1. Introduction
Viruses are underrepresented as targets in pharmacological screening efforts. This is because cell-based assays of viruses are innately complex and require biochemical assay counterscreens causing them to be time consuming and expensive. These challenges cause antiviral assays to be prioritized in the industry setting in favor of the most pressing viruses such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV) [
1]. West Nile fever is considered a neglected tropical disease. Its causative agent, West Nile Virus (WNV), belongs to the Flaviviridae family together with the hepatitis C virus (HCV). WNV is transmitted by
Culex spp. mosquitoes. Symptoms of the disease include fever, headache, body aches, skin rash, swollen lymph glands and in extreme cases, encephalitis or meningitis [
2]. The virus is mainly endemic to Africa, the Middle East and the area around the Mediterranean Sea [
3], but remains a threat to other countries when the infected hosts, both mosquitoes and humans, travel. HCV currently has more than two dozen prospective antivirals in various clinical phases, whereas WNV has none. However, WNV is well-defined biochemically and commercial biochemical assays are available for screening purposes [
4]. This allows for the rapid identification of novel inhibitors from new sources.
The replication cycle of the WNV begins as the 45–50 nm enveloped, icosahedral nucleocapsid binds to unknown receptors on the host cell. Receptor-mediated endocytosis brings the virion into the cell. The positive single-stranded 11 kb RNA genome is uncoated and can be directly translated by the host machinery into single long polyproteins in the rough endoplasmic reticulum (ER) [
5]. The polyprotein contains three structural proteins (capsid, pre-membrane, and envelope) and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5). The polyprotein must be cleaved by the NS3 protease in order to produce the individual proteins required for replication and particle maturation [
6]. A molecule that can inhibit the NS3 protease will prohibit subsequent replication, packaging, and spread of the virus within the host organism. Viral proteases are good drug targets as exemplified by the ten clinically approved HIV protease inhibitors [
7] and two approved inhibitors of the HCV NS3 protease [
8]. As a result, the WNV NS3 protease is a promising target for screening efforts directed at inhibiting WNV replication [
9]. The WNV NS3 protease is a serine protease with a marked homology to the four serotypes of the dengue virus (DENV) NS3 protease, where all have been described to have an active site that is relatively flat and highly exposed [
6], two features that pose a problem for identifying inhibitors. As a result, the non-competitive binding site has also become a targeted domain.
A well-represented group of marine natural products with unique structures are the halitoxins. These 3-alkylpyridinium molecules have acquired a number of hyponyms since the first isolation of the parent molecule, halitoxin, from a
Haliclona sp. sponge [
10]. The first compound from
Haliclona was investigated for its characteristic ichthyotoxicity. Accounts of the halitoxin family bioactivity since the first isolation include epidermal growth factor (EGF) receptor activation [
11], histone deacetylase inhibition [
12], acetylcholinesterase inhibition and selective toxicity toward non-small cell lung cancer cells [
13,
14], dorsal root ganglion (DRG) neuron activity [
15], antifungal [
16], antimycobacterial, and antimicrobial [
17] activity as well as cytotoxicity [
10,
18]. The caveat to cytotoxicity is that 3-alkylpyridiniums can be found as cyclic or linear compounds, as monomers or as polymeric alkylpyridiniums (poly-APS), where the cytotoxicity of the molecules is both size- and dose-dependent [
17]. Interestingly, macrocylic compounds in general are noted for their flexibility, high potency, selectivity, solubility, lipophilicity, membrane permeability, oral bioavailability, and metabolic stability and function as chemotherapeutics, immunosuppressants, antifungals, antiparasitics, antibacterials, and antivirals [
19]. However, to date none of the various forms of a 3-alkylpyridinium natural product have been reported to display any antiviral activity.
