2. Results and Discussion
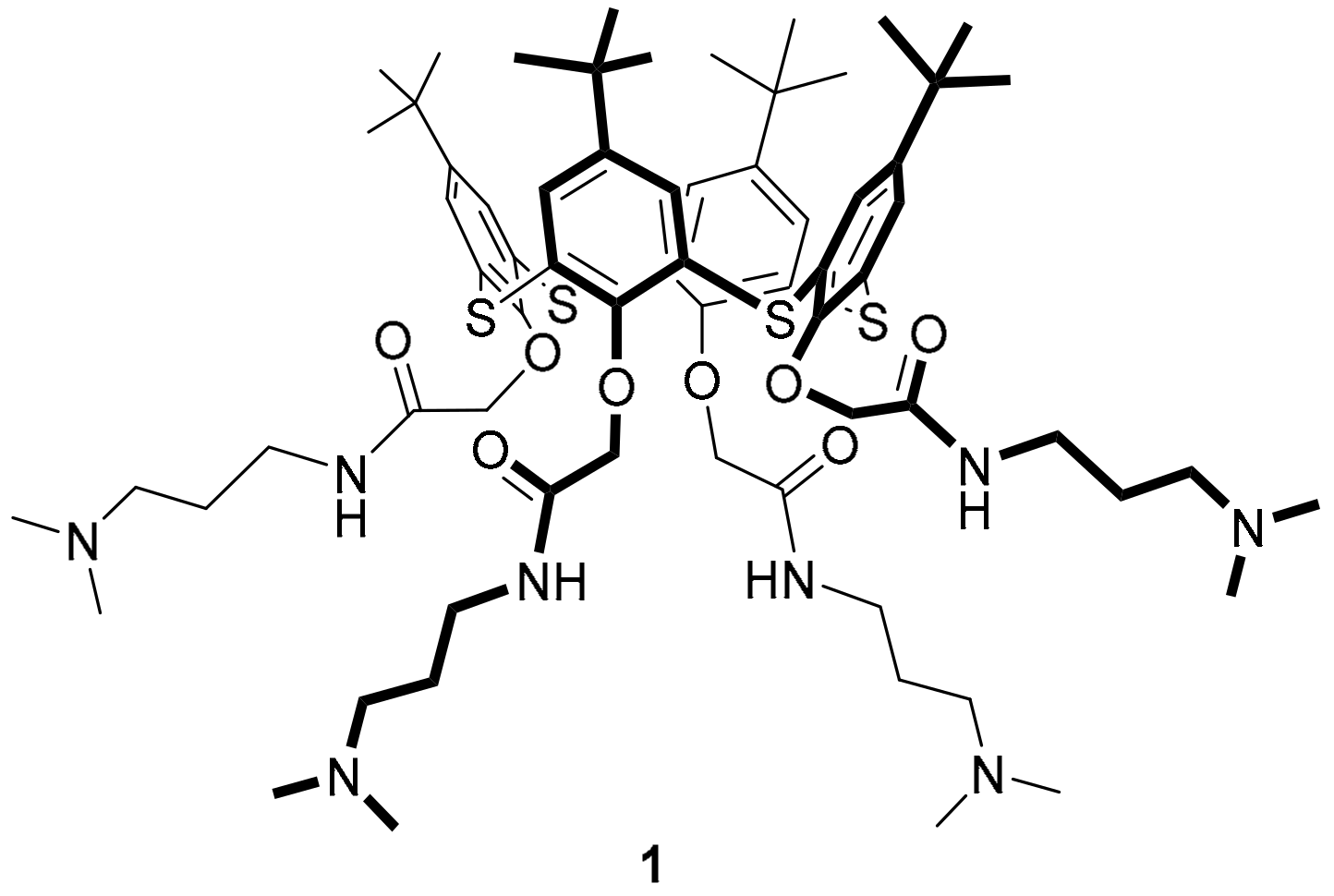
As the core of the multithiacalix[4]arenes, we selected previously synthesized tetrasubstituted
p-tert-butylthiacalix[4]arene
1 in the
cone configuration (
Figure 1). It contains polar tertiary amino groups on one side of the macrocycle and lipophilic
tert-butyl groups on the opposite side [
35]. The choice of this
p-tert-butylthiacalix[4]arene as a core is primarily due to its synthetic availability, as well as the presence of reactive tertiary amine groups on sufficiently long spacers, which exclude the influence of the steric factors on the formation of products of incomplete alkylation. As a peripheral macrocycle, we decided to synthesize a differently substituted
p-tert-butylthiacalix[4]arene in a
1,3-alternate conformation containing phthalimide groups in addition to the bromoacetamide moiety. Phthalimide groups are convenient synthons of amino groups that can be easily converted into the corresponding primary amines in the presence of hydrazine hydrate or under conditions of acid catalysis. In addition, a less steric loading of the reaction center of the
p-tert-butylthiacalix[4]arene derivative in the
1,3-alternate conformation reduces the possibility of the formation of byproducts through partial alkylation of the core.
