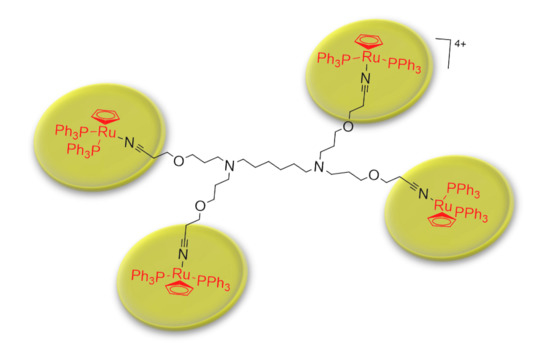
Poly(alkylidenimine) Dendrimers Functionalized with the Organometallic Moiety [Ru(η5-C5H5)(PPh3)2]+ as Promising Drugs Against Cisplatin-Resistant Cancer Cells and Human Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results and Discussion
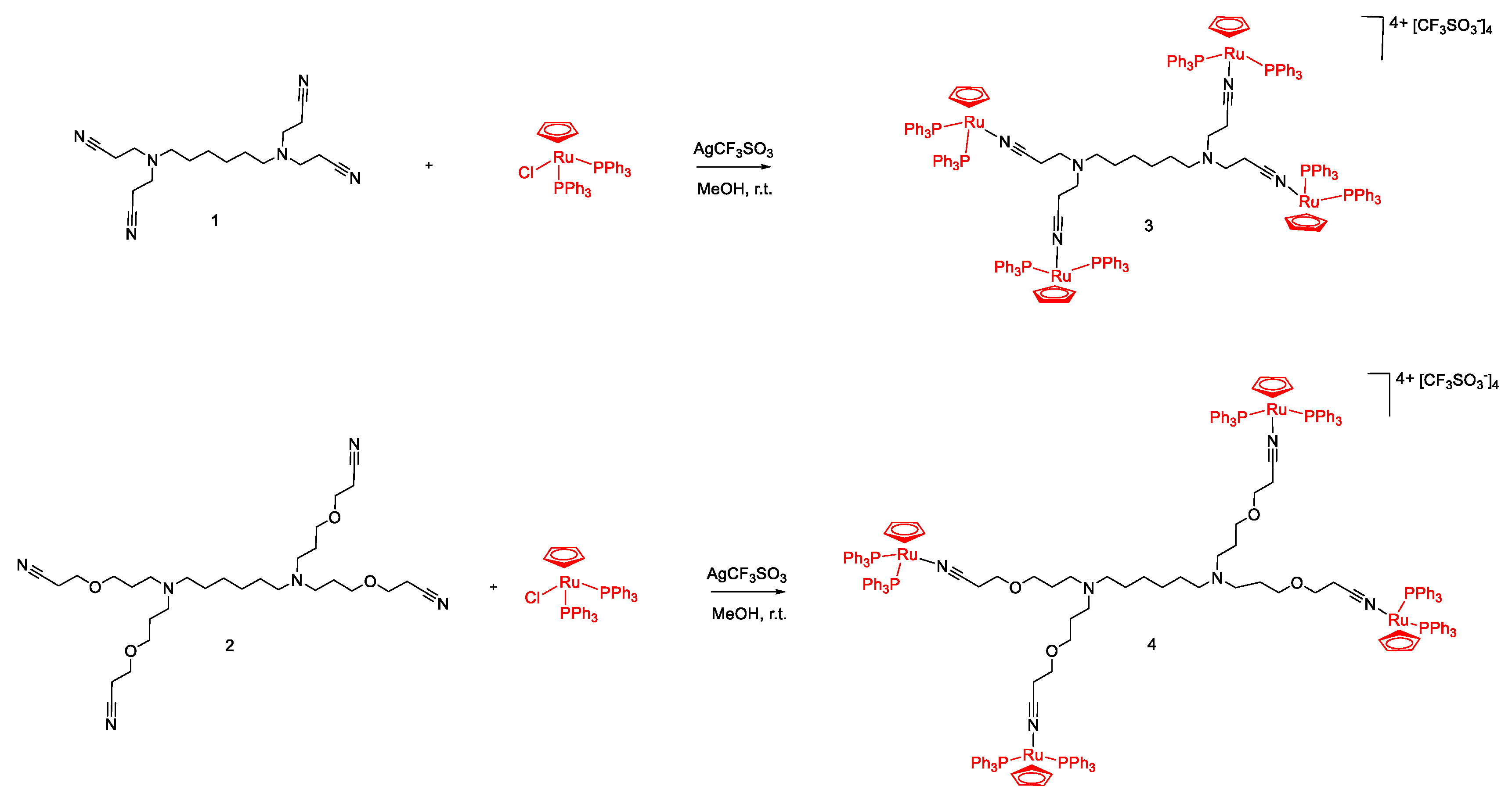
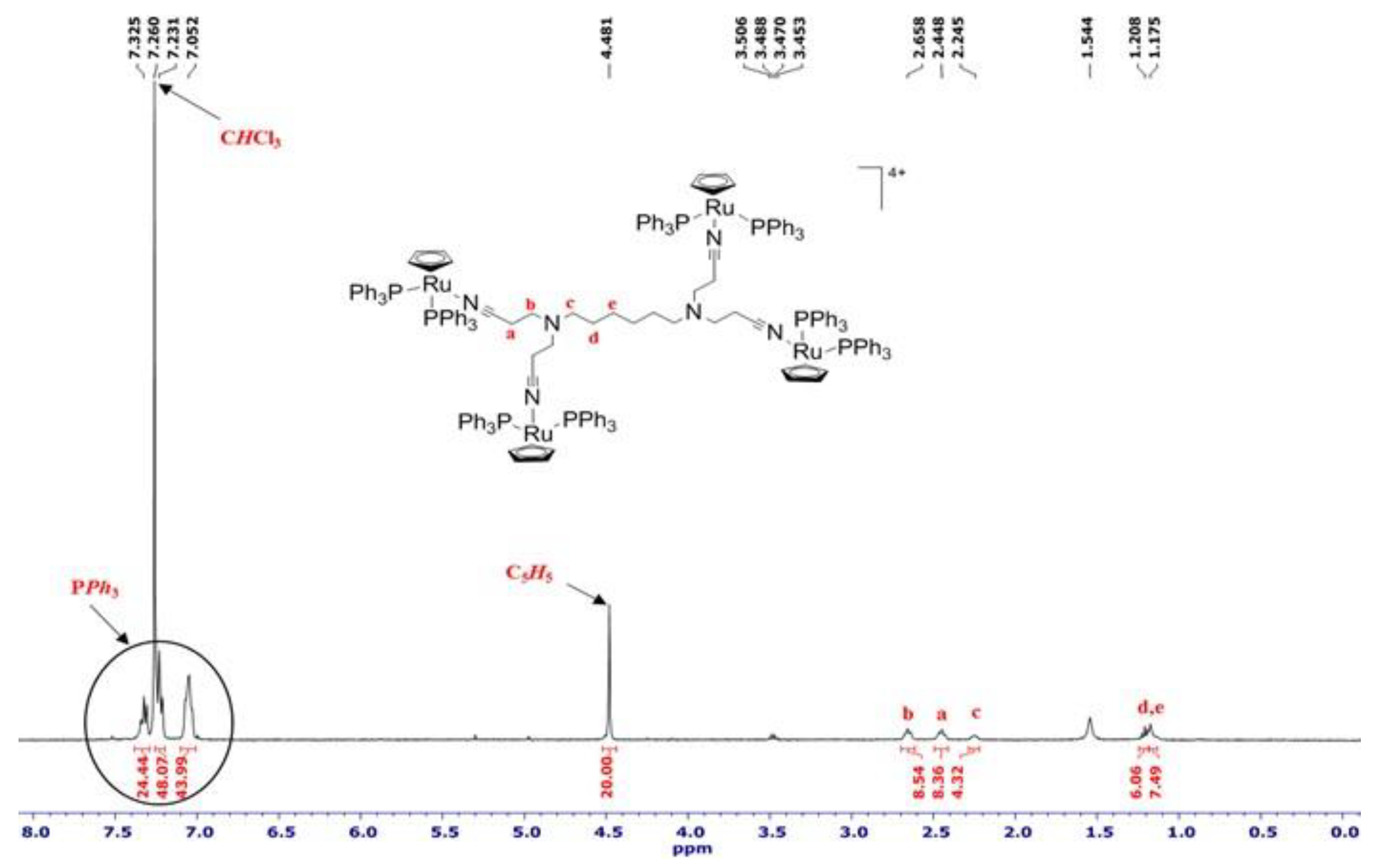
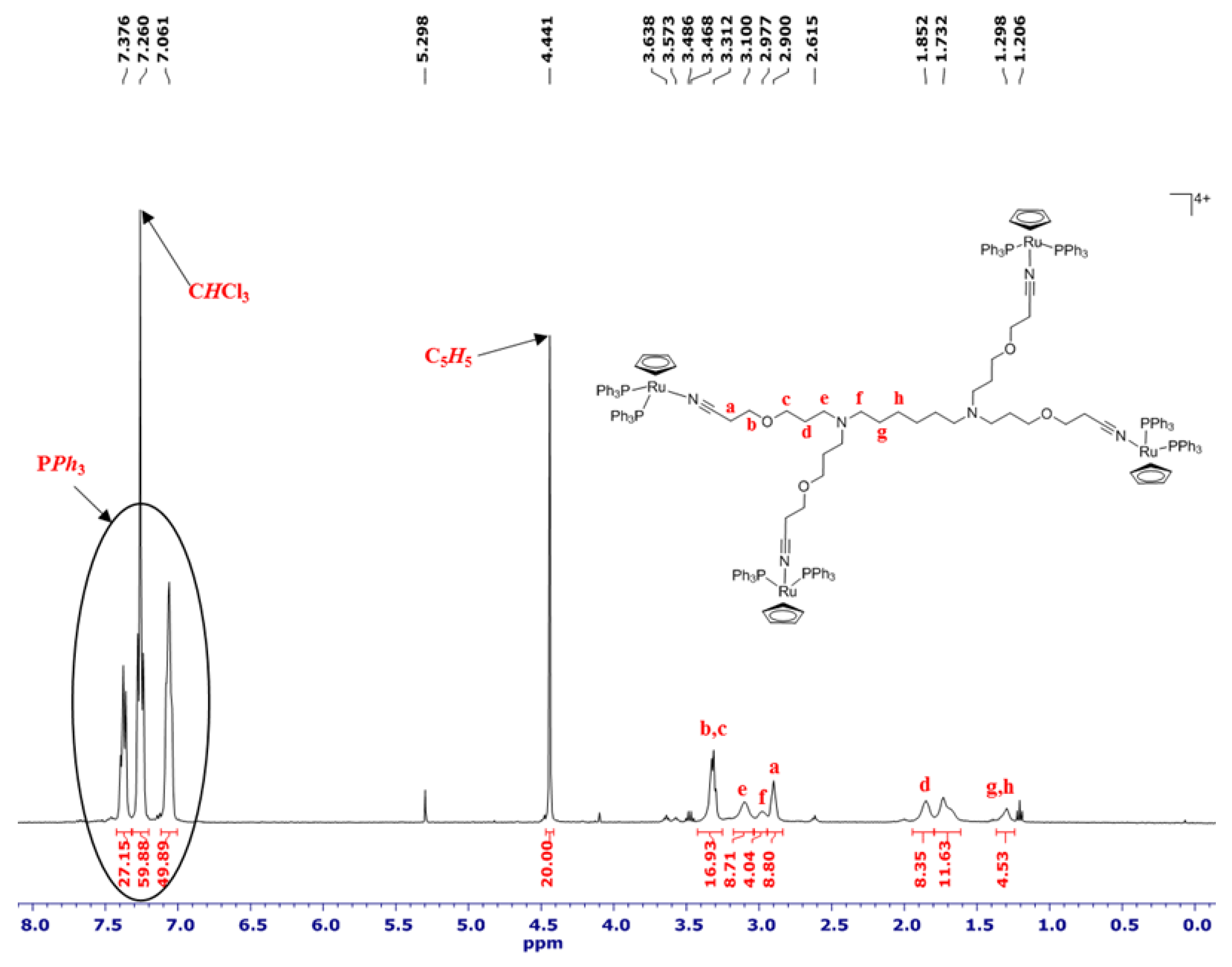
2.1. Synthesis and Characterization of [Ru(η5-C5H5)(PPh3)2]+ Functionalized Poly(alkylidenimine) Dendrimers
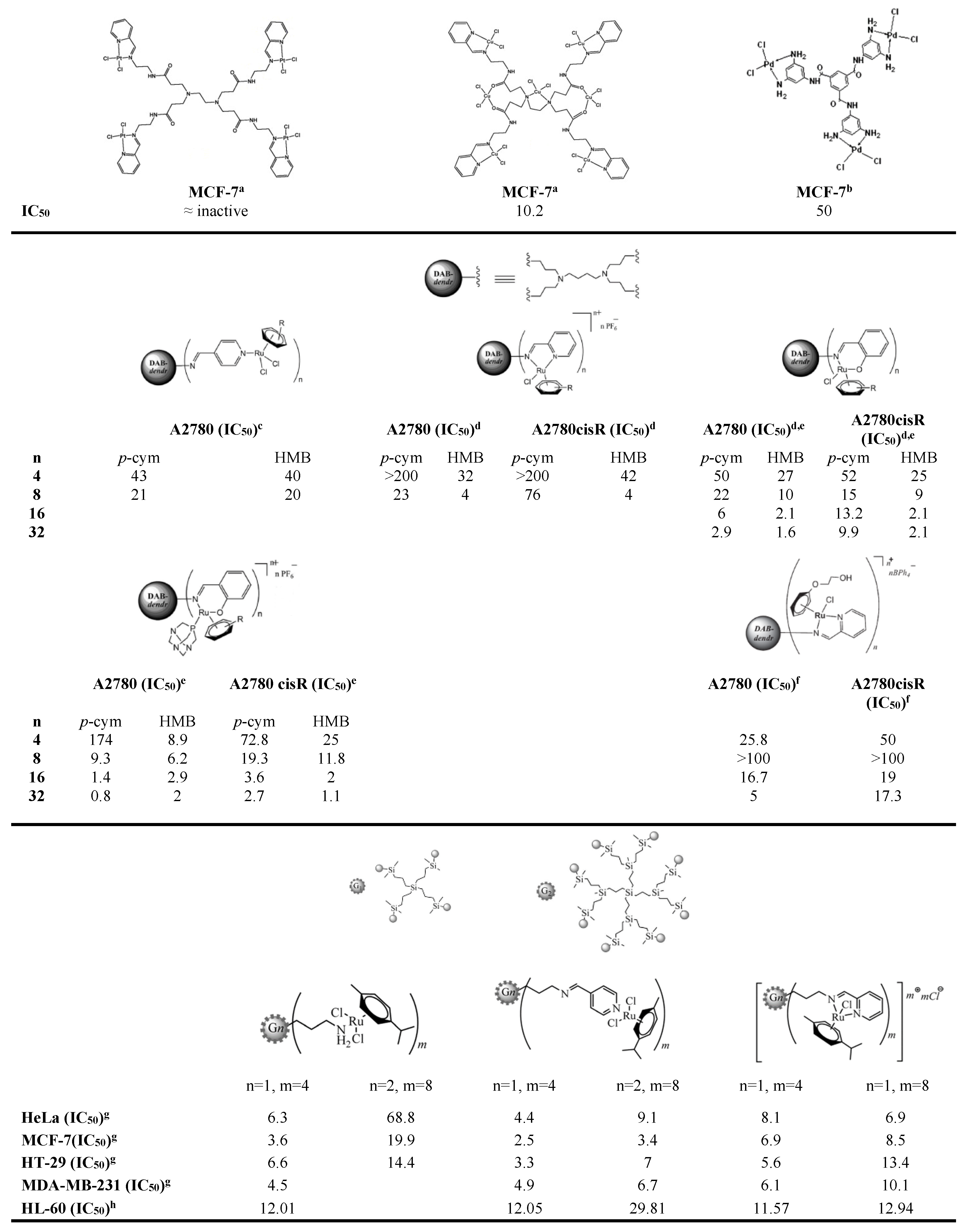
2.2. Biological Activity Assays
3. Materials and Methods
3.1. General Remarks
3.2. Physical Measurements
3.3. Synthesis
3.3.1. Synthesis of [{(η5-C5H5)(PPh3)2Ru}4(1)][CF3SO3]4 (3)
3.3.2. Synthesis of [{(η5-C5H5)(PPh3)2Ru}4(2)][CF3SO3]4 (4)
3.4. Cytotoxicity Studies
3.4.1. Cell Culture
3.4.2. Cell Viability Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ruddon, R.W. Cancer Biology, 4th ed.; Oxford University Press: New York, NY, USA, 2007; ISBN 978-0195175448. [Google Scholar]
- Hesketh, R. Introduction to Cancer Biology: A Concise Journey from Epidemiology Through Cell and Molecular Biology to Treatment and Prospects; Cambridge University Press: New York, NY, USA, 2013; ISBN 978-1-107-60148-2. [Google Scholar]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute—Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 26 April 2018).
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Trudu, F.; Amato, F.; Vaňhara, P.; Pivetta, T.; Peña-Méndez, E.M.; Havel, J. Coordination Compounds in Cancer: Past, Present and Perspectives. J. Appl. Biomed. 2015, 13, 79–103. [Google Scholar] [CrossRef]
- Monneret, C. Platinum Anticancer Drugs. From Serendipity to Rational Design. Ann. Pharm. Françaises 2011, 69, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Adjei, A.; Baas, P.; Meldgaard, P.; Nicolson, M.; Paz-Ares, L.; Reck, M.; Smit, E.F.; Syrigos, K.; Stahel, R.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Non-Small-Cell Lung Cancer First-Line/second and Further Lines of Treatment in Advanced Disease. Ann. Oncol. 2014, 25, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Gahart, B.L.; Nazareno, A.R. 2015 Intravenous Medications: A Handbook for Nurses and Health Professionals; Intravenous Medications, 31st ed.; Elsevier Health Sciences: St. Louis Missouri, USA, 2014. [Google Scholar]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and Targeted Therapy for Women With Human Epidermal Growth Factor Receptor 2—Negative (or Unknown) Advanced Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.H.; Einhorn, L.H. Testicular Cancer—Discoveries and Updates. N. Engl. J. Med. 2014, 371, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, M.; Kourie, H.R.; El Rassy, E.; Chebib, R.; El Karak, F.; Hanna, C.; Nasr, D. Optimum Chemotherapy for the Management of Advanced Biliary Tract Cancer. World J. Gastroenterol. 