UPLC-ESI-QTOF-MS2-Based Identification and Antioxidant Activity Assessment of Phenolic Compounds from Red Corn Cob (Zea mays L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolics Extraction by UAE
2.2. Antioxidant Activity of Red Corn Cob Extracts
2.3. Identification of Compounds by UPLC-ESI-QTOF-MS2
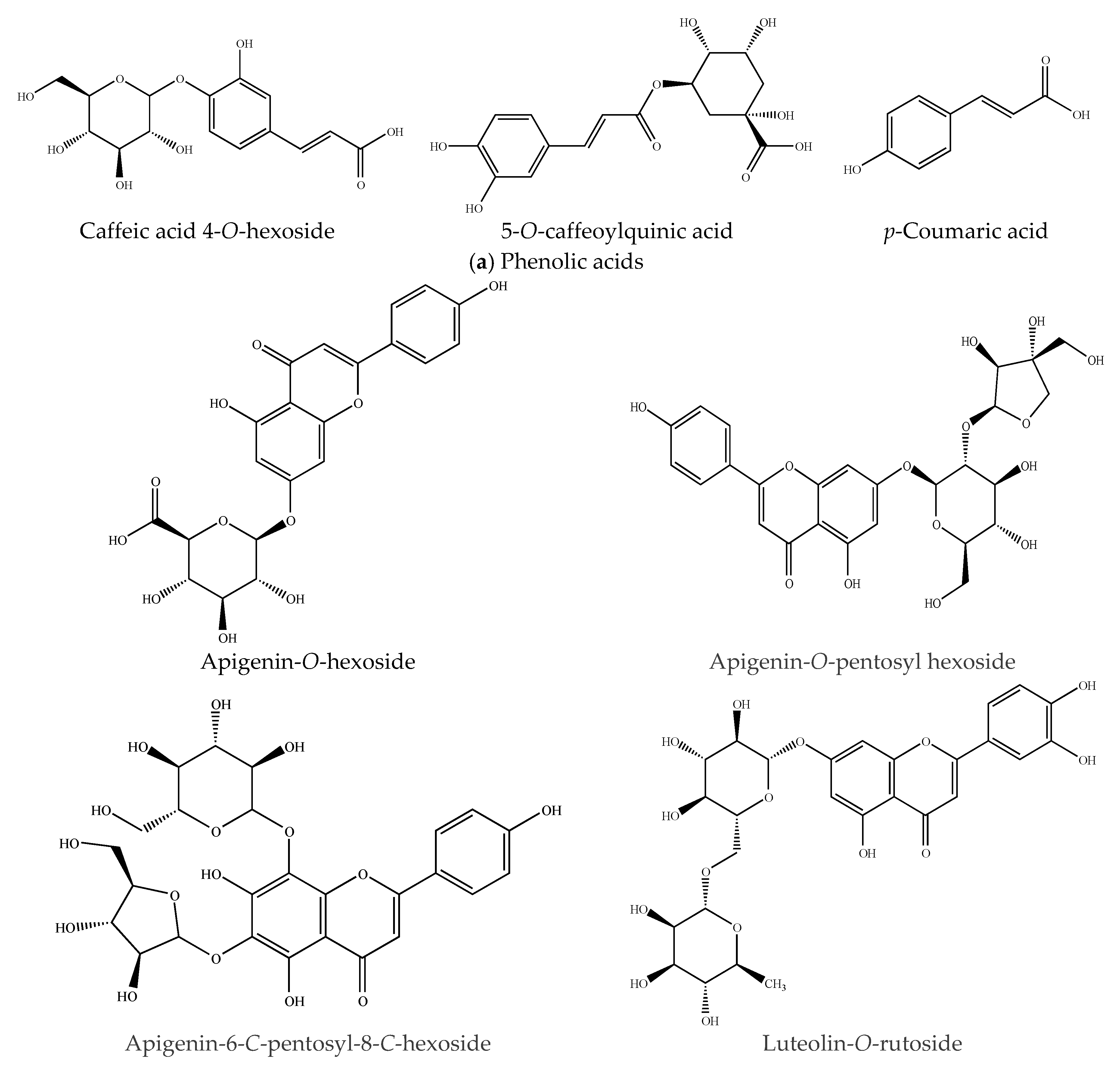
2.3.1. Phenolic Acids
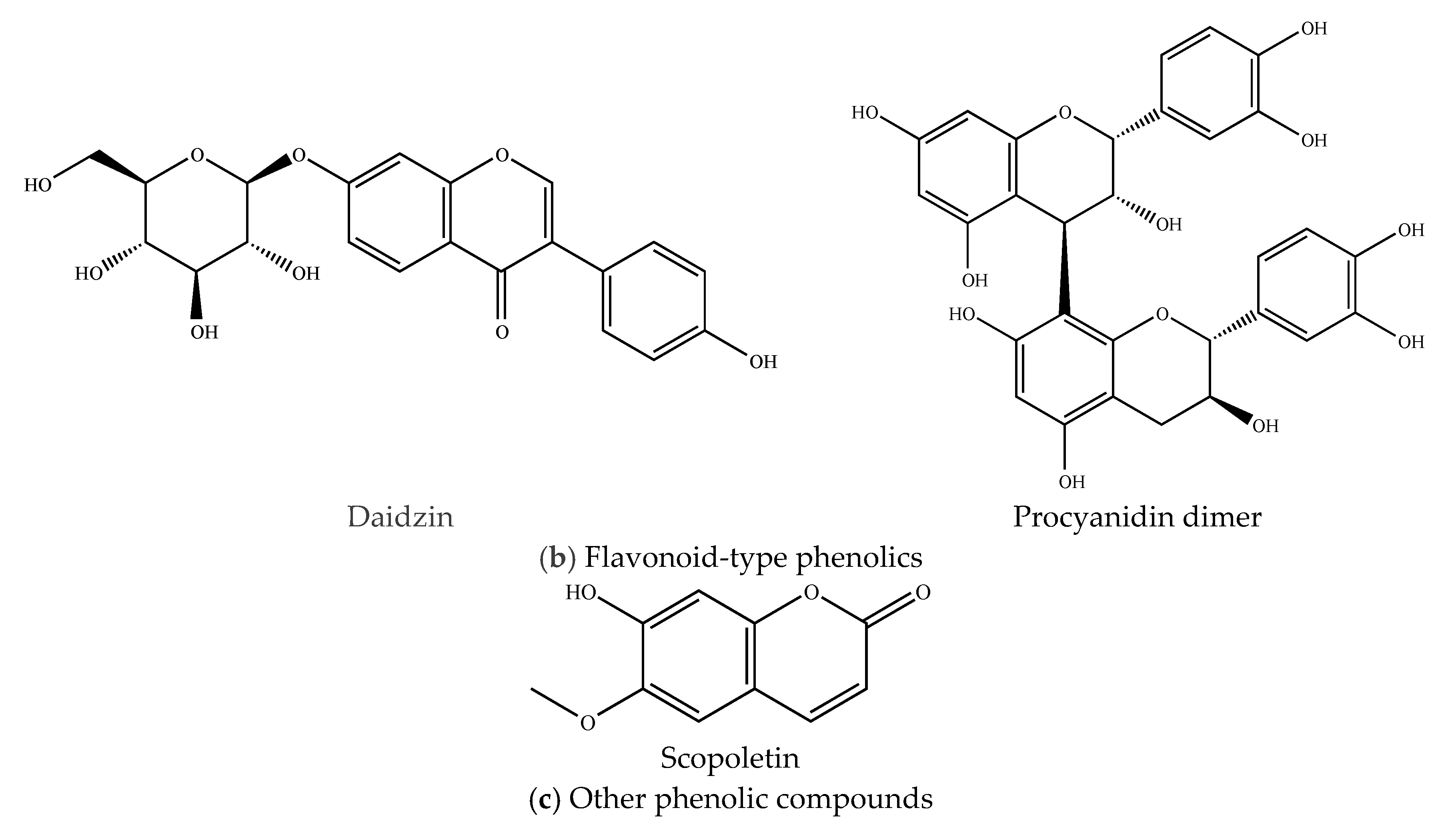
2.3.2. Flavonoid-Type Phenolics
2.3.3. Other Phenolic Compounds
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction of Phenolic Compounds
3.4. Phenolic Content Analysis
3.5. Antioxidant Activity Assays
3.5.1. DPPH• Free Radical Scavenging
3.5.2. ABTS•+ Free Radical Scavenging
3.6. UPLC-ESI-QTOF-MS2 Assay
3.7. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterization of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Afanasev, I.B.; Dcrozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and quantification of 56 targeted phenols in wines, spirits, and vinegars by online solid-phase extraction-ultrahigh-performance liquid chromatography-quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Costa, C.A.; Coleman, W.; Dube, M.; Rodrigues, A.E.; Rodrigues Pinto, P.C. Assessment of key features of lignin from lignocellulosic crops: Stalks and roots of corn, cotton, sugarcane, and tobacco. Ind. Crop. Prod. 2016, 92, 136–148. [Google Scholar] [CrossRef]
- Ferruz, E.; Loran, S.; Herrera, M.; Gimenez, I.; Bervis, N.; Barcena, C.; Carramiñana, J.J.; Juan, T.; Herrera, A.; Ariño, A. Inhibition of Fusarium growth and mycotoxin production in culture medium and in maize kernels by natural phenolic acids. J. Food Prot. 2016, 79, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.G.; Belmares-Cerda, R.E.; Aguilar, C.N. Ultrasound-assisted extraction of polyphenols from native plants in the Mexican desert. Ultrason. Sonochem. 