In this study a sponge-derived natural product library was screened for the ability to inhibit the WNV NS3 protease. This led to the identification of a fraction from the Red Sea sponge,
Amphimedon chloros, that displays the ability to inhibit the WNV NS3 protease
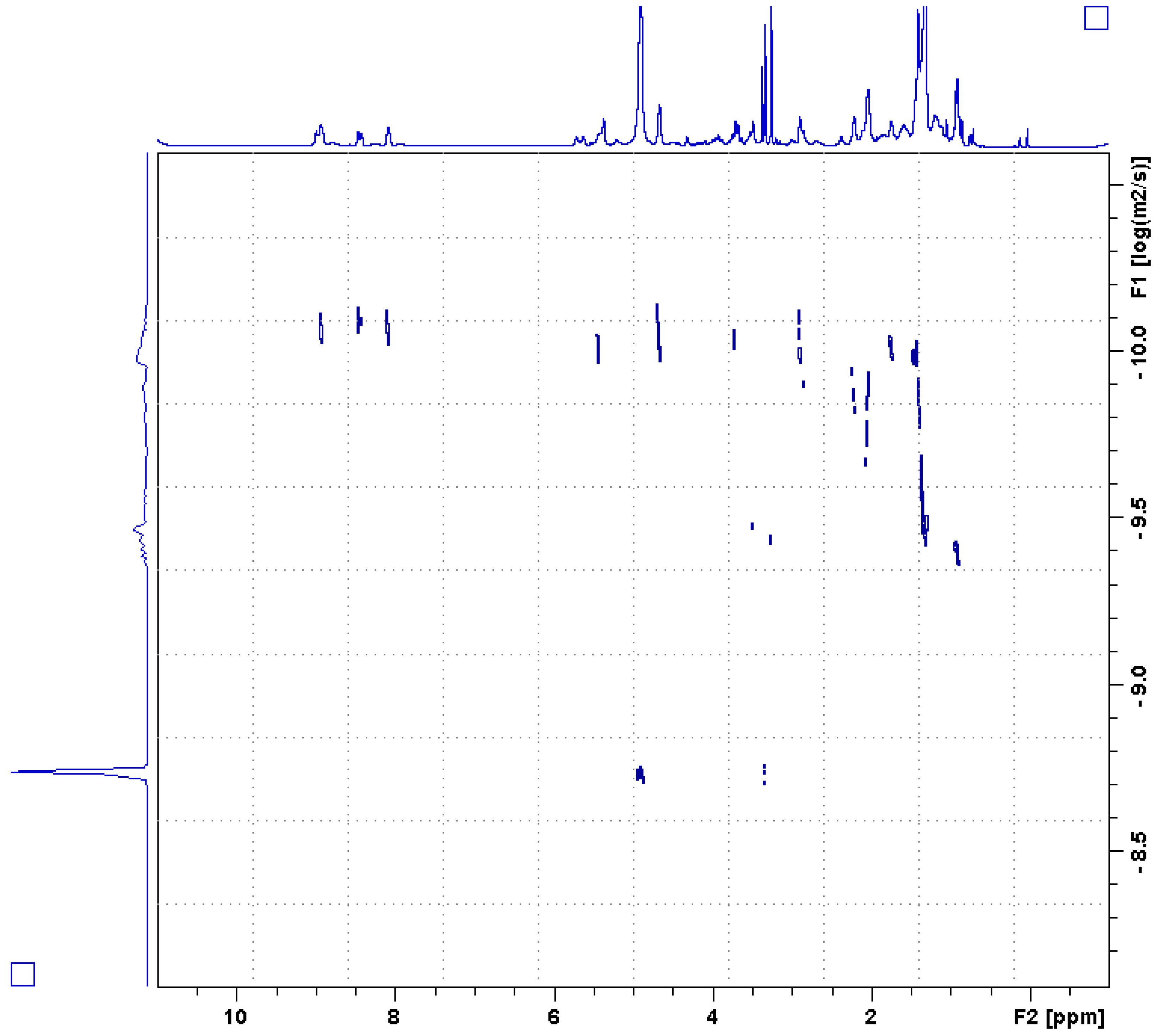
in vitro. This activity is attributed to the 3-alkylpyridinium derivative present in the active fraction, which in its simplest form, consists of an alkyl group connected to C-3 of a pyridine ring. An investigation into halitoxin and the 3-alkyl- pyridinium class of compounds revealed the long-standing problem of how to ascertain the relative molecular size of these compounds. This question was addressed by employing the 2D NMR DOSY technique. The technique in combination with an algorithm reported by Evans et al. [
20] provides a method by which to determine the molecular weight of a compound by its rate of diffusion. Knowing the molecular weight of the compound isolated from
A. chloros allowed for the direct comparison of the cytotoxicity of the isolated halitoxin to cytotoxicity reports for other halitoxins of various sizes. Provided here is the first account of the antiviral potential for the halitoxin family of compounds.