2015, 21, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus Paclitaxel Once a Week versus Every 3 Weeks in Patients with Advanced Ovarian Cancer (MITO-7): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Liao, B.-C.; Shao, Y.-Y.; Chen, H.-M.; Shau, W.-Y.; Lin, Z.-Z.; Kuo, R.N.; Lai, C.-L.; Chen, K.-H.; Cheng, A.-L.; Yang, J.C.-H.; et al. Comparative Effectiveness of First-Line Platinum-Based Chemotherapy Regimens for Advanced Lung Squamous Cell Carcinoma. Clin. Lung Cancer 2015, 16, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Garcia-Aguilar, J. Advances and Challenges in Treatment of Locally Advanced Rectal Cancer. J. Clin. Oncol. 2015, 33, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular Mechanisms of Resistance and Toxicity Associated with Platinating Agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; am Zehnhoff-Dinnesen, A.; Radtke, S.; Meitert, J.; Zolk, O. Understanding Platinum-Induced Ototoxicity. Trends Pharmacol. Sci. 2013, 34, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-Induced Neurotoxicity and Preventive Strategies: Past, Present, and Future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Thomale, J.; Stojanović, N.; Osmak, M.; Henninger, C.; Bormann, S.; Fritz, G. Platinum-Induced Kidney Damage: Unraveling the DNA Damage Response (DDR) of Renal Tubular Epithelial and Glomerular Endothelial Cells Following Platinum Injury. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 685–698. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum Neurotoxicity Pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum Resistance: The Role of DNA Repair Pathways. Clin. Cancer Res. 2008, 14, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum Resistant” Ovarian Cancer: What Is It, Who to Treat and How to Measure Benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, C.; Martinez-Cardús, A.; Santos, C.; Navarro-Pérez, V.; Martínez-Balibrea, E.; Musulen, E.; Carmona, F.J.; Sartore-Bianchi, A.; Cassingena, A.; Siena, S.; et al. Epigenetic Inactivation of the BRCA1 Interactor SRBC and Resistance to Oxaliplatin in Colorectal Cancer. J. Natl. Cancer Inst. 2014, 106, djt322. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Honecker, F. Cisplatin Resistance in Germ Cell Tumours: Models and Mechanisms. Andrology 2015, 3, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Horibe, S.; Matsuda, A.; Tanahashi, T.; Inoue, J.; Kawauchi, S.; Mizuno, S.; Ueno, M.; Takahashi, K.; Maeda, Y.; Maegouchi, T.; et al. Cisplatin Resistance in Human Lung Cancer Cells Is Linked with Dysregulation of Cell Cycle Associated Proteins. Life Sci. 2015, 124, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.; Beijnen, J.H.; Sava, G. Approaching Tumour Therapy beyond Platinum Drugs: Status of the Art and Perspectives of Ruthenium Drug Candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The Development of Anticancer Ruthenium(II) Complexes: From Single Molecule Compounds to Nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in Medicine: Current Clinical Uses and Future Prospects. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar] [CrossRef]
