H), 7.11 (br d, J = 8.4 Hz, 1H), 7.12 (br s, 1H), 7.24–7.31 (m, 5H), 7.42 (d, J = 8.5 Hz, 2H), 7.63 (br d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.5 Hz, 2H); 13C-NMR δ 16.5, 18.2, 36.0, 36.4, 42.9, 47.9, 48.8, 52.3, 57.5, 63.1, 64.6, 127.8, 128.7, 128.86, 128.93, 130.3, 132.0, 133.9, 138.2, 167.1, 168.2, 171.2, 172.6, 173.3; HRMS (TOF ES+) m/z calculated for C28H31ClN4O6SNa (M + Na) 609.1551; found 609.1565. Compound β-36c was further purified by reverse-phase HPLC on a Dynamax Microsorb 5-micron C18 column (21.4 × 250 mm) using a step gradient beginning with 70/30 of 1:1 MeCN/MeOH with 5.0 mM of ammonium acetate/water with 5.0 mM of ammonium acetate to afford 3.2 mg (2% over 13 steps) of β-36c; 1HNMR δ 1.39 (d, J = 7.3 Hz, 3H), 1.50 (d, J = 6.9 Hz, 3H), 2.57 (dd, J = 13.5 Hz and 7.0 Hz, 1H), 3.02 (dd, J = 13.5 Hz and 6.8 Hz, 1H), 3.09 (d, J = 13.0 Hz, 1H), 3.24 (d, J = 13.0 Hz, 1H), 3.32 (dd, J = 11.6 Hz and 6.9 Hz, 1H), 3.61 (dd, J = 11.7 Hz and 5.5 Hz, 1H), 3.79 (s, 3H), 4.09 (t, J = 6.9 Hz, 1H), 4.57 (quintet, J = 7.3 Hz, 1H), 4.69 (quintet, J = 7.1 Hz, 1H), 4.85 (t, J = 6.2 Hz, 1H), 6.70 (br d, J = 7.2 Hz, 1H), 6.84 (br s, 1H), 6.88 (br d, J = 7.6 Hz, 1H), 7.21–7.22 (m, 2H), 7.27–7.28 (m, 3H), 7.42 (d, J = 8.5 Hz, 2H), 7.73 (d, J = 8.5 Hz, 2H); 13C-NMR δ 18.2, 18.3, 34.4, 40.6, 42.4, 48.2, 49.2, 52.6, 60.1, 62.1, 65.7, 128.0, 128.5, 128.7, 128.9, 130.1, 131.9, 133.9, 138.3, 166.1, 167.4, 171.5, 172.7, 174.3; HRMS (TOF ES+) m/z calculated for C28H31ClN4O6SNa (M + Na) 609.1551; found 609.1572.
(2S)-Methyl2-((3R)-6-benzyl-6-((S)-2-(4-nitrobenzamido)propanamido)-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxamido)propanoate (β-30c and α-30c): Partial separation was achieved by chromatography on 1.0 g of silica gel using CH2Cl2 and CH2Cl2/EtOAc mobile phases. Compound β-30c was then purified by reverse-phase HPLC on a Dynamax Microsorb 5-micron, C18 column (21.4 × 250 mm) using 65/35 of 1:1 MeCN/MeOH with 5.0 mM of ammonium acetate/water with 5.0 mM of ammonium acetate to afford 9.1 mg (4% over 13 steps) of β-30c; 1H-NMR δ 1.40 (d, J = 7.2 Hz, 3H), 1.54 (d, J = 7.0 Hz, 3H), 2.59 (dd, J = 13.4 Hz and 7.0 Hz, 1H), 3.06 (dd, J = 13.5 Hz and 6.8 Hz, 1H), 3.11 (d, J = 13.1 Hz, 1H), 3.25 (d, J = 13.1 Hz, 1H), 3.32 (dd, J = 11.7 Hz and 7.0 Hz, 1H), 3.60 (dd, J = 11.7 Hz and 5.6 Hz, 1H), 3.79 (s, 3H), 4.14 (t, J = 6.9 Hz, 1H), 4.57 (quintet, J = 7.3 Hz, 1H), 4.75 (quintet, J = 7.0 Hz, 1H), 4.85 (t, J = 6.3 Hz, 1H), 6.84 (br s, 1H), 6.92 (br d, J = 7.6 Hz, 1H), 7.07 (br