3. Discussion
The halitoxins, presumed polymers of 3-alkylpyridinium fragments, are a group of structurally diverse natural products that have mainly been isolated from marine sponges. These molecules can be found as cyclic or linear compounds and as monomeric or as polymeric alkylpyridiniums (poly-APS). They were first characterized in 1978 [
10] for their characteristic toxicity to fish, and since then, a diversity of biological activities has been observed [
11,
12,
15,
16,
17,
18]. In this study, we have identified and characterized a 3-alkylpyridinium compound (compound
1), which shows inhibitory activity against the WNV NS3 protease. Davies-Coleman et al. [
11] synthesized the cyclic alkylpyridinium salt and found that on thin-layer chromatography (TLC) it ran much faster than the natural product they had isolated, leading them to conclude that the natural product was an oligomer or polymer. To accurately assess the molecular size of our active compound, we used DOSY and relaxation rate analysis. Our results suggest that the molecule is not a polymer but rather an oligomer, or assembly of up to 90 molecules. Notably, alkylpyridinum salts are used as surfactants and can form micelles with as few as 10 or as many as 100 molecules [
25].
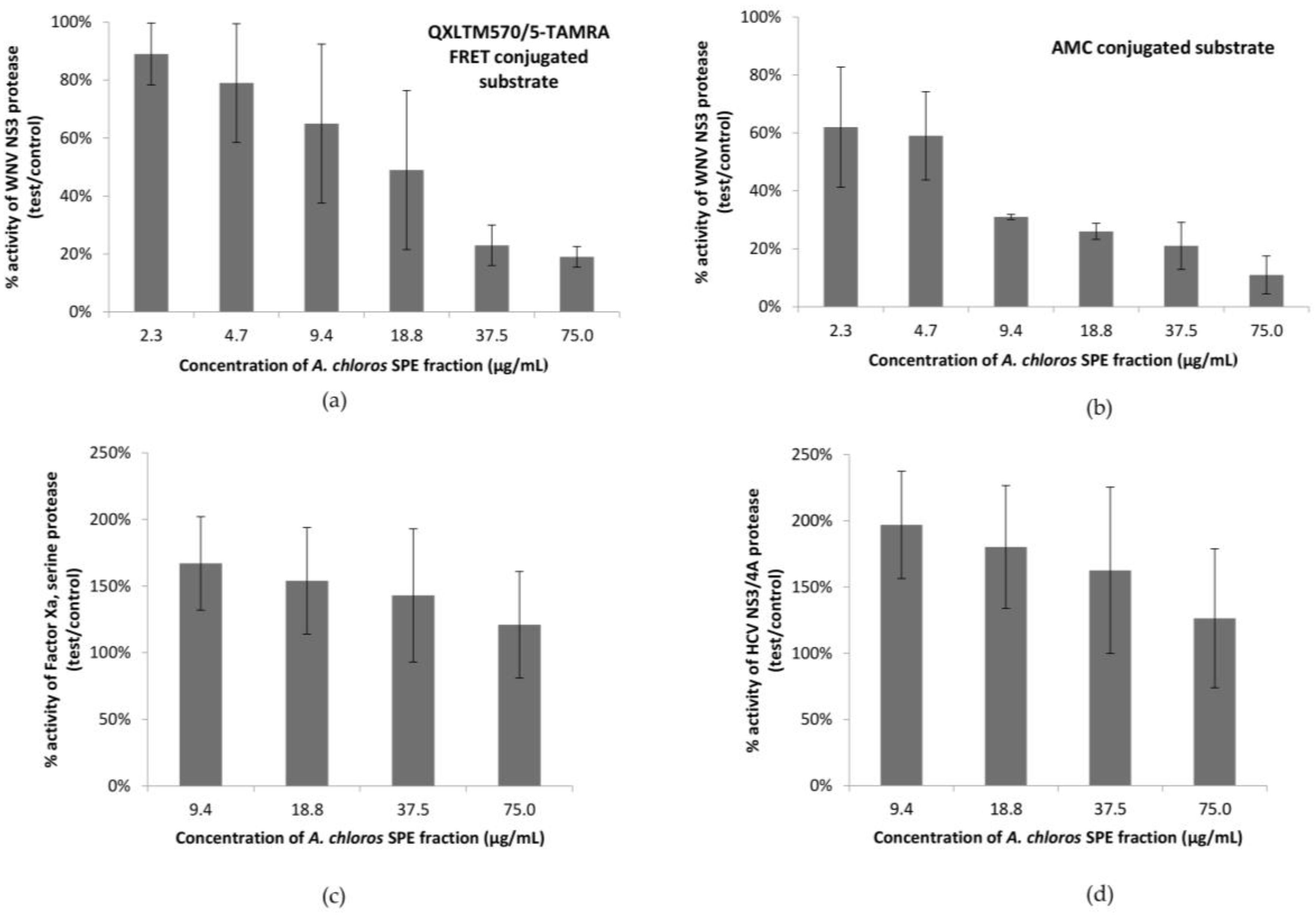
In our most sensitive assay, using an AMC conjugated substrate, the SPE fraction 2 that contained compound
1 showed 90% inhibition of the WNV NS3 protease at 75 μg/mL. The same set of samples was screened for the ability to inhibit the HCV protease, as well as Factor Xa (
Figure 1c,d), a serine protease which leads to blood clot formation by converting prothrombin to thrombin. All replicates of fraction 2 failed to show inhibition of either of the additional serine protease targets suggesting specificity for the WNV NS3 serine protease. Instead, in both the HCV and Factors Xa assays there was an enhancement of the protease activity on the substrate, which suggests that the active compound could be increasing the activity of these proteases, possibly through a surfactant effect [
26,
27,
28]. Like other surfactants, compound
1 most likely exhibits a critical micelle concentration (CMC) where the molecule shows an abrupt change in solution state according to temperature and electrolyte concentration. Additional serine protease targets should be screened to determine an exclusive specificity of the active compound for the WNV NS3 protease.
