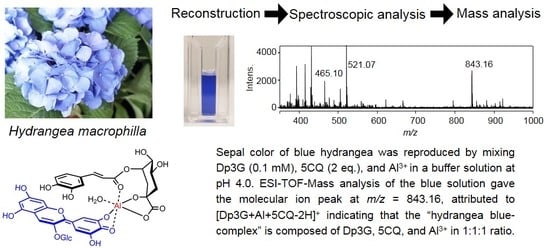
Direct Observation of Hydrangea Blue-Complex Composed of 3-O-Glucosyldelphinidin, Al3+ and 5-O-Acylquinic Acid by ESI-Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
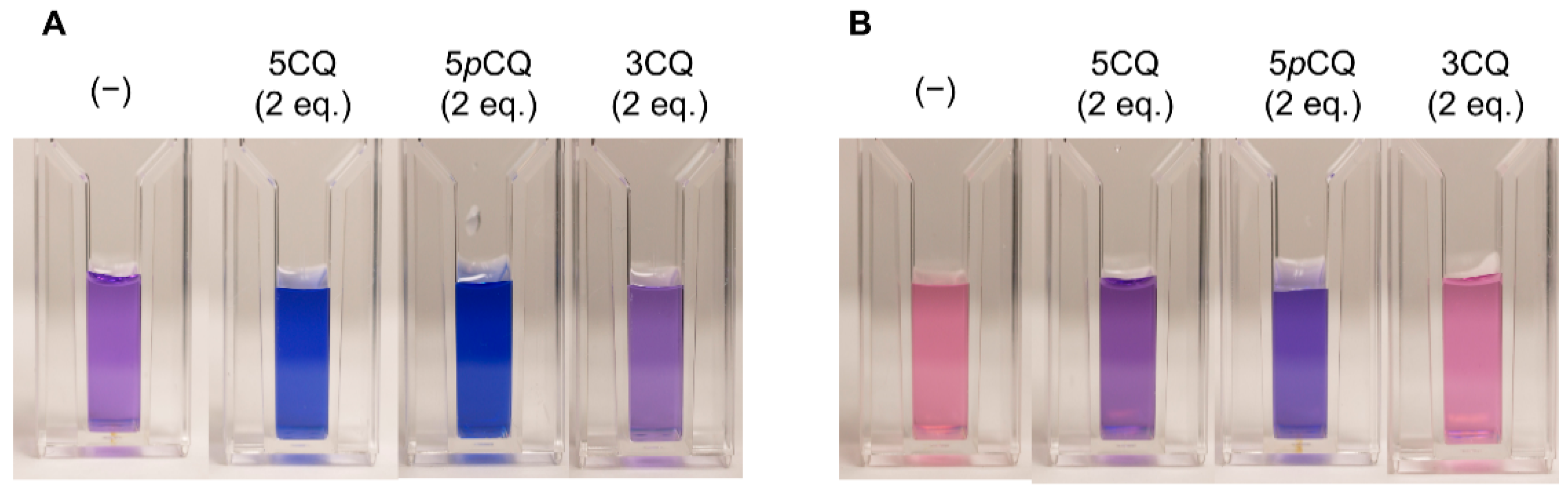
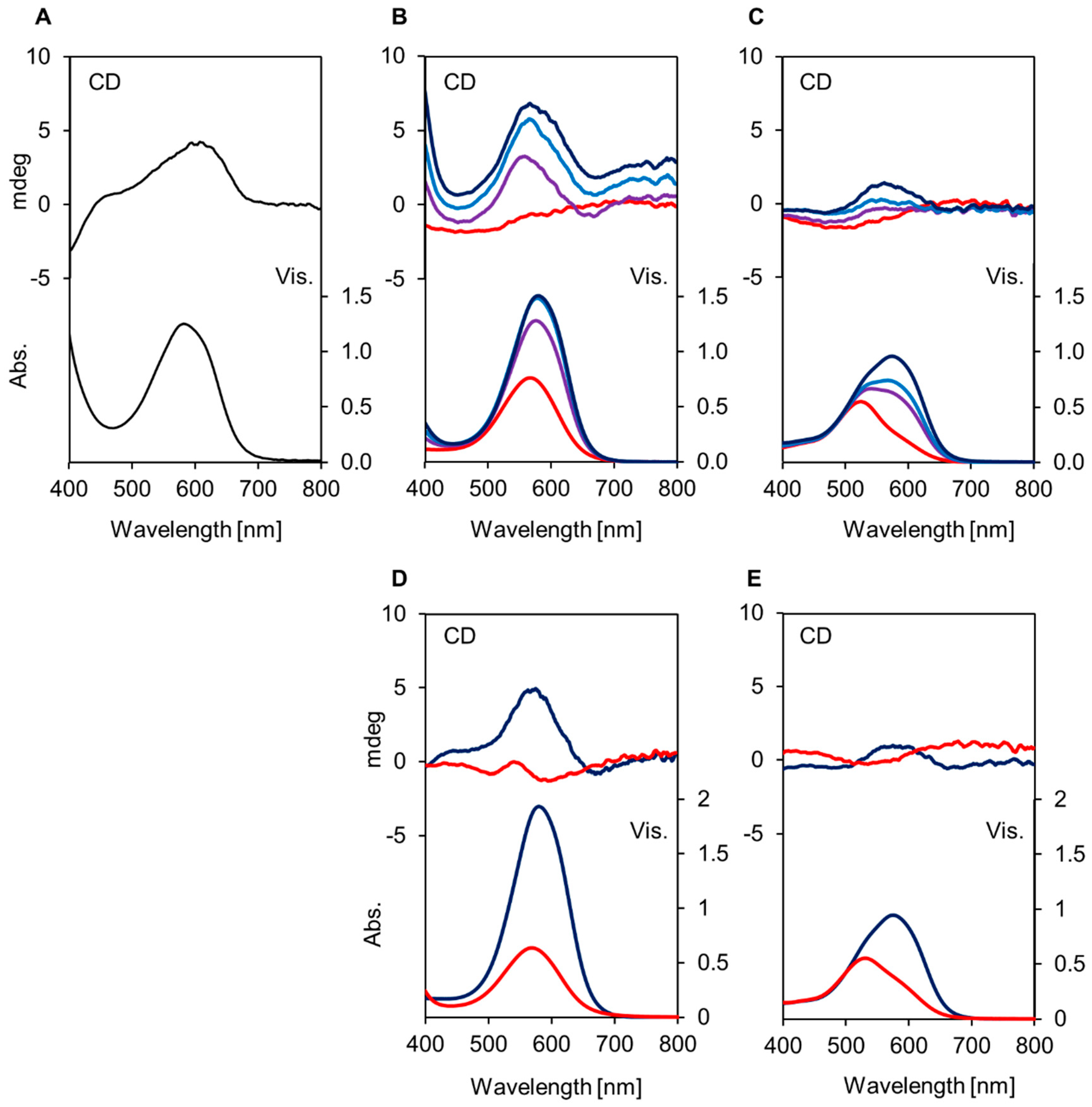
2.1. Reproduction of Blue and Red Sepal Colors In Vitro
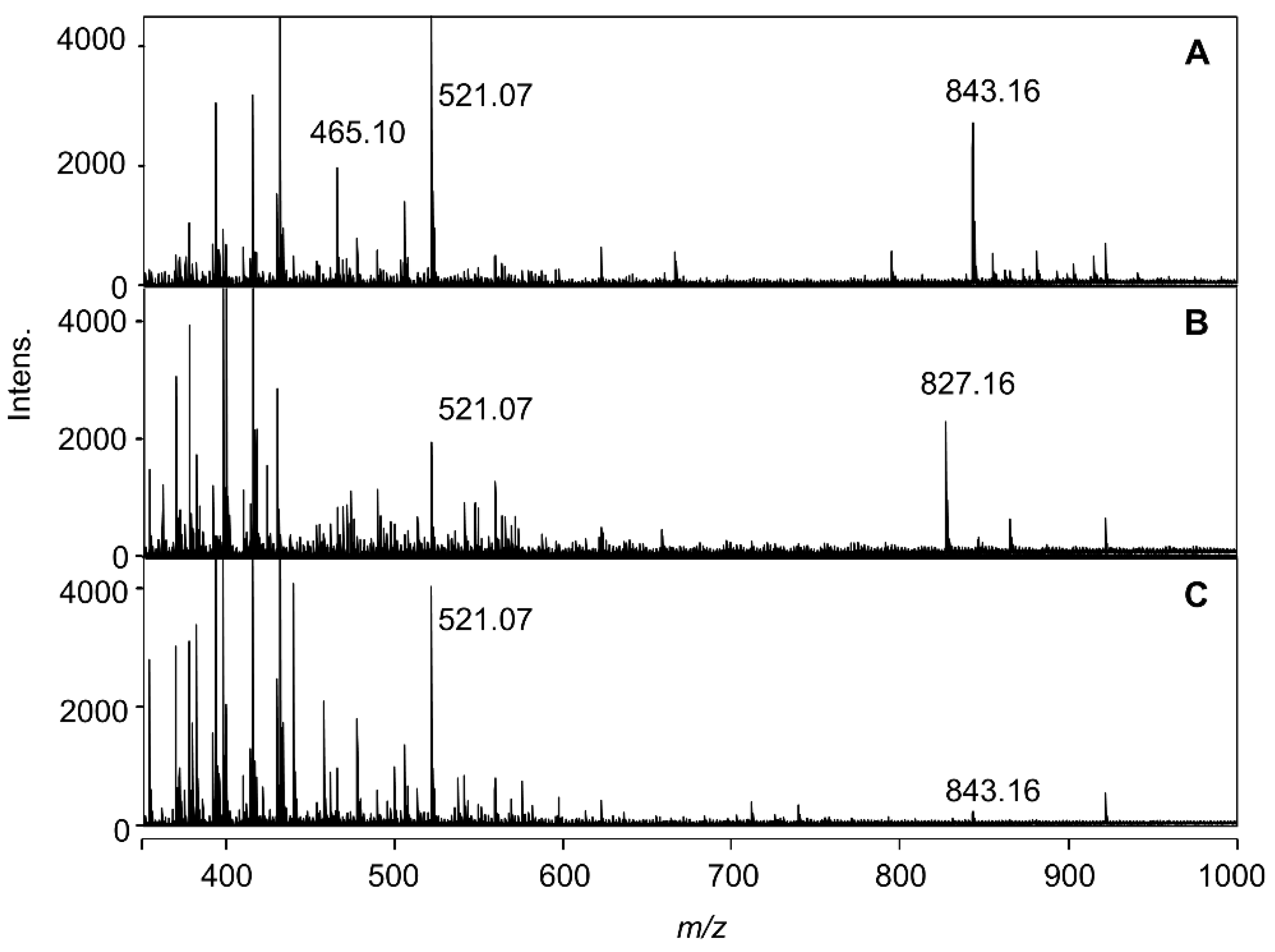
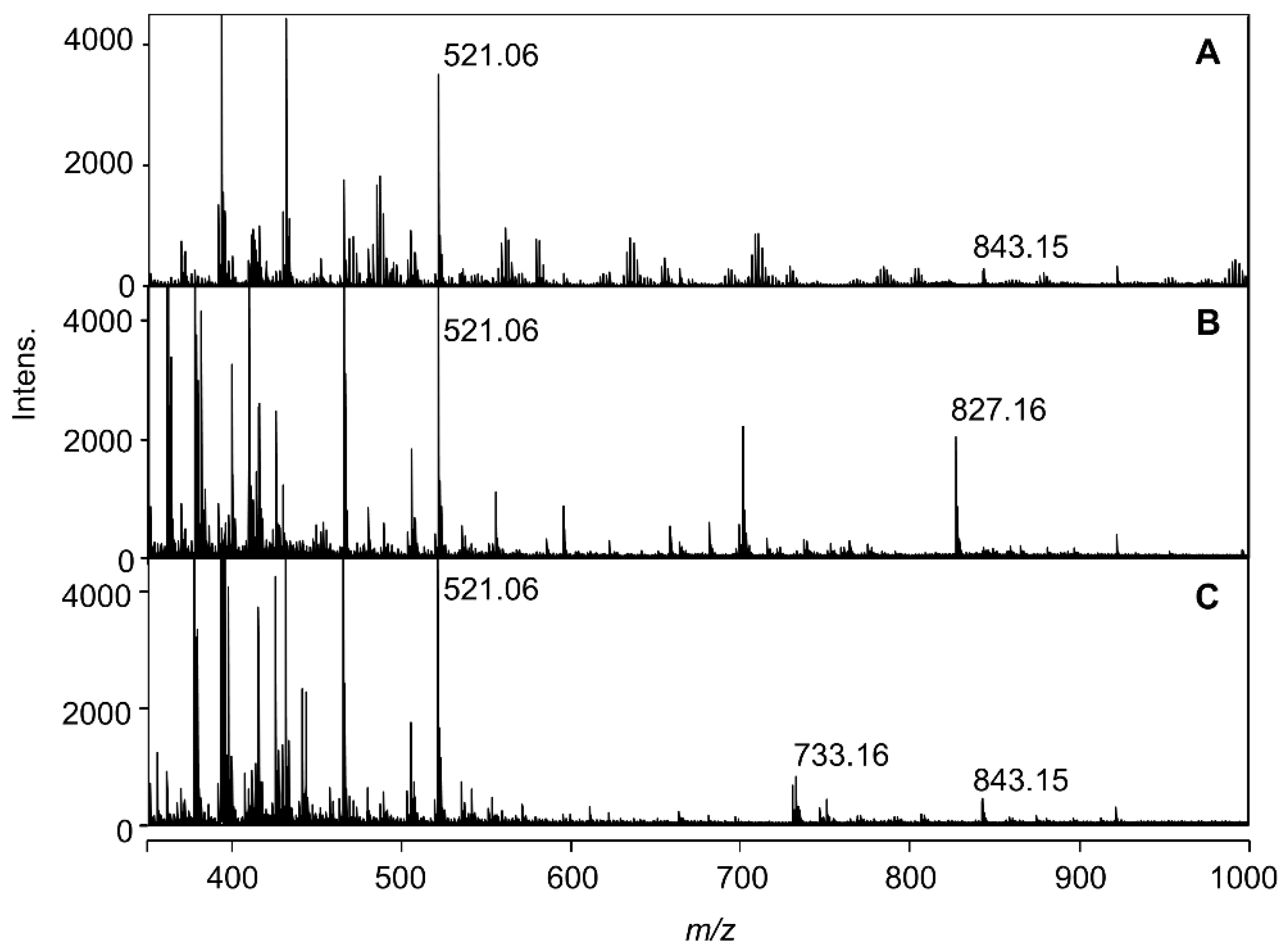
2.2. ESI-Mass Analysis of Reproduced Blue and Red Solutions
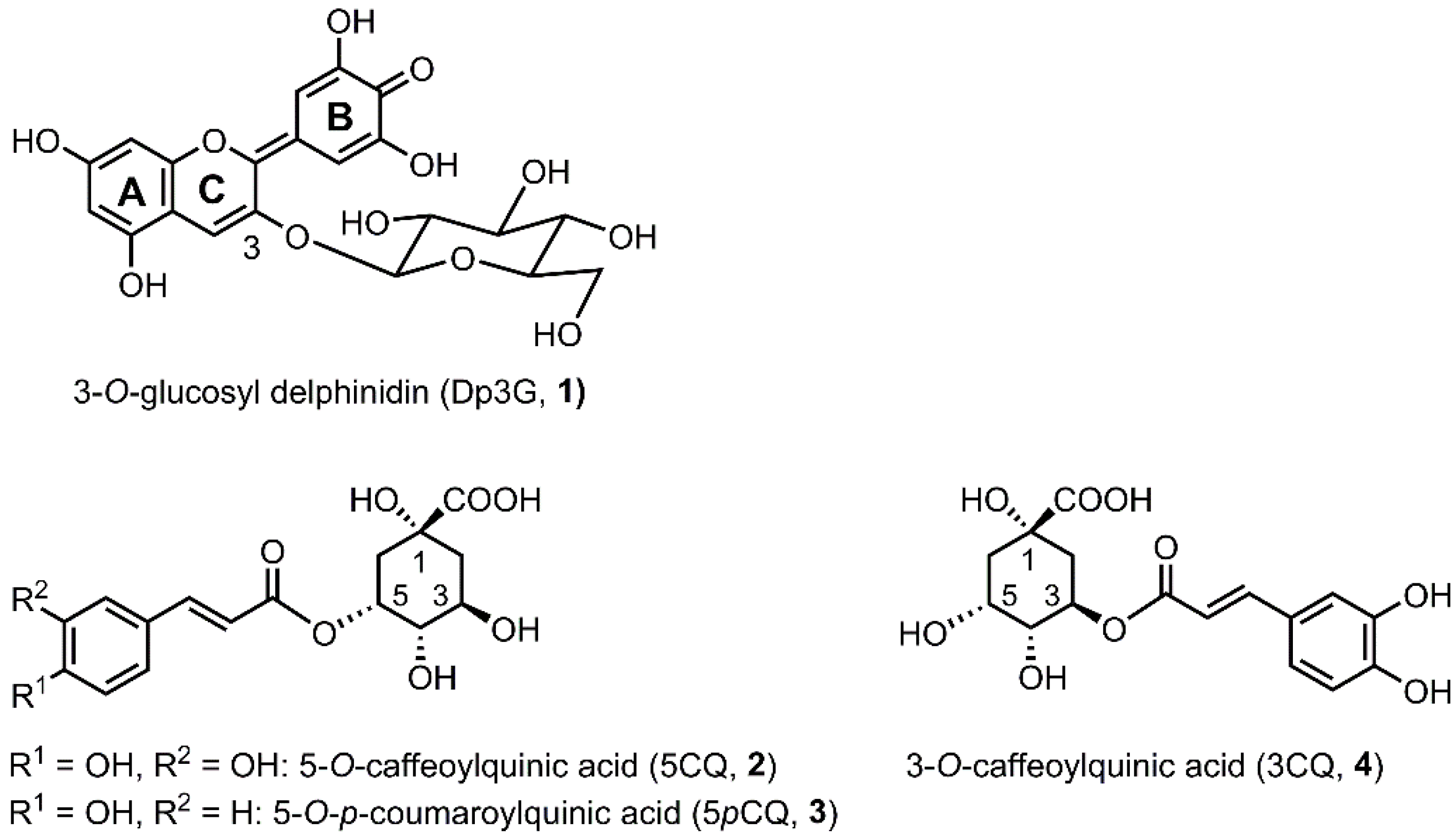
2.3. Chemical Structure of the Hydrangea Blue-Complex
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Cell Sap Preparation
3.4. Reproduction of Hydrangea Blue Color
3.5. Measurement of Vis and CD Spectra
3.6. ESI-TOF-Mass Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, D.; Shinkai, Y.; Kondo, T. Change of color and components in sepals of chameleon hydrangea during maturation and senescence. Phytochemistry 2008, 69, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Chenery, E.M. The Problem of the blue hydrangea. J. R. Hort. Soc. 1937, 62, 604–620. [Google Scholar]