2014, 22, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Urías-Orona, V.; Heredia, J.B.; Muy-Rangel, D.; Niño-Medina, G. Phenolic acids with antioxidant activity in maize bran and wheat bran. Ecosyst. Agric. Resour. 2016, 3, 43–50. [Google Scholar]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Influence of ethanol, water, and their mixtures as co-solvents of the supercritical carbon dioxide in the extraction of phenolics from purple corn cob (Zea mays L.). J. Supercrit. Fluids 2016, 118, 11–18. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic compounds and other polar compounds in the seed and seed coat of avocado by HPLC-DA-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Antioxidant activity of phenolic compounds obtained by autohydrolysis of corn residues. Ind. Crop. Prod. 2012, 36, 164–171. [Google Scholar] [CrossRef]
- Al-Owaisi, M.; Al-Hadiwi, N.; Khan, S.A. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed. 2014, 4, 964–970. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- Dal Prá, V.; Lunelli, F.C.; Vendruscolo, R.G.; Martins, R.; Wagner, R.; Lazzaretti, A.P.; da Rosa, M.B. Ultrasound-assisted extraction of bioactive compounds from palm pressed fiber with high antioxidant and photoprotective activities. Ultrason. Sonochem. 2017, 36, 362–366. [Google Scholar] [CrossRef]
- Floegel, A.; Kim D Chung, S.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Guan, Y.; Wei, Z.F.; Peng, X.; Li, T.T.; Qi, X.L.; Zu, Y.G.; Fu, Y.J. Flavonoid C-glycosides from pigeon pea leaves as color and anthocyanin stabilizing agent in blueberry juice. Ind. Crop. Prod. 2014, 58, 142–147. [Google Scholar] [CrossRef]