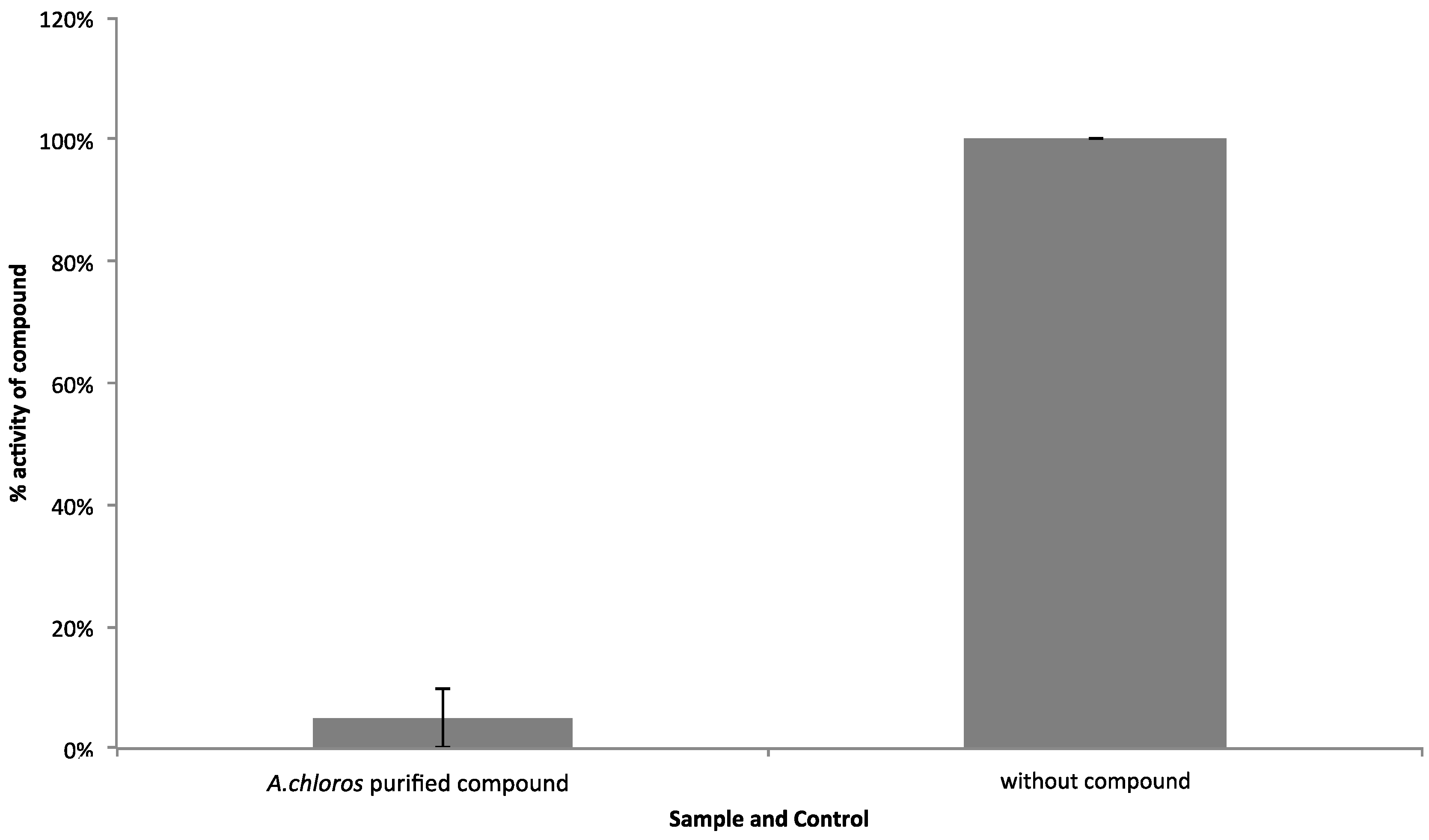
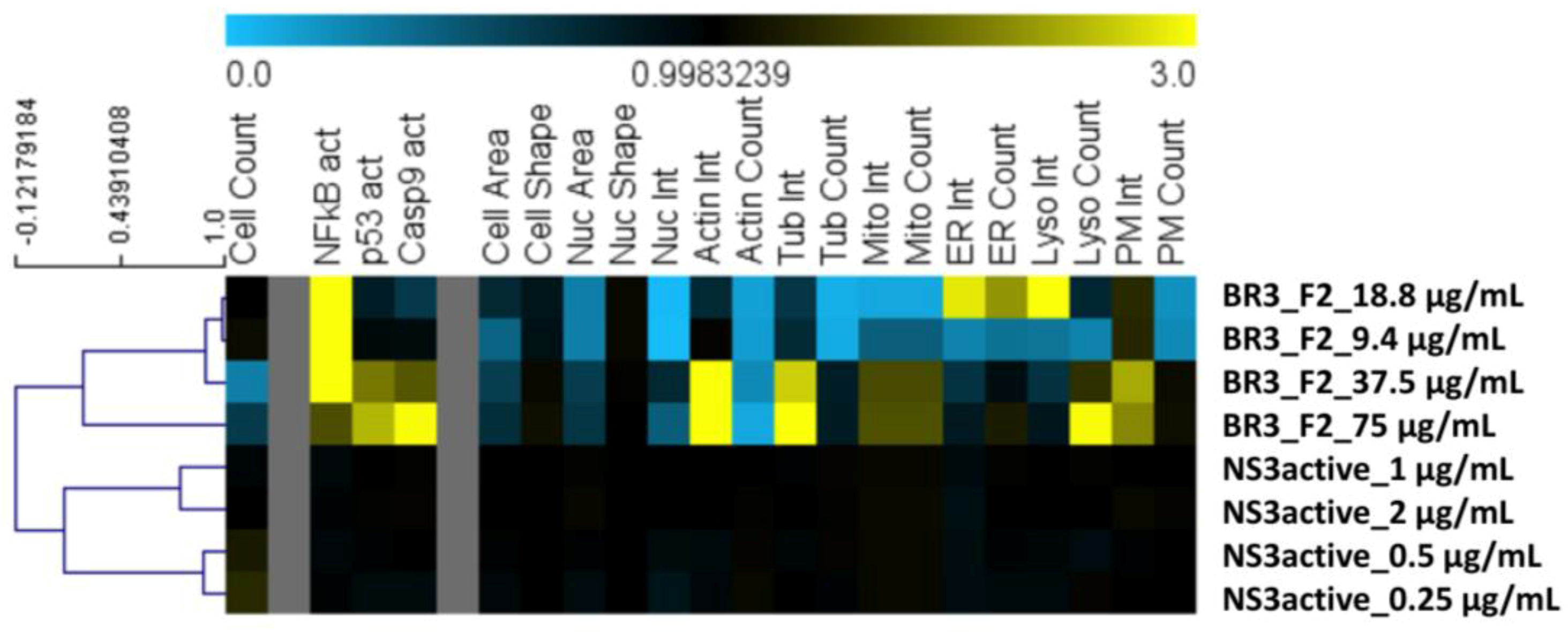
To gain insight into toxicity of compound
1, we employed a High-Content Screening (HCS) cytological profiling strategy on HeLa cells assaying SPE parent fraction dilutions and dilutions of compound
1 (
Figure 5). The parent fraction that contains compound
1 showed cytotoxicity for the four dilutions of 75 μg/mL to 9.4 μg/mL when screened using the HCS platform that assesses a number of cellular features commonly affected by toxic compounds (
Figure 5). After the LC-MS flow-through focused on the time point where compound
1 is present in the greatest relative abundance (
Figure S1), and testing again in the WNV NS3 assay with an AMC conjugated substrate, we observed that the compound
1 focused fraction, screened at 2 μg/mL, is most likely responsible for the observed WNV NS3 inhibition (
Figure 4). This same focused sample was then screened on the HCS platform and no longer showed the toxicity profile associated with the SPE parent fraction (
Figure 5). Our HCS results show that the fraction collected from LC-MS is nontoxic at concentrations below 9.4 µg/mL (screened at approximately 2 μg/mL), whereas, the parent fraction is toxic (
Figure 5). This is in accordance with previous reports relating compound size and treatment amount to toxicity for the halitoxin family [
15,
17,
29]. Interestingly, the parent fraction strongly affects a variety of cellular processes, even at concentrations where no reductions in cell numbers were observed, including the induction of several well-known markers of toxicity, including activation of NFkB, p53, caspase 9 as well as effects on mitochondria, ER, and the cytoskeleton, whereas the focused fraction does not. In addition, at the higher concentrations of the parent fraction with markedly reduced cell counts, we observed an increased intensity of the membrane marker which might indicate membrane-targeting activity of the active compound. The observed reduction in toxicity of the focused fraction could be due to the reduced concentration of the NS3 protease-inhibiting material; or this concentration may not be achieving the critical micelle concentration and thereby generating a large enough oligomer capable of permeating cell membranes. Future work to evaluate this compound for its potential as a WNV antiviral would require a cell-based assay to determine compound efficacy in addition to X-ray crystallography work to fully understand the mechanism of binding for this compound to the WNV NS3 protease.