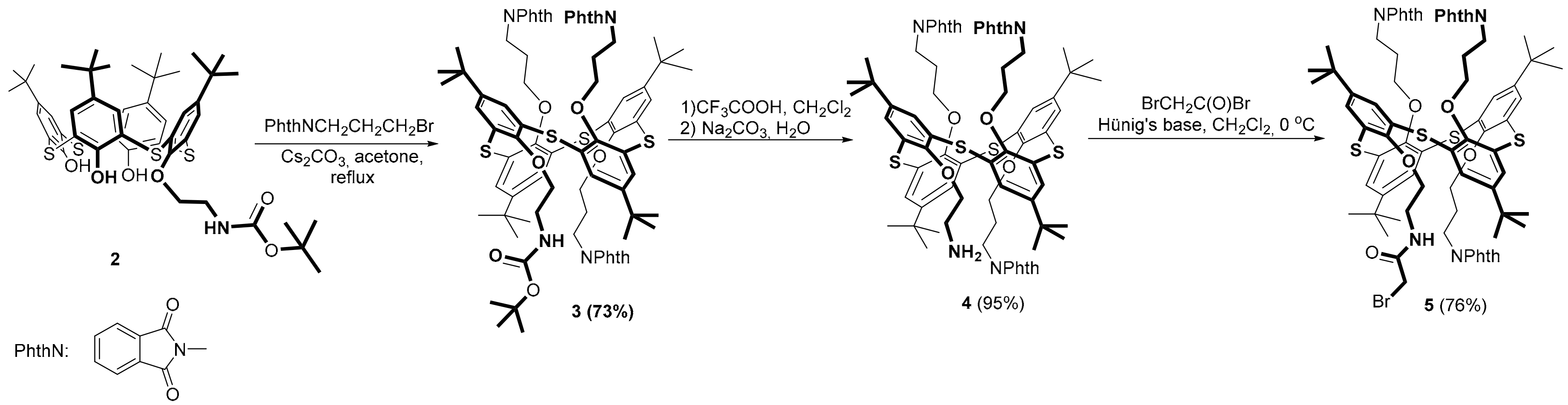
As the initial macrocycle for the synthesis of the precursor of multithiacalix[4]arenes, we chose mono-substituted
p-tert-butylthiacalix[4]arene
2 [
36] containing a
tert-butyloxycarbonyl (BOC) protected aminoethoxy group. The presence of a BOC-protected amino group in macrocycle
2 will yield a differently substituted macrocycle containing phthalimide fragments and an amine group required to introduce a bromoacetamide moiety into the macrocycle structure. To introduce phthalimide fragments into the structure of the thiacalix[4]arene
2, the interaction of macrocycle
2 with
N-(3-bromopropyl)phthalimide in acetone was studied (
Scheme 1). It is known that, depending on the metal carbonate (Na, K, or C), tetrafunctional derivatives of
p-tert-butylthiacalix[4]arenes in the
cone,
partial cone and
1,3-alternate conformations can be obtained [
27]. Therefore, in order to obtain the tetrasubstituted derivative of
p-tert-butylthiacalix[4]arene in the
1,3-alternate configuration, cesium carbonate was chosen as the base in the reaction of macrocycle
2 with
N-(3-bromopropyl)phthalimide. As a result of the reaction, macrocycle
3 was obtained with a yield of 73%. Based on
1H-
1H Nuclear Overhauser Effect Spectroscopy (NOESY) NMR experiment (see
Supporting Materials), macrocycle
3 is in a
1,3-alternate conformation.