- Fulcrand, H.; Mane, C.; Preys, S.; Cheynier, V. Direct mass spectrometry approaches to characterize polyphenol composition of complex samples. Phytochemistry 2008, 69, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Rohloff, J. Analysis of phenolic and cyclic compounds in plants using derivatization techniques in combination with GC-MS-based metabolite profiling. Molecules 2015, 20, 3431–3462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Lina, F.; Zhanga, H.; Xiao, R. Structural elucidation of industrial bioethanol residual lignin from corn stalk: A potential source of vinyl phenolics. Fuel Process. Technol. 2018, 169, 50–57. [Google Scholar] [CrossRef]
- Piasecka, A.; Sawikowska, A.; Krajewski, P.; Kachlicki, P. Combined mass spectrometric and chromatographic methods for in-depth analysis of phenolic secondary metabolites in barley leaves. J. Mass Spectrom. 2015, 50, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal. Bioanal. Chem. 2015, 408, 3125–3143. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; de Camargo, A.C.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted Camelina and Sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Khamis, M.M.; Saeid, W.M.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrason. Sonochem. 2015, 27, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Van Den Berg, R.; Haenen, G.R.M.M.; Van Den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Kumari, S.; Elancheran, R.; Kotoky, J.; Devi, R. Rapid screening and identification of phenolic antioxidants in Hydrocotyle sibthorpioides Lam. by UPLC-ESI-MS/MS. Food Chem. 2016, 203, 521–529. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Independent Variable | Antioxidant Activity | Total Phenolic Content | ||