4. Materials and Methods
4.1. A. chloros Sponge Collection
The Amphimedon chloros specimens were collected using gardening shears from Inner Fsar reef (22°14′37.61″ N; 39°00′28.03″ E) off the coast of KAUST (Red Sea, Saudi Arabia) at 12 meters depth using SCUBA. The samples were briefly rinsed with PBS, wrapped in foil and placed on ice, then frozen to −80 °C until processing.
4.2. A. chloros Sponge Extraction
Small sponge specimens of 4–10 grams in weight were ground using a mortar and pestle, and then extracted with 15 mL of methanol overnight at 4 °C. The following day, the methanol extract was dried onto 150 mg of Diaion HP20SS beads in a CentriVap complete vacuum concentrator (Labconco, Kansas City, MO, USA) on low, the beads were loaded into a 25 mL Flash Cartridge (Sorbtech, Norcross, GA, USA) with 0.795 diameter Frits (Sorbtech), desalted with deionized water (15 mL, FW1, FW2), and then eluted with 15 mL of solvent in the following series: 25% IPA/H
2O, 50% IPA/H
2O, 75% IPA/H
2O, 100% MeOH [
27].
4.3. Liquid Chromatography-Mass Spectrometry (LC-MS) of A. chloros SPE Fraction
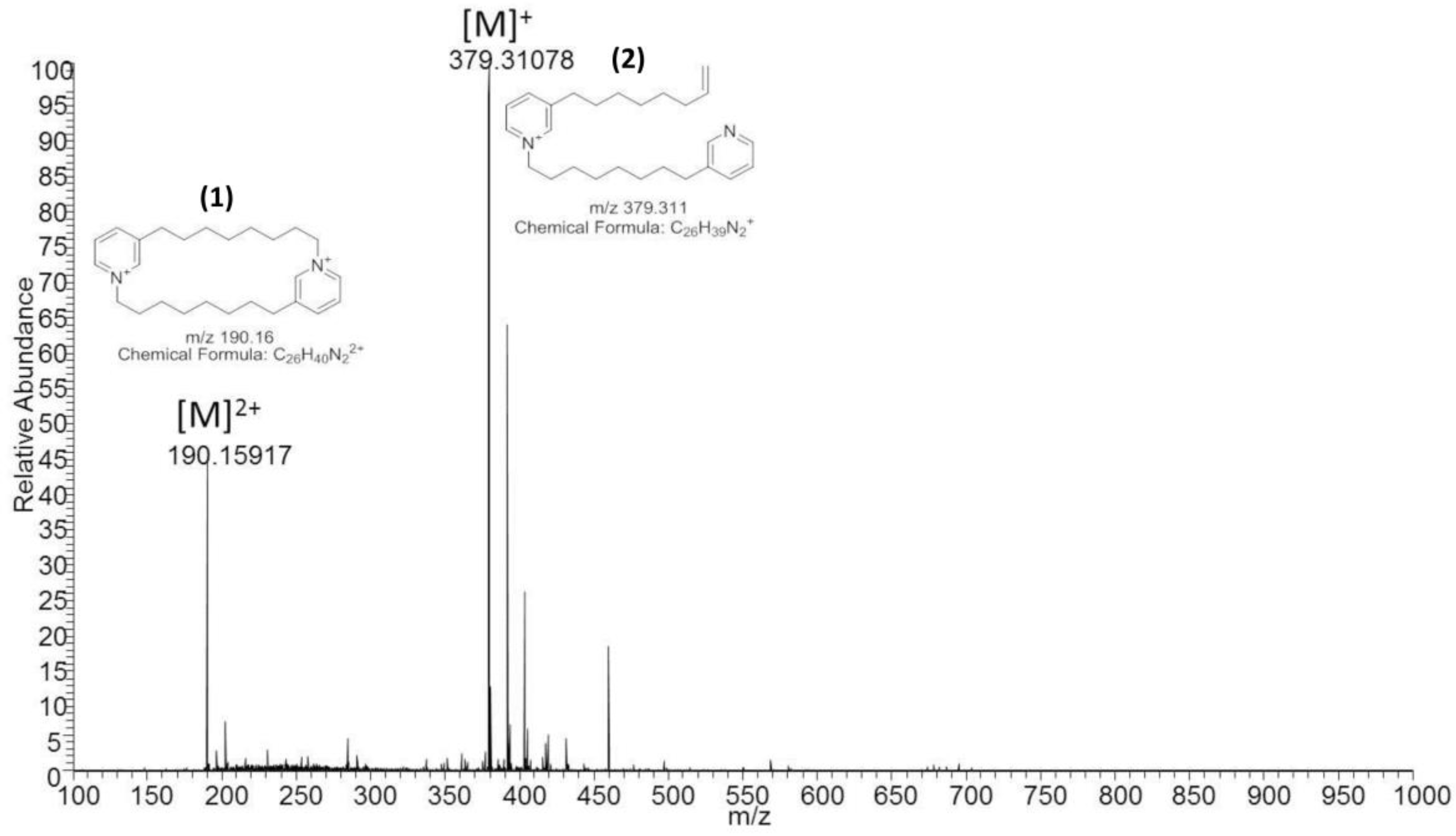
Liquid chromatography-mass spectrometry (LC-MS) was carried out on the Thermo LTQ Orbitrap instrument (Thermo Fisher Scientific, Waltham, MA, USA) in positive mode using electrospray ionization. The 10 μL of solid phase extracted (SPE) sponge material was separated using a ZORBAX Eclipse XDB-C18 LC column (Agilent Technologies, Santa Clara, CA, USA), 4.6 mm, 150 mm, 5 µm with the gradient displayed in
Table S1 and a flow rate of 0.800 mL/min. 10 μL of solid phase extract was also directly injected and the desired compound was collected at a specific run time to obtain a concentrated sample of