- Allen, R.C. Influence of aluminum on the flower color of Hydrangea macrophylla DC. Boyce Thompson Inst. 1943, 13, 221–242. [Google Scholar]
- Asen, S.; Siegelman, H.W. Effect of aluminum on absorption spectra of the anthocyanin and flavonols from sepals of Hydrangea macrophylla var. Merveille. Proc. Am. Soc. Hort. Sci. 1957, 70, 478–481. [Google Scholar]
- Kinraide, T. Identity of the rhizotoxic aluminium species. Plant Soil 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Lawrence, W.J.C.; Price, J.R.; Robinson, G.M.; Robinson, R. CCXV. A survey of anthocyanins. V. Biochem. J. 1938, 32, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.M.; Robinson, R. The colloid chemistry of leaf and flower pigments and the precursors of the anthocyanins. J. Am. Chem. Soc. 1939, 61, 1606–1607. [Google Scholar] [CrossRef]
- Hayashi, K.; Abe, Y. Studien über anthocyane, XXIII. Papier-chromatographische übersicht der anthocyane im pflanzeneich. I. Misc. Rep. Res. Inst. Nat. Resour. 1958, 29, 1–8. [Google Scholar]
- Asen, S.; Siegelman, H.W.; Stuart, N.W. Anthocyanin and other phenolic compounds in red and blue sepals of Hydrangea Macrophylla var. Merveille. Proc. Am. Soc. Hort. Sci. 1957, 69, 561–569. [Google Scholar]
- Takeda, K.; Yamashita, T.; Takahashi, A.; Timberlake, C.F. Stable blue complexes of anthocyanin-aluminium-3-p-coumaroyl- or 3-caffeoyl-quinic acid involved in the blueing of Hydrangea flower. Phytochemistry 1990, 29, 1089–1091. [Google Scholar] [CrossRef]
- Yoshida, K.; Toyama-Kato, Y.; Kameda, K.; Kondo, T. Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective microelectrode. Plant Cell Physiol. 2003, 44, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Toyama-Kato, Y.; Yoshida, K. Essential structure of co-pigment for blue sepal-color development of hydrangea. Tetrahedron Lett. 2005, 46, 6645–6649. [Google Scholar] [CrossRef]
- Toyama-Kato, Y.; Kondo, T.; Yoshida, K. Synthesis of designed acylquinic acid derivatives involved in blue color development of hydrangea and their co-pigmentation effect. Heterocylces 2007, 72, 239–254. [Google Scholar] [CrossRef]