|---|---|---|---|---|
| Extraction Time (min) | Solid:Liquid Ratio (w/v) | ABTS•+ (TE mg/100g) | DPPH• (GAE mg/100 g) | F. Ciocalteau (GAE mg/100 g) |
| 60 | 10 | 148.35 ± 8.70 a,b,c | 38.96 ± 7.82 c | 215.17 ± 33.49 c |
| 60 | 20 | 155.14 ± 3.02 a | 44.02 ± 1.33 b,c | 441.95 ± 31.82 a,b |
| 60 | 30 | 151.41 ± 2.50 a,b | 65.20 ± 2.25 a | 348.39 ± 5.02 b,c |
| 90 | 10 | 133.60 ± 3.38 c,d,e | 34.29 ± 3.51 c | 385.5 ± 49.50 a,b |
| 90 | 20 | 127.56 ± 6.96 d,e | 50.93 ± 7.38 a,b,c | 242.44 ± 3.09 b,c |
| 90 | 30 | 139.44 ± 3.51 b,c,d | 56.82 ± 5.54 a,b | 453.63 ± 6.19 a,b |
| 120 | 10 | 114.63 ± 1.31 e | 14.39 ± 1.20 d | 388.00 ± 12.03 a,b |
| 120 | 20 | 121.05 ± 4.72 e | 35.10 ± 5.38 c | 474.15 ± 4.51 a,b |
| 120 | 30 | 123.73 ± 6.42 e | 30.03 ± 4.19 c,d | 527.33 ± 103.79 a |
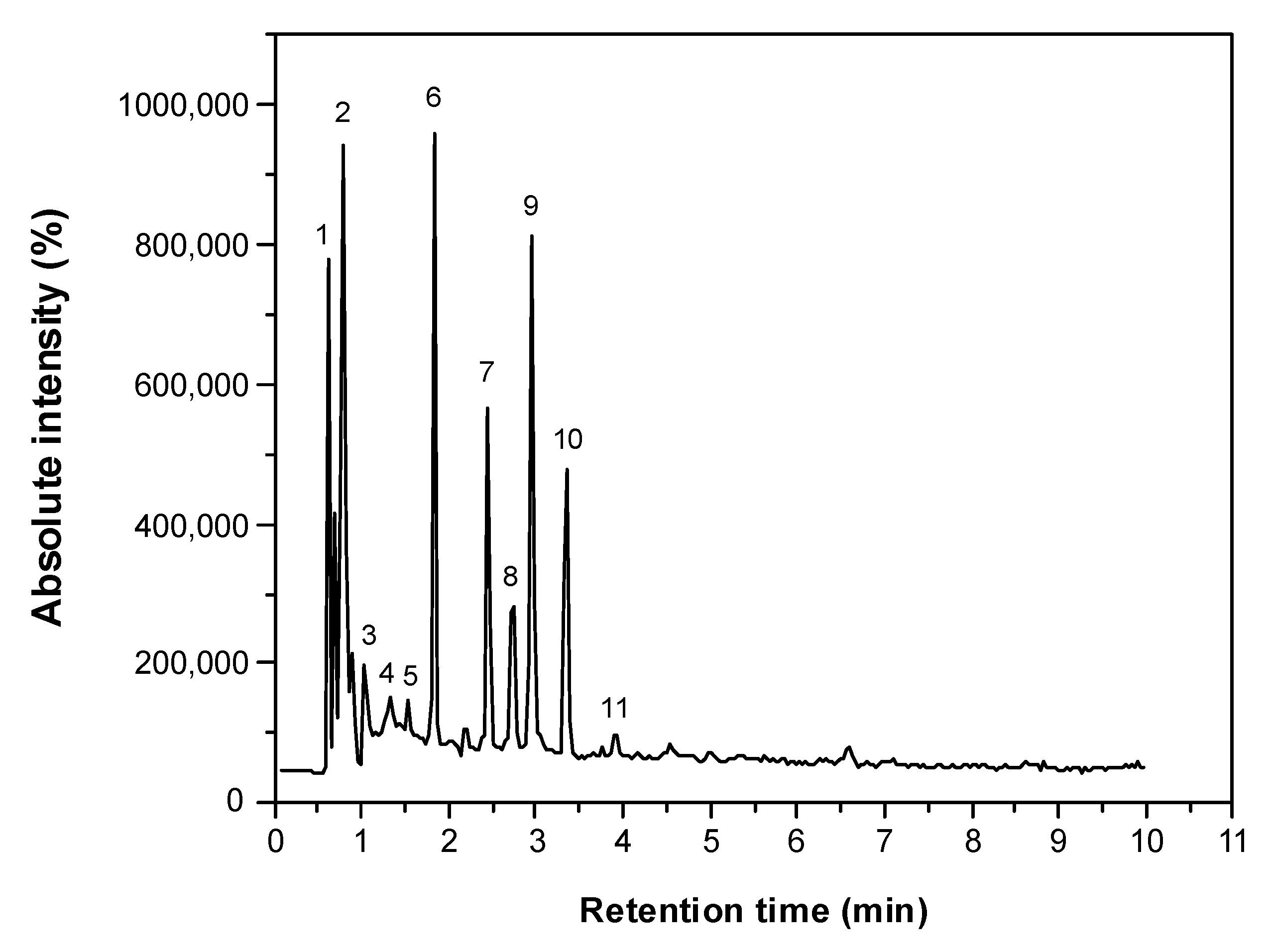
| N° of Signal | RT (min) | [M − H]− (m/z) | Tentative Identity | Phenol Type | Molecular Formula | Dominant Fragment Ion (MS2) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 0.78 | 387.0324 | Caffeic acid 4-O-hexoside | Phenolic acid | C15H18O9 | 135.0156 | [25] |
| 2 | 0.88 | 191.0487 | Scopoletin | Hydroxycoumarin | C10H8O4 | 176.0112 | [5] |
| 3 | 1.32 | 445.0452 | Apigenin-O-hexoside | Flavonoid | C21H18O11 | 269.0238 | [25] |
| 4 | 1.52 | 353.0301 | 5-O-caffeoylquinic acid | Phenolic acid | C16H18O9 | 191.0182 | [29] |
| 5 | 1.83 | 593.0031 | Luteolin-O-rutinoside | Flavonoid | C27H30O15 | 285.0391 | [27] |
| 6 | 2.16 | 415.0813 | Daidzin | Isoflavonoid | C15H10O4 | 253.0213 | [28] |