Removal of the BOC group from the aminoethoxyl fragment of the thiacalix[4]arene
3 was a further step of our work. The synthesis was carried out in dichloromethane at room temperature in the presence of trifluoroacetic acid. As a result of the reaction, the monoamine
4 was synthesized in a high yield after neutralization with aqueous sodium carbonate. To introduce the bromoacetamide moiety into the structure of macrocycle
4, the interaction of the monoamine
4 with bromoacetyl bromide in the presence of Hünig’s base (
N,
N-diisopropylethylamine) was studied. Macrocycle
5 was obtained with a yield of 76%. Based on the
1H-
1H NOESY NMR spectra (see
Supplementary Materials), macrocycle
5 had a
1,3-alternate conformation. According to thin-layer chromatography (TLC), no other products were formed in the reaction.
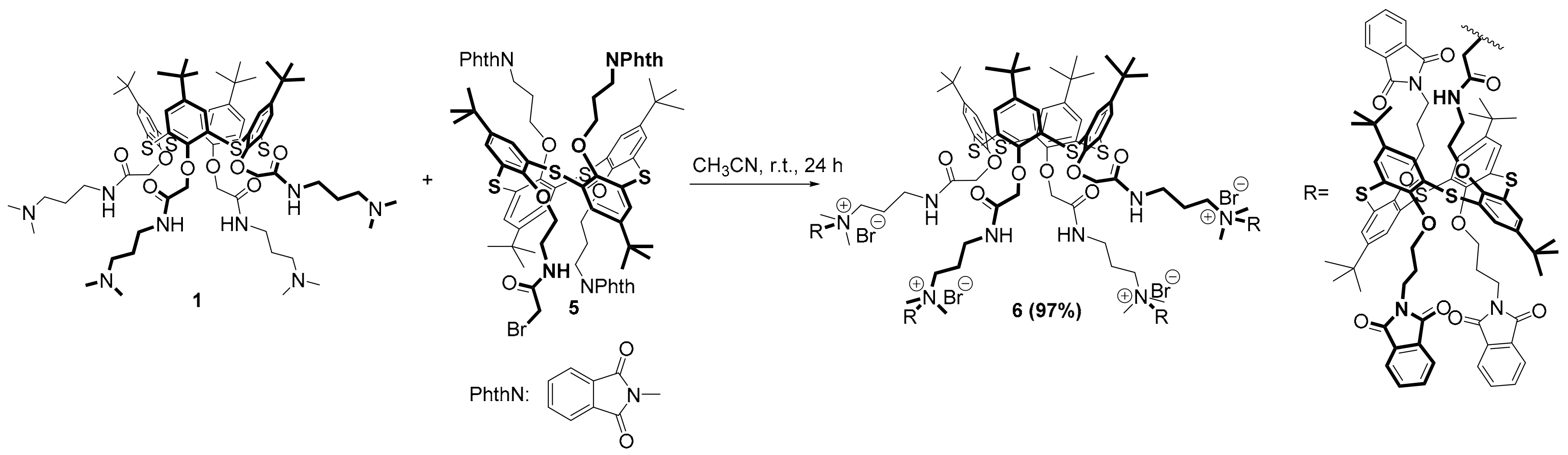
Multithiacalix[4]arene containing phthalimide groups was obtained in the next step of the work. The interaction of macrocycle
1 with the thiacalix[4]arene
5 was carried out over 24 h (
Scheme 2). The synthesis was carried out at room temperature in acetonitrile in order to reduce the possibility of the side reaction of the decomposition of quaternary ammonium derivatives. The advantage of using acetonitrile as a solvent was that the multithiacalix[4]arene
6 was poorly soluble and precipitated during the reaction. For the greatest degree of conversion of the tertiary amino groups, a 20% excess of macrocycle
5 was used against each functional group of the core
1. It should be noted that a complex mixture of alkylation products of tertiary amino groups and of the products of the cleavage of quaternary ammonium salts by the Hoffmann reaction was formed when the reaction temperature was raised to 60 °C.
Multithiacalix[4]arene
6 was synthesized in a high yield, and its structure was characterized by NMR
1Н,
13С, and IR spectroscopy and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry.
