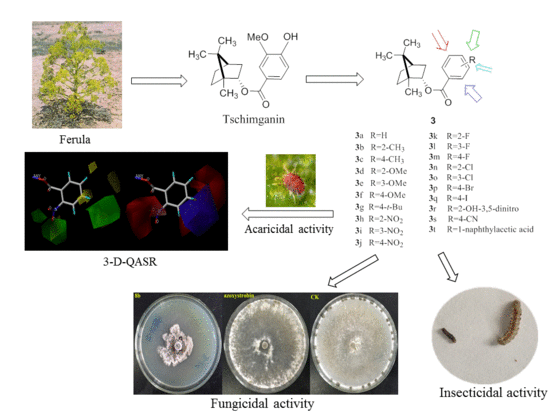
Synthesis, Insecticidal, Fungicidal Activities and Structure–Activity Relationships of Tschimganin Analogs
Abstract
1. Introduction
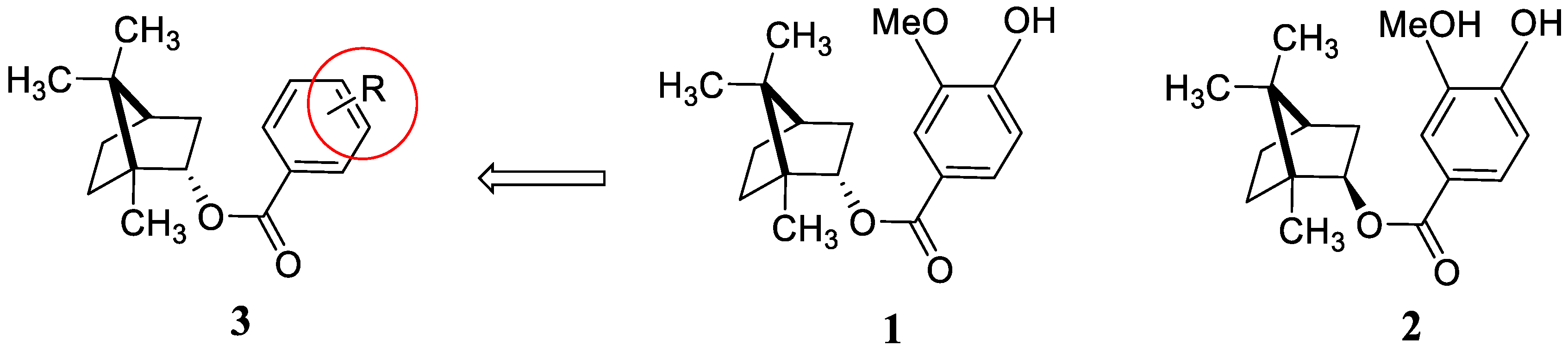
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Measurements
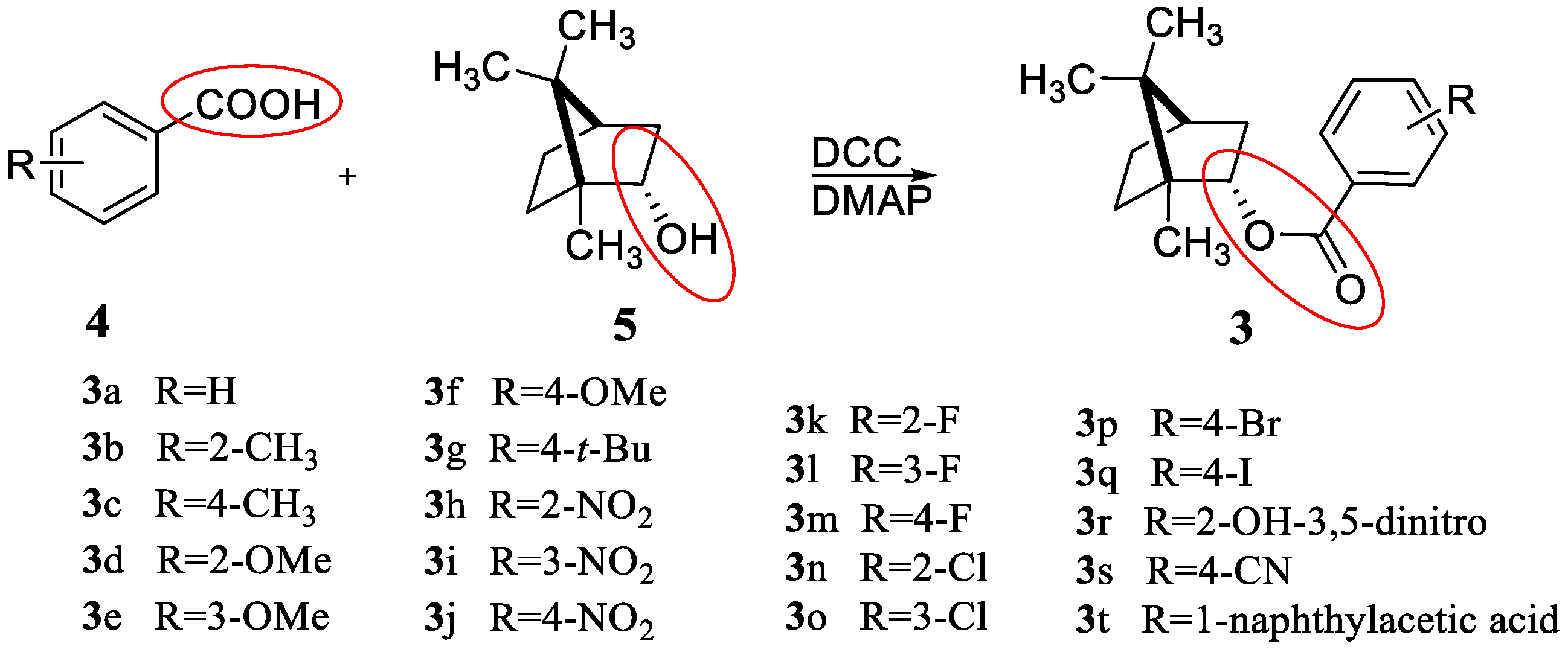
3.2. General Synthetic Method for Compounds 3
3.3. Insecticidal Activity
3.4. Enzyme Activity
3.5. Antifungal Activity
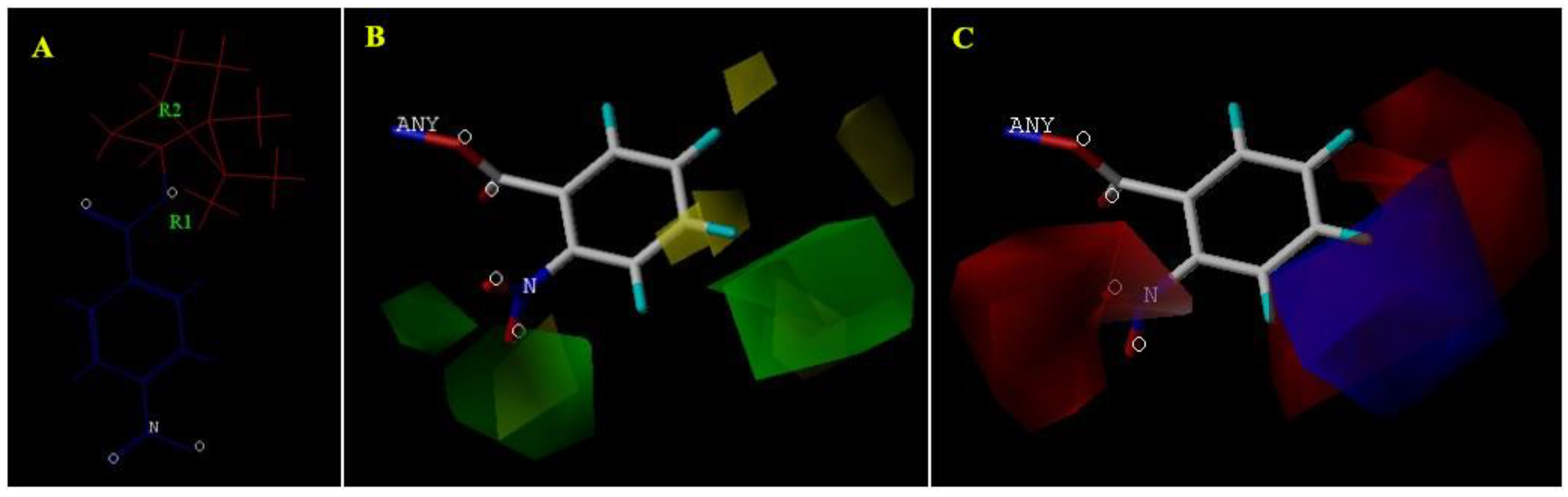
3.6. Quantitative Structure–Activity Relationship (QSAR) Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; Li, Z.; Wang, K.J.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Synthesis and antiviral, insecticidal, and fungicidal activities of gossypol derivatives containing alkylimine, oxime or hydrazine moiety. Bioorg. Med. Chem. 2016, 24, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Su, J. Dictionary of Chinese Traditional Medicines; Shanghai People’s Press: Shanghai, China, 1998; pp. 1186–1187. [Google Scholar]
- Zhou, Y.T.; Xin, F.; Zhang, G.Q.; Qu, H.X.; Yang, D.S.; Han, X.Q. Recent Advances on Bioactive Constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Hegazy, M.E.; Zellagui, A.; Rhouati, S.; Mohamed, T.A.; Sayed, A.A.; Mohamed, A.A.; Shinji, O.; Toshifumi, H. Ferulsinaic acid, a sesquiterpene coumarin with a rare carbon skeleton from Ferula species. Phytochemistry 2007, 68, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Wang, J.C.; Li, X.J.; Cao, L.; Gao, L.; Lv, N.; Si, J.Y. An unusual sesquiterpene coumarin from the seeds of Ferula sinkiangensis. J. Asian Nat. Prod. Res. 2016, 18, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Maxia, L.; Bascope, M.; Houghton, P.J.; Gonzalo, S.D.; Munoz, E.; Sterner, O. A Meroterpenoid NF-κB Inhibitor and Drimane Sesquiterpenoids from Asafetida. J. Nat. Prod. 2006, 69, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Han, H.Y.; Li, G.Y.; Wang, H.Y.; Zhang, C.; Zhang, K.; Tan, Y.; Li, P.Y.; Wang, J.H. Two new terpenoid benzoates with antitumor activity from the roots of Ferula dissecta. J. Asian Nat. Prod. Res. 2013, 15, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.C.