- Alessio, E.; Guo, Z. Metal Anticancer Complexes—Activity, Mechanism of Action, Future Perspectives. Eur. J. Inorg. Chem. 2017, 1549–1560. [Google Scholar] [CrossRef]
- Reedijk, J. Metal-Ligand Exchange Kinetics in Platinum and Ruthenium Complexes. Platin. Met. Rev. 2008, 52, 2–11. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Sadler, P.J. Controlling Platinum, Ruthenium, and Osmium Reactivity for Anticancer Drug Design. Adv. Inorg. Chem. 2009, 61, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, M.; Schluga, P.; Jakupec, M.A.; Arion, V.B.; Hartinger, C.G.; Allmaier, G.; Keppler, B.K. Transferrin Binding and Transferrin-Mediated Cellular Uptake of the Ruthenium Coordination Compound KP1019, Studied by Means of AAS, ESI-MS and CD Spectroscopy. J. Anal. At. Spectrom. 2004, 19, 46–51. [Google Scholar] [CrossRef]
- Guo, W.; Zheng, W.; Luo, Q.; Li, X.; Zhao, Y.; Xiong, S.; Wang, F. Transferrin Serves As a Mediator to Deliver Organometallic Ruthenium(II) Anticancer Complexes into Cells. Inorg. Chem. 2013, 52, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Emadi, A. Ruthenium-Based Chemotherapeutics: Are They Ready for Prime Time? Cancer Chemother. Pharmacol. 2010, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.H.; Dyson, P.J. Classical and Non-Classical Ruthenium-Based Anticancer Drugs: Towards Targeted Chemotherapy. Eur. J. Inorg. Chem. 2006, 2006, 4003–4018. [Google Scholar] [CrossRef]
- Levina, A.; Mitra, A.; Lay, P.A. Recent Developments in Ruthenium Anticancer Drugs. Metallomics 2009, 1, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Rademaker-Lakhai, J.M.; van den Bongard, D.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M. A Phase I and Pharmacological Study with Imidazolium-Trans-DMSO-Imidazole-Tetrachlororuthenate, a Novel Ruthenium Anticancer Agent. Clin. Cancer Res. 2004, 10, 3717–3727. [Google Scholar] [CrossRef] [PubMed]
- Leijen, S.; Burgers, S.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.; Schellens, J.M. Phase I/II Study with Ruthenium Compound NAMI-A and Gemcitabine in Patients with Non-Small Cell Lung Cancer after First Line Therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Sava, G. Linking the Future of Anticancer Metal-Complexes to the Therapy of Tumour Metastases. Chem. Soc. Rev. 2015, 44, 8818–8835. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, A New Redox-Active Anticancer Agent—Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Lentz, F.; Drescher, A.; Lindauer, A.; Henke, M.; Hilger, R.A.; Hartinger, C.G.; Scheulen, M.E.; Dittrich, C.; Keppler, B.K.; Jaehde, U. Research-EWIV, in collaboration with C. E. S. for A. D. Pharmacokinetics of a Novel Anticancer Ruthenium Complex (KP1019, FFC14A) in a Phase I Dose-Escalation Study. Anticancer Drugs 2009, 20, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, the First Ruthenium-Based Anticancer Drug on the Edge to Clinical Application. Chem. Sci. 2014, 5, 2925–2932. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. The Elements of Life and Medicines. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2015, 373. [Google Scholar] [CrossRef] [PubMed]
- Dömötör, O.; Hartinger, C.; Bytzek, A.; Kiss, T.; Keppler, B.; Enyedy, E. Characterization of the Binding Sites of the Anticancer ruthenium(III) Complexes KP1019 and KP1339 on Human Serum Albumin via Competition Studies. JBIC J. Biol. Inorg. Chem. 2013, 18, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Blunden, B.M.; Rawal, A.; Lu, H.; Stenzel, M.H. Superior Chemotherapeutic Benefits from the Ruthenium-Based Anti-Metastatic Drug NAMI-A through Conjugation to Polymeric Micelles. Macromolecules 2014, 47, 1646–1655. [Google Scholar] [CrossRef]
- Montesarchio, D.; Mangiapia, G.; Vitiello, G.; Musumeci, D.; Irace, C.; Santamaria, R.; D’Errico, G.; Paduano, L. A new design for nucleolipid-based Ru(III) complexes as anticancer agents. Dalton Trans. 2013, 42, 16697–16708. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Luchini, A.; D’Errico, G.; Santamaria, R.; Capuozzo, A.; Irace, C.; Montesarchio, D.; Paduano, L. Cationic Liposomes as Efficient Nanocarriers for the Drug Delivery of an Anticancer Cholesterol-Based Ruthenium Complex. J. Mater. Chem. B 2015, 3, 3011–3023. [Google Scholar] [CrossRef]
- Irace, C.; Misso, G.; Capuozzo, A.; Piccolo, M.; Riccardi, C.; Luchini, A.; Caraglia, M.; Paduano, L.; Montesarchio, D.; Santamaria, R. Antiproliferative effects of ruthenium-based nucleolipidic nanoaggregates in human models of breast cancer in vitro: Insights into their mode of action. Sci. Rep. 2017, 7, 45236. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Musumeci, D.; Irace, C.; Paduano, L.; Montesarchio, D. RuIII Complexes for Anticancer Therapy: The Importance of Being Nucleolipidic. Eur. J. Org. Chem. 2017, 1100–1119. [Google Scholar] [CrossRef]
- Blunden, B.M.; Chapman, R.; Danial, M.; Lu, H.; Jolliffe, K.A.; Perrier, S.; Stenzel, M.H. Drug Conjugation to Cyclic Peptide-Polymer Self-Assembling Nanotubes. Chem. Eur. J. 2014, 20, 12745–12749. [Google Scholar] [CrossRef] [PubMed]