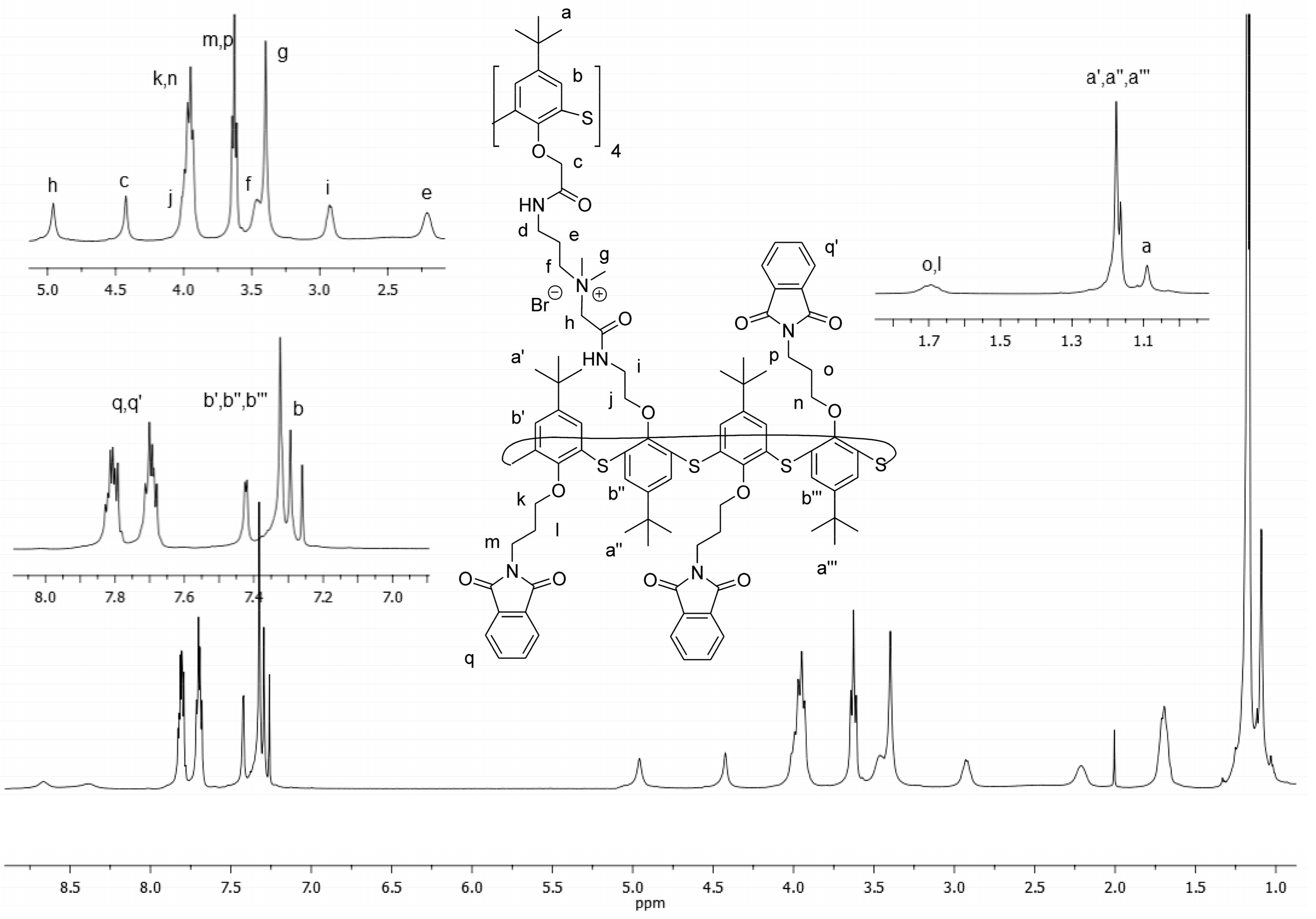
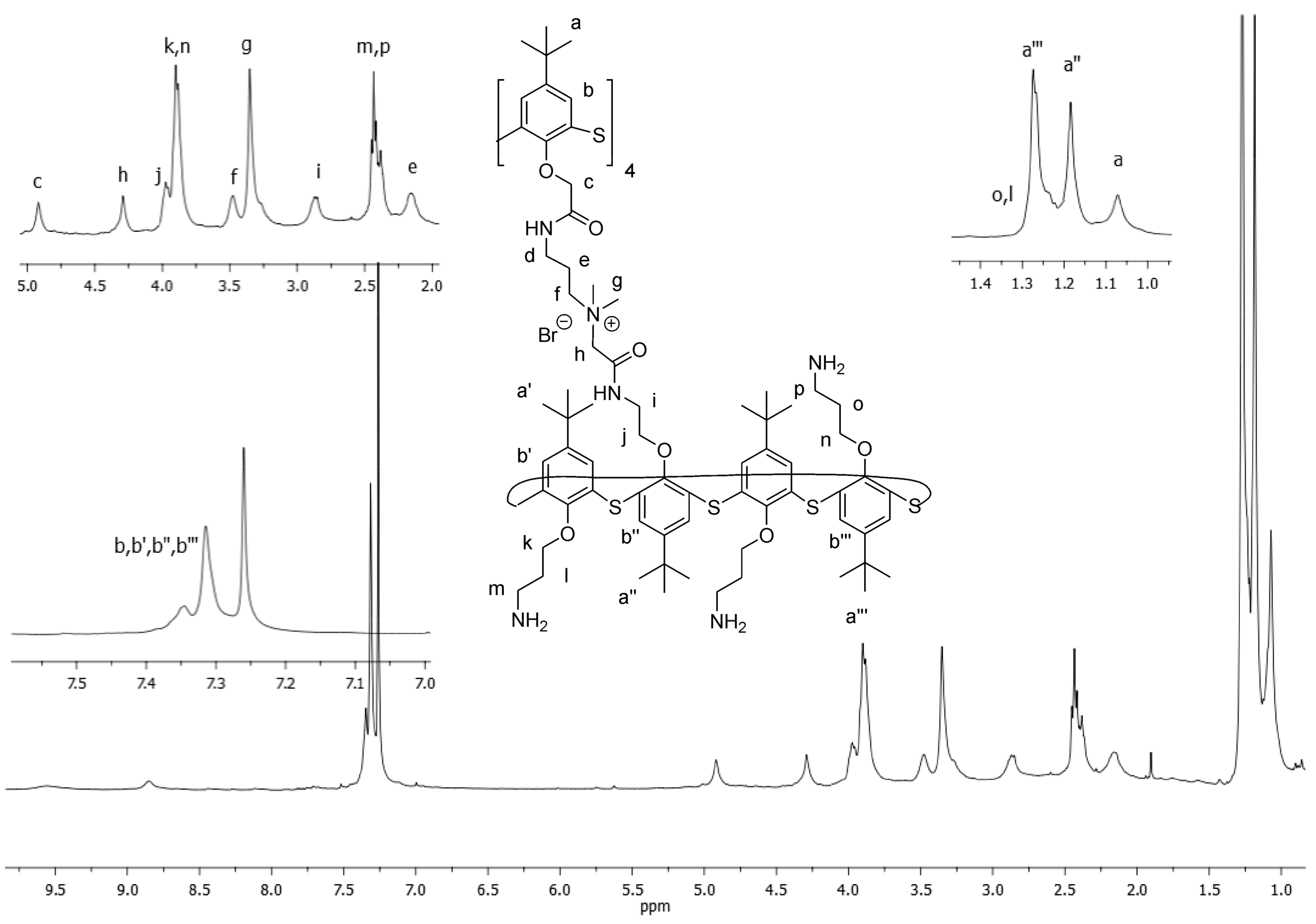
Figure 2 shows the NMR
1H spectrum of the synthesized multimacrocycle
6.
In the
1H NMR spectrum of macrocycle 6, individual assignments of the proton signals were made based on integrated intensity, multiplicity, chemical shifts, and comparison of spectral data of macrocycles
1 and
5 (see
Supplementary Materials). A shift of signals to lower fields of methyl protons
g (CH
3-) 3.39 ppm, oxymethylene protons of the core
c (-O-CH
2-C (O)) 4.44 ppm and peripheral macrocycle
h (-O-CH
2-N
+) 4.97 ppm against those observed in macrocycles
1 and
5 unequivocally indicates that the tertiary amino groups of macrocycle
1 were alkylated. Similar multiplicity of the signals in the
1H NMR spectrum of macrocycle
6 compared with those in macrocycles
1 and
5, indicates the symmetrical structure of the multithiacalix[4]arene
6.