m/
z 190.16 (
Figure 2, compound
1).
4.4. Nuclear Magnetic Resonance (NMR) of A. chloros 3-Alkyl Pyridinium
The isolated 3-alkylpyridinium compound (12 mg) dissolved in methanol-d
4 was used to collect NMR spectra at 298 K on Avance III 600 MHz spectrometer (Bruker, Billerica, MA, USA). Standard Bruker pulse sequences were employed. Spectra were referenced to the residual methanol solvent resonance (3.31 ppm for
1H and 49.0 for
13C). The HMBC was optimized for 8 Hz couplings, the NOESY used a 500 ms mixing time, and the ROESY used a 300 ms mixing time. The DOSY experimental data were obtained using bipolar gradient pulse pairs and two spoil gradients (
ledbpgp2s in the standard Bruker pulse sequence library). A smoothed square shape of 2.4 ms duration was used for the bipolar gradients; its strength was increased linearly from 2% to 95% of the maximum gradient strength of 53 G/cm, acquiring 32 steps of gradient levels. A gradient recovery delay of 2 ms was used and the diffusion time was set to 300 ms (D20 of 300 ms and a P30 of 2400 µs). Each spectrum collected 128 transients with a 5 s recycle delay for a total experimental time of 7.5 h. The spectrum was processed and analyzed using TopSpin 2.1 (Bruker).
13C relaxation rate spectra were measured in an interleaved fashion. For T
1s 13 delays were used (10, 20, 30, 40, 50, 70, 100, 150, 200, 300, 500, 700 and 1000 ms). For the T
2s 11 delays were used (22.4, 44.8, 67.2, 89.6, 112.0, 134.0, 179.0, 246.0, 291.0, 381.0 and 493.0 ms). Spectra were processed with the software NMRpipe [
22] and rates were extracted using the software Sparky [
23].