- Ito, D.; Shinkai, Y.; Kato, Y.; Kondo, T.; Yoshida, K. Chemical studies on different color development in blue- and red-colored sepal cells of Hydrangea macrophylla. Biosci. Biotechnol. Biochem. 2009, 73, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.-I.; Yamada, T.; Ito, D.; Kondo, T.; Yoshida, K. Metal-complex pigment involved in the blue sepal color development of hydrangea. J. Agric. Food. Chem. 2015, 63, 7630–7635. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ueda, M.; Yoshida, K.; Titani, K.; Isobe, M.; Goto, T. Direct observation of a small-molecule associated supramolecular pigment, commelinin, by electrospray ionization mass spectroscopy. J. Am. Chem. Soc. 1994, 116, 7457–7458. [Google Scholar] [CrossRef]
- Kondo, T.; Ueda, M.; Tamura, H.; Yoshida, K.; Isobe, M.; Goto, T. Composition of protocyanin, a self-assembled supramolecular pigment form the blue cornflower Centaurea cyanus. Angew. Chem. Int. Ed. 1994, 33, 978–979. [Google Scholar] [CrossRef]
- Kondo, T.; Oyama, K.; Yoshida, K. Chiral molecular recognition on formation of a metalloanthocyanin: A supramolecular metal complex pigment from blue flower of Salvia patens. Angew. Chem. Int. Ed. 2001, 40, 894–897. [Google Scholar] [CrossRef]
- Mori, M.; Kondo, T.; Yoshida, K. Cyanosalvianin, a supramolecular blue metalloanthocyanin, from petals of Salvia uliginosa. Phytochemistry 2008, 69, 3151–3158. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.-I.; Watanabe, N.; Yamada, T.; Suzuki, M.; Sekiguchi, Y.; Kondo, T.; Yoshida, K. Efficient and versatile synthesis of 5-O-acylquinic acids with a direct esterification using a p-methoxybenzyl quinate as a key intermediate. Tetrahedron 2015, 71, 3120–3130. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |

| Co-Pigment | pH | λmax (Amax) | |||
|---|---|---|---|---|---|
| 0 eq. | 1 eq. | 2 eq. | 3 eq. | ||
| 5CQ (2) | 566 (0.77) | 576 (1.28) | 579 (1.49) | 581 (1.51) | |
| 5pCQ (3) | 4.0 | 578 (1.56) | 580 (1.94) | 582 (1.99) | |
| 3CQ (4) | 569 (0.91) | 569 (0.64) | 569 (0.61) | ||
| 5CQ (2) | 525 (0.55) | 543 (0.67) | 567 (0.74) | 576 (0.96) | |
| 5pCQ (3) | 3.2 | 569 (0.73) | 575 (0.95) | 578 (1.22) | |