- Suss-Fink, G. Arene Ruthenium Complexes as Anticancer Agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.H.; Morais, T.S.; Florindo, P.; Piedade, M.F.; Moreno, V.; Ciudad, C.; Noe, V. Inhibition of Cancer Cell Growth by ruthenium(II) Cyclopentadienyl Derivative Complexes with Heteroaromatic Ligands. J. Inorg. Biochem. 2009, 103, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, A.I.; Jakusch, T.; Morais, T.S.; Marques, F.; de Almeida, R.F.M.; Mendes, F.; Enyedy, É.A.; Santos, I.; Pessoa, J.C.; Kiss, T.; et al. [RuII(η5-C5H5)(bipy)(PPh3)]+, a Promising Large Spectrum Antitumor Agent: Cytotoxic Activity and Interaction with Human Serum Albumin. J. Inorg. Biochem. 2012, 117, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.S.; Santos, F.C.; Jorge, T.F.; Côrte-Real, L.; Madeira, P.J.A.; Marques, F.; Robalo, M.P.; Matos, A.; Santos, I.; Garcia, M.H. New Water-Soluble ruthenium(II) Cytotoxic Complex: Biological Activity and Cellular Distribution. J. Inorg. Biochem. 2014, 130, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.F.A.; Sadler, P.J. Medicinal Organometallic Chemistry: Designing Metal Arene Complexes as Anticancer Agents. Chem. An Asian J. 2008, 3, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.S.; Silva, T.J.L.; Marques, F.; Robalo, M.P.; Avecilla, F.; Madeira, P.J.A.; Mendes, P.J.G.; Santos, I.; Garcia, M.H. Synthesis of Organometallic ruthenium(II) Complexes with Strong Activity against Several Human Cancer Cell Lines. J. Inorg. Biochem. 2012, 114, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as Nanocarrier for Drug Delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging Polymers for Drug-Delivery Systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, T.; Roy, R.; Shi, X.; Majoral, J.-P. Bench-to-bedside translation of dendrimers: Reality or utopia? A concise analysis. Adv. Drug Deliv. Rev. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Iyer, A.K. Recent Advances in Dendrimer-Based Nanovectors for Tumor-Targeted Drug and Gene Delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Govender, P.; Therrien, B.; Smith, G.S. Bio-Metallodendrimers—Emerging Strategies in Metal-Based Drug Design. Eur. J. Inorg. Chem. 2012, 2012, 2853–2862. [Google Scholar] [CrossRef]
- Jansen, B.A.J.; van der Zwan, J.; Reedijk, J.; den Dulk, H.; Brouwer, J. A Tetranuclear Platinum Compound Designed to Overcome Cisplatin Resistance. Eur. J. Inorg. Chem. 1999, 1999, 1429–1433. [Google Scholar] [CrossRef]
- Hurley, A.L.; Mohler, D.L. Organometallic Photonucleases: Synthesis and DNA-Cleavage Studies of Cyclopentadienyl Metal-Substituted Dendrimers Designed To Increase Double-Strand Scission. Org. Lett. 2000, 2, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Govender, P.; Antonels, N.C.; Mattsson, J.; Renfrew, A.K.; Dyson, P.J.; Moss, J.R.; Therrien, B.; Smith, G.S. Anticancer Activity of Multinuclear Arene Ruthenium Complexes Coordinated to Dendritic Polypyridyl Scaffolds. J. Organomet. Chem. 2009, 694, 3470–3476. [Google Scholar] [CrossRef]