To obtain amino derivatives of the multithiacalix[4]arene, the hydrazinolysis of the multithiacalix[4]arene
6 (
Scheme 3) was studied. It is known that quaternary ammonium derivatives can undergo various transformations in the presence of bases. Thus, removal of the phthalimide groups from multithiacalix[4]arene
6 was carried out at room temperature. Control over the conversion of phthalimide groups was performed by disappearance of the proton signals of the phthalimide groups in the
1H NMR spectra.
Multithiacalix[4]arene
7 was synthesized at a high yield, and its structure was characterized by NMR
1Н,
13С, IR spectroscopy, and MALDI-TOF mass spectrometry.
Figure 3 shows the
1H NMR spectrum of the synthesized macrocycle
7.
The disappearance of signals in the 7.5–8.0 ppm range clearly indicates complete conversion of the phthalimide groups to primary amino groups. In addition, the signals of the methylene protons
l, m and
o, p significantly shifted to strong fields. This can be explained by a change in their environment. In comparison with the chemical shifts of the methylene protons
o,
l (CH
2-CH
2-CH
2-NPhth) 1.69 ppm and
m,
p (CH
2-CH
2-CH
2-NPhth) 3.62 ppm of macrocycle
6, in multimacrocycle
7 the signals of these groups are located in a strong field at 1.28 ppm and 2.45 ppm, respectively. It should also be noted that the absence of the proton signals of the alkene derivatives in the range of 5.5–6.5 ppm, as well as the symmetry of the signals in the
1H NMR spectrum of the multithiacalix[4]arene
7, indicate that no byproducts of the cleavage of the multithiacalix[4]arene structure are formed during the hydrazinolysis. The appearance of a deformation vibration band of the NH bond of the primary amino group in the 1576 cm