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.L.; Si, Y.Y.; Hou, Y. Sesquiterpene Coumarins from Ferula sinkiangensis Act as Neuroinflammation Inhibitors. Planta Med. 2017, 83, 135–142. [Google Scholar] [CrossRef]
- Alam, M.; Khan, A.; Wadood, A.; Ayesha, K.; Bashir, S.; Aman, A.; Jan, A.K.; Rauf, A.; Ahmad, B.; Khan, A.R.; et al. Bioassay-Guided Isolation of Sesquiterpene Coumarins from Ferula narthex Bioss: A New Anticancer Agent. Front. Pharmacol. 2016, 7, 16. [Google Scholar] [CrossRef]
- Li, X.J.; Jiang, L.; Da, P. Preparation and investigation of the pharmacodynamics of effective antiulcerative composition in ferula sinkiangensis K. M. Shen. Mod. Chin. Med. 2007, 9, 8–10. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Iranshahi, M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)—A review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dastan, D.; Salehi, P.; Aliahmadi, A.; Gohari, A.R.; Maroofi, H.; Ardalan, A. New coumarin derivatives from Ferula pseudalliacea with antibacterial activity. Nat. Prod. Res. 2016, 30, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Mansour, Z.; Insaf, F.; Aymen, J.; Joseph, C.; Jalloul, B.; Hichem, B.J. Chemical Composition and In Vitro Evaluation of Antimicrobial, Antioxidant and Antigerminative Properties of the Seed Oil from the Tunisian Endemic Ferula tunetana POMEL ex BATT. Chem. Biodivers. 2017, 14, e1600116. [Google Scholar] [CrossRef]
- Samaneh, F.; Habib, A.; Ayatallah, S. Phytochemical and Acaricidal Study of the Galbanum, Ferula gumosa Boiss. (Apiaceae) Essential Oil against Tetranychus urticae Koch (Tetranychidae). J. Essent. Oil Bear. Plants 2017, 20, 185–195. [Google Scholar] [CrossRef]