- Zhao, X.; Loo, S.C.J.; Lee, P.P.-F.; Tan, T.T.Y.; Chu, C.K. Synthesis and Cytotoxic Activities of chloropyridylimineplatinum(II) and chloropyridyliminecopper(II) Surface-Functionalized Poly(amidoamine) Dendrimers. J. Inorg. Biochem. 2010, 104, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Govender, P.; Renfrew, A.K.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. Antiproliferative Activity of Chelating N,O- and N,N-Ruthenium(II) Arene Functionalised Poly(propyleneimine) Dendrimer Scaffolds. Dalton Trans. 2011, 40, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Robilotto, T.J.; Alt, D.S.; von Recum, H.A.; Gray, T.G. Cytotoxic Gold(I)-Bearing Dendrimers from Alkyne Precursors. Dalton Trans. 2011, 40, 8083–8085. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Mapolie, S.F.; Alshehri, S. Synthesis and Characterization of Polyamide Metallodendrimers and Their Anti-Bacterial and Anti-Tumor Activities. Med. Chem. Res. 2012, 21, 2023–2031. [Google Scholar] [CrossRef]
- Govender, P.; Sudding, L.C.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. The Influence of RAPTA Moieties on the Antiproliferative Activity of Peripheral-Functionalised Poly(salicylaldiminato) Metallodendrimers. Dalton Trans. 2013, 42, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.; Govender, P.; Therrien, B.; Clavel, C.M.; Dyson, P.J.; Smith, G.S. Neutral and Cationic Multinuclear Half-Sandwich Rhodium and Iridium Complexes Coordinated to Poly(propyleneimine) Dendritic Scaffolds: Synthesis and Cytotoxicity. J. Organomet. Chem. 2013, 729, 20–27. [Google Scholar] [CrossRef]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.M.; Aubert, G.; Laurent, R.; Caminade, A.-M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.-P. Original Multivalent Copper(II)-Conjugated Phosphorus Dendrimers and Corresponding Mononuclear Copper(II) Complexes with Antitumoral Activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Sudding, L.C.; Payne, R.; Govender, P.; Edafe, F.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. Evaluation of the in Vitro Anticancer Activity of Cyclometalated Half-Sandwich Rhodium and Iridium Complexes Coordinated to Naphthaldimine-Based Poly(propyleneimine) Dendritic Scaffolds. J. Organomet. Chem. 2014, 774, 79–85. [Google Scholar] [CrossRef]
- Govender, P.; Edafe, F.; Makhubela, B.C.E.; Dyson, P.J.; Therrien, B.; Smith, G.S. Neutral and Cationic osmium(II)-Arene Metallodendrimers: Synthesis, Characterisation and Anticancer Activity. Inorg. Chim. Acta 2014, 409, 112–120. [Google Scholar] [CrossRef]
- Govender, P.; Riedel, T.; Dyson, P.J.; Smith, G.S. Higher Generation Cationic N,N-ruthenium(II)-Ethylene-Glycol-Derived Metallodendrimers: Synthesis, Characterization and Cytotoxicity. J. Organomet. Chem. 2015, 799–800, 38–44. [Google Scholar] [CrossRef]