| 3CQ (4) | 531 (0.54) | 530 (0.55) | 531 (0.58) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Oyama, K.-i.; Yoshida, K. Direct Observation of Hydrangea Blue-Complex Composed of 3-O-Glucosyldelphinidin, Al3+ and 5-O-Acylquinic Acid by ESI-Mass Spectrometry. Molecules 2018, 23, 1424. https://doi.org/10.3390/molecules23061424
Ito T, Oyama K-i, Yoshida K. Direct Observation of Hydrangea Blue-Complex Composed of 3-O-Glucosyldelphinidin, Al3+ and 5-O-Acylquinic Acid by ESI-Mass Spectrometry. Molecules. 2018; 23(6):1424. https://doi.org/10.3390/molecules23061424
Chicago/Turabian StyleIto, Takaaki, Kin-ichi Oyama, and Kumi Yoshida. 2018. "Direct Observation of Hydrangea Blue-Complex Composed of 3-O-Glucosyldelphinidin, Al3+ and 5-O-Acylquinic Acid by ESI-Mass Spectrometry" Molecules 23, no. 6: 1424. https://doi.org/10.3390/molecules23061424
APA StyleIto, T., Oyama, K.-i., & Yoshida, K. (2018). Direct Observation of Hydrangea Blue-Complex Composed of 3-O-Glucosyldelphinidin, Al3+ and 5-O-Acylquinic Acid by ESI-Mass Spectrometry. Molecules, 23(6), 1424. https://doi.org/10.3390/molecules23061424