- Adnan, A.; Emmy, T.; Paul, C.; Louis, M.; Vassiliki, E.; Sandra, A.; Luc, P. Antiprotozoal and Antiglycation Activities of Sesquiterpene Coumarins from Ferula narthex Exudate. Molecules 2016, 21, 1287. [Google Scholar] [CrossRef]
- Nazrullaev, S.; Saidkhodzhaev, S.; Akhmedkhodzhaeva, A.I.; Syrov, V.N.; Rasulev, B.F.; Khushbaktova, Z.A. Estrogen activity of terpenoids from plants of the genus Ferula. Chem. Nat. Compd. 2008, 44, 572–577. [Google Scholar] [CrossRef]
- Trusheva, B.; Todorov, I.; Ninova, M.; Najdenski, H.; Daneshmand, A.; Bankova, V. Antibacterial mono- and sesquiterpene esters of benzoic acids from Iranian propolis. Chem. Cent. J. 2010, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.X.; Song, H.J.; Li, Y.Q.; Wang, Q.M. Additive effects on the improvement of insecticidal activity: Design, synthesis, and insecticidal activity of novel pymetrozine derivatives. Bioorg. Med. Chem. 2016, 24, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.Y.; Guo, L.; Chen, X.F.; Zhou, Y.T.; Zhang, J.; Han, X.Q.; Dai, B. Design, Synthesis, and Antifungal Activity of Novel Aryl-1,2,3-Triazole-β-Carboline Hybrids. Molecules 2018, 23, 1344. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Lu, H.Z.; Yang, D.Y.; Li, H.; Gu, X.L.; Wan, C.; Jia, C.Q.; Wang, M.; Li, X.Y.; Qin, Z.H. Synthesis, Antifungal Activity and QSAR of Some Novel Carboxylic Acid Amides. Molecules 2015, 20, 4071–4087. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 8a–8t are available from the authors. |

| Compound | H. armigera (Hübner) | T. truncatus | T. turkestani | |||
|---|---|---|---|---|---|---|
| Mortality Rate % (48 h) | Growth and Development | Mortality Rate % (24 h) | Mortality Rate % (48 h) | Mortality Rate % (24 h) | Mortality Rate % (48 h) | |
| 3a | 0 | ND | 15.56 | 63.33 | 10.41 | 40.62 |