- Maroto-Diaz, M.; Elie, B.T.; Gomez-Sal, P.; Perez-Serrano, J.; Gomez, R.; Contel, M.; Javier de la Mata, F. Synthesis and Anticancer Activity of Carbosilane Metallodendrimers Based on Arene Ruthenium(II) Complexes. Dalton Trans. 2016, 45, 7049–7066. [Google Scholar] [CrossRef] [PubMed]
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Gomez Ramirez, R.; Javier de la Mata, F.; Bryszewska, M. Ruthenium Metallodendrimers with Anticancer Potential in an Acute Promyelocytic Leukemia Cell Line (HL60). Eur. Polym. J. 2017, 87, 39–47. [Google Scholar] [CrossRef]
- Reagan, M.R.; Kaplan, D.L. Concise Review: Mesenchymal Stem Cell Tumor-Homing: Detection Methods in Disease Model Systems. Stem Cells 2011, 29, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.I.; Schwertschkow, A.H.; Nolta, J.A.; Wu, J. Involvement of Mesenchymal Stem Cells in Cancer Progression and Metastases. Curr. Cancer Drug Targets 2015, 15, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.-J.; Lee, I.J.; Eom, W.Y. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033. [Google Scholar] [CrossRef] [PubMed]
- Jardim, M.G.; Rissanen, K.; Rodrigues, J. Preparation and Characterization of Novel Poly(alkylidenamine) Nitrile Ruthenium Metallodendrimers. Eur. J. Inorg. Chem. 2010, 2010, 1729–1735. [Google Scholar] [CrossRef]
- Van Geelen, C.M.M.; de Vries, E.G.E.; Le, T.K.P.; van Weeghel, R.P.; de Jong, S. Differential Modulation of the TRAIL Receptors and the CD95 Receptor in Colon Carcinoma Cell Lines. Br. J. Cancer 2003, 89, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Tolan, D.; Gandin, V.; Morrison, L.; El-Nahas, A.; Marzano, C.; Montagner, D.; Erxleben, A. Oxidative Stress Induced by Pt(IV) Pro-drugs Based on the Cisplatin Scaffold and Indole Carboxylic Acids in Axial Position. Sci. Rep. 2016, 6, 29367. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F. Concise Review: Dissecting a Discrepancy in the Literature: Do Mesenchymal Stem Cells Support or Suppress Tumor Growth? Stem Cells 2011, 29, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ullah, M.; Zeitouni, S.; Beaver, J.; Prockop, D.J. Cancer Cells Enter Dormancy after Cannibalizing Mesenchymal Stem/stromal Cells (MSCs). Proc. Natl. Acad. Sci. USA 2016, 113, E6447–E6456. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.-S.; Lee, H.-Y.; Kang, K.-S. Mesenchymal Stem Cells and Cancer: Friends or Enemies? Mutat. Res. 2014, 768, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Houthuijzen, J.M.; Daenen, L.G.M.; Roodhart, J.M.L.; Voest, E.E. The Role of Mesenchymal Stem Cells in Anti-Cancer Drug Resistance and Tumour Progression. Br. J. Cancer 2012, 106, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Jardim, M.G.; Figueira, J.; Gouveia, M.; Tomas, H.; Rissanen, K. Poly(alkylidenamines) Dendrimers as Scaffolds for the Preparation of Low-Generation Ruthenium Based Metallodendrimers. New J. Chem. 2011, 35, 1938–1943. [Google Scholar] [CrossRef]