d, J = 7.1 Hz, 1H), 7.21–7.23 (m, 2H), 7.26–7.29 (m, 3H), 7.97 (d, J = 8.6 Hz, 2H), 8.29 (d, J = 8.7 Hz, 2H); 13C-NMR δ 18.2, 18.7, 34.6, 40.7, 42.4, 48.3, 49.4, 52.6, 60.0, 62.2, 65.8, 123.8, 128.0, 128.4, 128.8, 130.1, 133.8, 139.1, 149.8, 165.0, 167.4, 171.5, 172.7, 174.1; HRMS (TOF ES+) m/z calculated for C28H32N5O8SNa (M + H) 598.1966; found 598.1957. Compound α-30c was purified by reverse-phase HPLC on a Dynamax Microsorb 5-micron, C18 column (21.4 × 250 mm) using 65/35 of 1:1 MeCN/MeOH with 5.0 mM of ammonium acetate/water with 5.0 mM ammonium acetate to afford 4.7 mg (2% over 13 steps) of α-30c; 1H-NMR δ 1.39 (d, J = 6.9 Hz, 3H), 1.45 (d, J = 7.2 Hz, 3H), 2.56 (d, J = 6.2 Hz, 2H), 3.03 (d, J = 13.7 Hz, 1H), 3.12 (d, J = 13.7 Hz, 1H), 3.44 (dd, J = 11.6 Hz and 8.4 Hz, 1H), 3.48 (dd, J = 11.7 Hz and 5.4 Hz, 1H), 3.65 (s, 3H), 4.67 (quintet, J = 7.2 Hz, 1H), 4.74–4.77 (m, 2H), 5.25 (t, J = 6.1 Hz, 1H), 7.09 (br s, 1H), 7.25–7.26 (m, 2H), 7.29–7.34 (m, 3H), 7.43 (br d, J = 7.9 Hz, 1H), 7.62 (br d, J = 8.2 Hz, 1H), 8.04 (d, J = 8.7 Hz, 2H), 8.29 (d, J = 8.8 Hz, 2H); 13C-NMR δ 16.3, 18.4, 35.7, 36.6, 42.9, 47.8, 49.0, 52.4, 57.4, 63.2, 64.8, 123.7, 127.9, 128.6, 129.0, 130.3, 133.9, 139.4, 149.8, 166.3, 168.1, 171.1, 172.5, 173.6; HRMS (TOF ES+) m/z calculated for C28H32N5O8SNa (M + H) 598.1966; found 598.1960.
(2S)-Methyl2-((3R)-6-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-propanamido)-6-methyl-5-oxohexahydropyrrolo[2,1-b]thiazole-3-carboxamido)-propanoate (α-37b and β-37b): To 252 μmol of resin 13b (R1 = Me) swelled with NMP contained in a 3.5-mL reaction vessel, 2 mL of 20% piperidine in NMP was added. The vessel was drained, charged with 3 mL of the 20% piperidine solution, and rotated at room temperature for 45 minutes. The vessel was drained, and the resin was washed with 7 × 3 mL of NMP to remove all of the piperidine. The resin was then treated with a solution of 1.25 mmol (5 equiv.) each of HBTU and Fmoc-Ala-OH and 2.50 mmol (10 equiv.) of DIEA in 3.75 mL of NMP. The vessels were rotated at room temperature for six days, drained, and the resins were washed with 3 × 3 mL each of NMP, 1;1 THF:MeOH, THF, and 4 × 3 mL of dichloromethane to give acetal resin 22b (R1 = Me), which was hydrolyzed using 3 mL of 4:4:1 TFA:CH2Cl2/water over 35 minutes at room temperature. The vessel was drained, and the resulting aldehyde resin was washed six times with dichloromethane. To this resin, a solution of 32 in 1 mL of acetic acid that was generated from 268 μmol (1.07 equiv.) of 