| 3b | 3 | dysplasia | 13.33 | 48.89 | 8.93 | 50.00 |
| 3c | 10 | dysplasia | 27.78 | 45.56 | 22.29 | 46.87 |
| 3d | 37 | dysplasia | 55.56 | 83.33 | 26.75 | 35.94 |
| 3e | 30 | dysplasia | 30.00 | 40.00 | 25.27 | 71.87 |
| 3f | 23 | dysplasia | 38.89 | 52.22 | 47.57 | 70.31 |
| 3g | 60 | dysplasia | 64.44 | 93.33 | 28.24 | 48.44 |
| 3h | 13 | dysplasia | 17.89 | 35.67 | 60.95 | 79.69 |
| 3i | 17 | dysplasia | 84.44 | 93.33 | 53.51 | 64.06 |
| 3j | 7 | dysplasia | 33.33 | 37.78 | 74.33 | 82.81 |
| 3k | 0 | ND | 18.89 | 33.33 | 11.89 | 37.50 |
| 3l | 0 | ND | 15.56 | 32.22 | 16.36 | 62.50 |
| 3m | 0 | ND | 65.56 | 82.22 | 13.37 | 31.25 |
| 3n | 10 | dysplasia | 15.56 | 30.00 | 13.37 | 62.50 |
| 3o | 10 | dysplasia | 12.22 | 16.67 | 5.94 | 18.75 |
| 3p | 7 | dysplasia | 11.11 | 17.78 | 25.27 | 21.87 |
| 3q | 10 | dysplasia | 13.23 | 15.36 | 41.62 | 59.37 |
| 3r | 0 | ND | 15.56 | 51.11 | - | - |
| 3s | 7 | dysplasia | 12.22 | 22.22 | 35.67 | 50.00 |
| 3t | 0 | ND | 17.78 | 41.11 | 5.95 | 0.00 |
| chlorantraniliprole | 100 | ND | - | - | - | - |
| biflenazate | - | - | 85.56 | 95.56 | 61.11 | 65.62 |
| pyridaben | - | - | 61.11 | 71.21 | 97.78 | 98.44 |
| hexythiazox | - | - | 83.33 | 83.33 | 67.19 | 71.11 |
| Compound | Inhibition Ratio (%) | ||||||
|---|---|---|---|---|---|---|---|
| CL | RS | FF | PG | AK | SSR | RSR | |
| 3a | 17.89 | 20.87 | 31.74 | 21.09 | 37.32 | 32.32 | 56.75 |
| 3b | 0.00 | −4.81 | 4.63 | 32.43 | 11.97 | 55.49 | 67.86 |
| 3c | 0.00 | −9.40 | 8.37 | 23.36 | 37.32 | 39.23 | 36.31 |
| 3d | 0.00 | 40.04 | 29.64 | 37.19 | 40.85 | 49.39 | 22.62 |
| 3e | 0.00 | 10.02 | 50.44 | 60.09 | 57.04 | 49.59 | 29.56 |
| 3f | 1.42 | −3.76 | 15.38 | 19.73 | 25.35 | 1.22 | 11.71 |
| 3g | 0.00 | −2.72 | 3.46 | 31.97 | 22.54 | 42.89 | 4.96 |
| 3h | 0.00 | 6.89 | 14.91 | 24.49 | 37.32 | 36.18 | 41.87 |
| 3i | 0.00 | −8.98 | 17.02 | 27.44 | 41.55 | 26.63 | 73.81 |