- Bruce, M.I.; Windsor, N.J. Cyclopentadienyl-Ruthenium and -Osmium Chemistry. IV. Convenient High-Yield Synthesis of Some Cyclopentadienyl Ruthenium or Osmium Tertiary Phosphine Halide Complexes. Aust. J. Chem. 1977, 30, 1601–1604. [Google Scholar] [CrossRef]
- Blunden, B.M.; Stenzel, M.H. Incorporating Ruthenium into Advanced Drug Delivery Carriers—An Innovative Generation of Chemotherapeutics. J. Chem. Technol. Biotechnol. 2015, 90, 1177–1195. [Google Scholar] [CrossRef]
- Thangavel, P.; Viswanath, B.; Kim, S. Recent developments in the nanostructured materials functionalized with ruthenium complexes for targeted drug delivery to tumors. Int. J. Nanomed. 2017, 12, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |

| Cell Type (IC50 Values in µM)1 | ||||||
|---|---|---|---|---|---|---|
| Compound | Caco-2 | CAL-72 | MCF-7 | A2780 | A2780cisR | hMSCs |
| RuCp | 14.7 | 2.4 | 4.4 | 0.3 | 2.3 | <0.05 |
| Metallodendrimer 3 | 3.4 | 0.6 | 2.5 | 0.1 | 0.3 | <0.05 |
| Metallodendrimer 4 | 3.2 | 1.4 | 3.0 | 0.2 | 0.3 | <0.05 |
| cisPt | 8.9 [84] | - 2 | 7.6 [85] | 1.1 | >50 | <0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, M.; Figueira, J.; Jardim, M.G.; Castro, R.; Tomás, H.; Rissanen, K.; Rodrigues, J. Poly(alkylidenimine) Dendrimers Functionalized with the Organometallic Moiety [Ru(η5-C5H5)(PPh3)2]+ as Promising Drugs Against Cisplatin-Resistant Cancer Cells and Human Mesenchymal Stem Cells. Molecules 2018, 23, 1471. https://doi.org/10.3390/molecules23061471
Gouveia M, Figueira J, Jardim MG, Castro R, Tomás H, Rissanen K, Rodrigues J. Poly(alkylidenimine) Dendrimers Functionalized with the Organometallic Moiety [Ru(η5-C5H5)(PPh3)2]+ as Promising Drugs Against Cisplatin-Resistant Cancer Cells and Human Mesenchymal Stem Cells. Molecules. 2018; 23(6):1471. https://doi.org/10.3390/molecules23061471
Chicago/Turabian StyleGouveia, Marisol, João Figueira, Manuel G. Jardim, Rita Castro, Helena Tomás, Kari Rissanen, and João Rodrigues. 2018. "Poly(alkylidenimine) Dendrimers Functionalized with the Organometallic Moiety [Ru(η5-C5H5)(PPh3)2]+ as Promising Drugs Against Cisplatin-Resistant Cancer Cells and Human Mesenchymal Stem Cells" Molecules 23, no. 6: 1471. https://doi.org/10.3390/molecules23061471
APA StyleGouveia, M., Figueira, J., Jardim, M. G., Castro, R., Tomás, H., Rissanen, K., & Rodrigues, J. (2018). Poly(alkylidenimine) Dendrimers Functionalized with the Organometallic Moiety [Ru(η5-C5H5)(PPh3)2]+ as Promising Drugs Against Cisplatin-Resistant Cancer Cells and Human Mesenchymal Stem Cells. Molecules, 23(6), 1471. https://doi.org/10.3390/molecules23061471