| 7 | 2.44 | 563.0032 | Apigenin-O-pentosyl hexoside | Flavonoid | C26H28O14 | 563, 269.0126 | [24] |
| 8 | 2.74 | 563.0036 | Apigenin 6-C-pentosyl-8-C-hexoside | Flavonoid | C26H28O14 | 563, 473 | [24] |
| 9 | 2.94 | 237.0558 | Unknown | - | - | - | - |
| 10 | 3.35 | 163.0404 | p-Coumaric acid | Phenolic acid | C9H8O3 | 119.0502, 93.1266 | [5] |
| 11 | 3.89 | 577.0161 | Procyanidin dimer | Flavonoid | - | 289.0143 | [28] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, M.; Ventura, J.; Castro, C.; Boone, V.; Rojas, R.; Ascacio-Valdés, J.; Martínez-Ávila, G. UPLC-ESI-QTOF-MS2-Based Identification and Antioxidant Activity Assessment of Phenolic Compounds from Red Corn Cob (Zea mays L.). Molecules 2018, 23, 1425. https://doi.org/10.3390/molecules23061425
Hernández M, Ventura J, Castro C, Boone V, Rojas R, Ascacio-Valdés J, Martínez-Ávila G. UPLC-ESI-QTOF-MS2-Based Identification and Antioxidant Activity Assessment of Phenolic Compounds from Red Corn Cob (Zea mays L.). Molecules. 2018; 23(6):1425. https://doi.org/10.3390/molecules23061425
Chicago/Turabian StyleHernández, María, Janeth Ventura, Cecilia Castro, Víctor Boone, Romeo Rojas, Juan Ascacio-Valdés, and Guillermo Martínez-Ávila. 2018. "UPLC-ESI-QTOF-MS2-Based Identification and Antioxidant Activity Assessment of Phenolic Compounds from Red Corn Cob (Zea mays L.)" Molecules 23, no. 6: 1425. https://doi.org/10.3390/molecules23061425
APA StyleHernández, M., Ventura, J., Castro, C., Boone, V., Rojas, R., Ascacio-Valdés, J., & Martínez-Ávila, G. (2018). UPLC-ESI-QTOF-MS2-Based Identification and Antioxidant Activity Assessment of Phenolic Compounds from Red Corn Cob (Zea mays L.). Molecules, 23(6), 1425. https://doi.org/10.3390/molecules23061425