31 was then added, followed by a solution of 2.0 mmol (8.0 equiv.) of potassium acetate in acetic acid. After exposure at room temperature for 18 h, the vessel was drained into a tared collection vial. The resin was washed with 2 × 2 mL of acetic acid, and the filtrates were combined. The resin was treated with 3 mL of acetic acid, and was placed in an oven heated at 45–52 °C for 66 h. The vessels were drained, and the resin was washed with 2 × 2 mL of acetic acid. This filtrate was evaporated to give 25.3 mg of α-37b and β-37b. The diastereomers were separated by reverse-phase HPLC on a Dynamax Microsorb 5-micron, C18 column (21.4 × 250 mm) using a step gradient beginning with 75/25 of 1:1 MeCN/MeOH with 5.0 mM of ammonium acetate/water with 5.0 mM of ammonium acetate to afford 3.7 mg (3% over 11 steps) of α-37b; 1H-NMR δ 1.35 (d, J = 6.6 Hz, 3H), 1.45 (d, J = 7.2 Hz, 3H), 1.49 (s, 3H), 2.24 (br dd, J = 14.0 Hz and 3.2 Hz, 1H), 2.75 (br dd, J = 13.1 Hz and 7.4 Hz, 1H), 3.55 (dd, J = 11.7 Hz and 8.7 Hz, 1H), 3.62 (dd, J = 11.7 Hz and 5.0 Hz, 1H), 3.69 (s, 3H), 4.21 (t, J = 7.0 Hz, 1H), 4.26 (br m, 1H), 4.39–4.47 (br m, 2H), 4.56 (quintet, J = 7.3 Hz, 1H), 4.86 (dd, J = 8.6 Hz and 5.1 Hz, 1H), 5.21 (br m, 1H), 5.58 (br d, J = 6.0 Hz, 1H), 6.75 (br s, 1H), 7.30 (q, J = 7.6 Hz, 2H), 7.40 (t, J = 7.4 Hz, 2H), 7.58 (d, J = 7.5 Hz, 2H), 7.59 (br d, J = 6.0 Hz, 1H), 7.76 (d, J = 7.6 Hz, 2H); 13C-NMR δ 17.8, 25.7, 36.3, 38.9, 47.1, 48.3, 50.4, 52.4, 57.7, 60.3, 62.6, 67.2, 120.0, 124.96, 125.04, 127.06, 127.12, 127.8, 141.3, 143.6, 143.7, 156.5, 168.5, 172.5, 172.6, 172.8; HRMS (TOF ES+) m/z calculated for C30H35N4O7S (M + H) 595.2221; found 595.2219 and 5.5 mg (4% over 11 steps) of β-37b: 1H-NMR δ 1.39 (d, J = 7.2 Hz, 6H), 1.56 (s, 3H), 2.62 (br dd, J = 12.6 Hz and 7.3 Hz, 1H), 2.78 (br dd, J = 12.8 Hz and 6.5 Hz, 1H), 3.42 (dd, J = 11.7 Hz and 7.3 Hz, 1H), 3.71 (dd, J = 11.8 Hz and 6.1 Hz, 1H), 3.75 (s, 3H), 4.22 (quintet, J = 7.1 Hz, 1H), 4.25 (br m, 1H), 4.40 (br d, J = 6.8 Hz, 2H), 4.51 (quintet, J = 7.1 Hz, 1H), 4.84 (t, J = 6.6 Hz, 1H), 5.06 (t, J = 7.1 Hz, 1H), 5.33 (br d, J = 6.5 Hz, 1H), 6.61 (br s, 1H), 7.19 (br d, J = 6.2 Hz, 1H), 7.32 (t, J = 7.6 Hz, 2H), 7.41 (t, J = 7.0 Hz, 2H), 7.58 (d, J = 7.4 Hz, 2H), 7.76 (d, J = 7.6 Hz, 2H); 13C-NMR δ 18.1, 23.5, 34.9, 41.6, 47.1, 48.4, 50.3, 52.6, 59.1, 61.5, 62.4, 67.2, 120.0, 125.03, 125.06, 127.1, 127.8, 141.3, 143.7, 156.0, 167.8, 171.6, 172.9, 174.9; HRMS (TOF ES+) m/z calculated for C30H34N4O7SNa (M + Na) 617.2046; found 617.2043.