4.5. West Nile Virus (WNV) NS3 Protease Inhibition Assay
The West Nile Virus NS3 protease inhibition assay was carried out using the commercial kit SensoLyte®) 440 West Nile Virus Protease Assay Kit (AnaSpec, San Jose, CA, USA). The protease is a truncated form of West Nile NS3 protease (residues 1–186). Connected to it is NS2B cofactor (residues 49–96) by the linker sequence, GGGGSGGGG. Protease activity was assessed by its ability to cleave the fluorogenic peptide Pyr-RTKR-AMC, and the subsequent production of free AMC (7-amino-4-methylcoumarin) fluorophores monitored at 354 nm excitation wavelength and 442 nm emission wavelength. As well, a second orthogonal substrate cleavage experiment was carried out, which used a FRET conjugated substrate. In this assay, West Nile Virus NS3 protease inhibition was assayed using the commercial kit SensoLyte® 570 West Nile Virus Protease Assay Kit (AnaSpec). Here, WNV protease inhibitors are assessed by their ability to inhibit the cleavage of the QXLTM570/5-TAMRA FRET substrate by the protease. Upon cleavage into two separate fragments by the WNV NS3 protease, the fluorescence of 5-TAMRA can be monitored at excitation/emission = 540 nm/575 nm. All extracts and controls were performed with three replicates in a 384-well plate format, each with a total reaction mixture of 33 μL. To begin, the test extracts and protease solution were incubated at 37 °C for 10 min before the addition of the pre-heated substrate. After substrate addition and gentle mixing the reaction was incubated at 37 °C for one h. The fluorophore was detected with the use of a SpectraMax® Paradigm® Multi-mode Microplate Detection Platform (Molecular Devices, Sunnyvale, CA, USA) by scanning at the wavelength associated with the respective assay.
4.6. HCV NS3/4A Protease Inhibition Assay
The HCV NS3/4A protease inhibition assay was carried out using the commercial SensoLyte® 520 HCV Protease Assay Kit (AnaSpec). The HCV NS3/4A protease is a 217 amino acid fusion protein (22.7 kDa) with NS4A co-factor fused to the N-terminus of NS3 protease domain. HCV NS3/4A protease activity was assessed by its ability to cleave the fluorogenic FRET peptide, and the subsequent unquenching by QXL 520 quencher of the 5-FAM fluorophore, which emits fluorescence. All extracts and controls were performed with three replicates in a 384-well plate format, each with a total reaction mixture of 18 μL. To begin, the test extracts and protease solution were incubated at room temperature for 10 min before the addition of the substrate. After substrate addition and gentle mixing the reaction was incubated at room temperature for one hour. The fluorophore was detected with the use of a SpectraMax® Paradigm® Multi-mode Microplate Detection Platform (Molecular Devices) by scanning at 490 nm excitation wavelength and 520 nm emission wavelength.
4.7. Thrombin Serine Protease Inhibition Assay
The Factor Xa inhibition assay was carried out using a commercial SensoLyte® 520 Factor Xa Assay Kit (AnaSpec). The Factor Xa activity was assessed by its ability to cleave the fluorogenic FRET peptide and the subsequent unquenching by QXL 520 quencher of the 5-FAM fluorophore, which emits fluorescence. All extracts and controls were performed with three replicates in a 384-well plate format, each with a total reaction mixture of 12.5 μL. To begin, the test extracts and Thrombin/Factor Xa solution were incubated for five minutes then the substrate was added. After substrate addition and gentle mixing the reaction was incubated at room temperature for one hour. The fluorophore was detected with the use of a SpectraMax® Paradigm® Multi-mode Microplate Detection Platform (Molecular Devices) by scanning at 490 nm excitation wavelength and 520 nm emission wavelength.
4.8. Cytological Profiling by High-Content Screening (HCS)
Cytological profiling was performed as recently described [
24,
30]. Briefly, HeLa cells were tested in black µClear CELLSTAR 384-well plates (Greiner Bio-One, Kremsmünster, Austria) at an approximate density of 2000 cells per well in a volume of 25 µL of cell culture medium with re-dissolved fractions in 4 replicates. 24 h after treatment, four different cell-staining protocols (panels) were used to stain for 10 cellular targets (cell count/cell loss; cell cycle; whole cell morphology; nuclear morphology; actin; tubulin; mitochondria; lysosomes; endoplasmic reticulum; plasma membrane; activation of NFkB, p53 and caspase 9). For High-Content Analysis, the CellomicsArrayScan VTI (Thermo Fisher Scientific, Waltham, MA, USA) platform equipped with a 10x objective (Zeiss Plan Neofluar, NA 0.3, ZEISS, Oberkochen, Germany) was used. Images were analyzed using the Compartmental Analysis Bio Application software (Cellomics, Thermo Fisher Scientific). At least 500 valid objects were analyzed per well. Cell cycle analysis and analysis of cell loss were accomplished by using the Cell Cycle Bio Application (Cellomics) using a minimum of 2000 valid objects.