| 3j | 0.00 | −8.98 | 8.60 | 29.71 | 31.69 | 7.72 | 80.95 |
| 3k | 19.51 | 4.17 | 22.86 | 24.94 | 40.85 | 13.21 | 18.85 |
| 3l | 15.04 | −5.22 | 31.98 | 32.43 | 33.10 | 46.34 | 16.27 |
| 3m | 28.46 | −6.06 | 26.60 | 35.83 | 41.55 | 57.93 | 50.60 |
| 3n | 0.00 | 17.32 | 32.21 | 36.51 | 11.97 | 22.97 | 16.27 |
| 3o | 0.00 | −10.03 | 15.61 | 24.94 | 26.76 | 17.80 | 8.13 |
| 3p | 0.00 | −5.43 | 13.51 | 30.61 | 38.03 | 2.85 | 14.29 |
| 3q | 0.00 | 7.30 | 4.63 | 31.29 | 63.38 | 55.49 | 78.57 |
| 3r | 73.17 | 57.41 | 50.44 | 56.43 | 60.14 | 65.04 | 84.33 |
| 3s | 7.11 | 5.22 | 22.86 | 34.92 | 42.25 | 31.10 | 84.13 |
| 3t | 6.30 | −2.72 | 17.02 | 33.33 | 7.04 | 3.46 | 4.96 |
| azoxystrobin | 66.54 | 18.37 | 55.61 | 73.92 | 61.97 | 89.63 | 90.48 |
| kresoxim-methyl | 0.00 | 1.25 | 25.20 | 21.54 | 19.25 | 2.24 | 75.00 |
| trifloxystrobin | 46.54 | 28.60 | 52.55 | 71.28 | 65.39 | 86.79 | 17.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, C.; Xin, F.; Han, X.; Zhang, J.; Sun, K. Synthesis, Insecticidal, Fungicidal Activities and Structure–Activity Relationships of Tschimganin Analogs. Molecules 2018, 23, 1473. https://doi.org/10.3390/molecules23061473
Zhou Y, Wang C, Xin F, Han X, Zhang J, Sun K. Synthesis, Insecticidal, Fungicidal Activities and Structure–Activity Relationships of Tschimganin Analogs. Molecules. 2018; 23(6):1473. https://doi.org/10.3390/molecules23061473
Chicago/Turabian StyleZhou, Yueting, Chunjuan Wang, Fang Xin, Xiaoqiang Han, Jie Zhang, and Ke Sun. 2018. "Synthesis, Insecticidal, Fungicidal Activities and Structure–Activity Relationships of Tschimganin Analogs" Molecules 23, no. 6: 1473. https://doi.org/10.3390/molecules23061473
APA StyleZhou, Y., Wang, C., Xin, F., Han, X., Zhang, J., & Sun, K. (2018). Synthesis, Insecticidal, Fungicidal Activities and Structure–Activity Relationships of Tschimganin Analogs. Molecules, 23(6), 1473. https://doi.org/10.3390/molecules23061473