−1 region, as well as an increase in the intensity of the broad bands in the 3260–3360 cm
−1 region vs. that in the spectrum of macrocycle
6 (see the
Supplementary Materials), are further confirmation of the formation of amino groups in macrocycle
7. Furthermore, the removal of the phthalimide groups led to a decrease in the absorption band of stretching vibrations of the carbonyl group (C=O)) in the region of 1683 cm
−1.
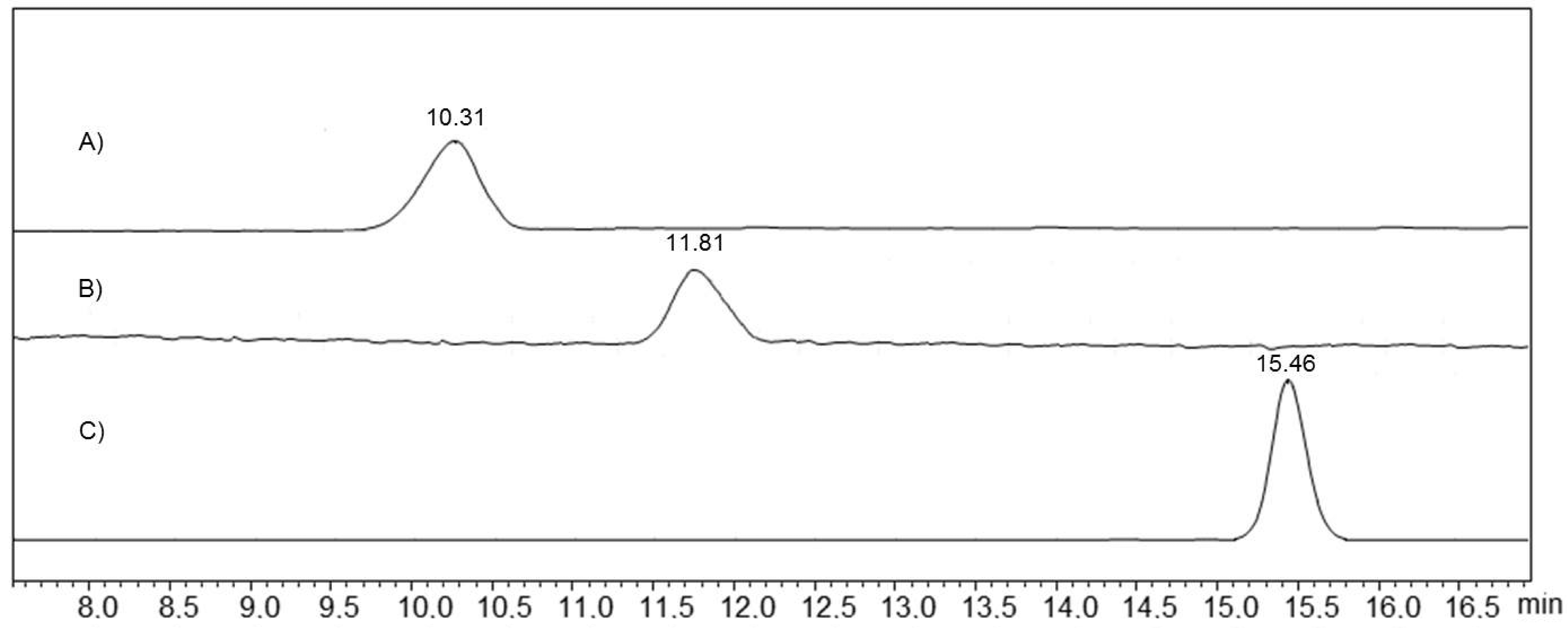
Byproducts of the non-complete alkylation of core
1 in the synthesized multithiacalix[4]arene were estimated by the gel permeation chromatography (
Figure 4). As follows from the data obtained, the synthesized multithiacalix[4]arenes
6 and
7 are represented by one peak at 10.31 min and 11.81 min, respectively, and the retention time of macrocycle
5 is equal to 15.46 min. The absence of extra peaks on the chromatograms of macrocycles
6 and
7 indicates that the multithiacalix[4]arenes obtained are free from the products of non-complete alkylation or cleavage of the quaternary ammonium salts.
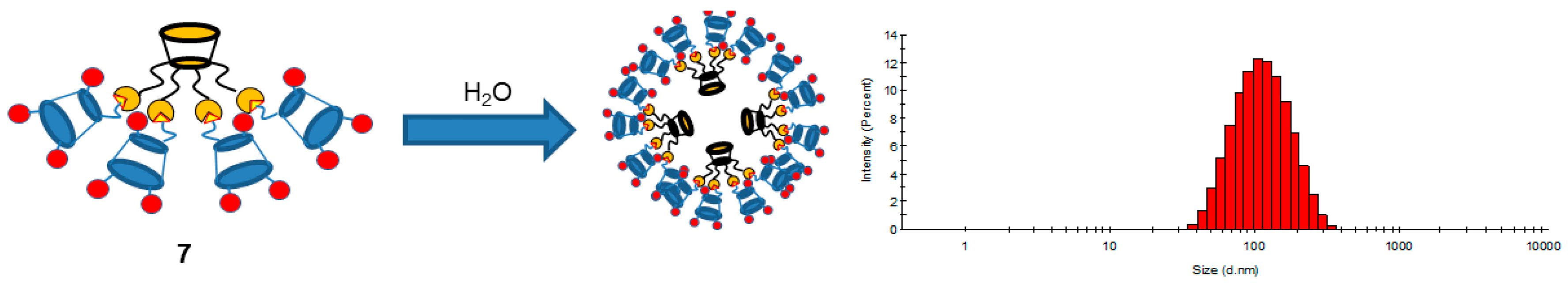
The solubility in water and the possibility of their self-assembly with the formation of various nanosized particles is one of the important aspects of the use of dendrimers for various biomedical purposes. To study the behavior of amino multithiacalix[4]arene
7 in water, dynamic light scattering (DLS) was used. The macrocycle concentration varied in the range from 1 × 10
−3 to 1 × 10
−5 mol/L. DLS experiments showed that macrocycle
7 formed nanosized aggregates in water (108.9 ± 3.2 nm, pdi = 0.266) with a low polydispersity index at a macrocycle concentration of 1 × 10
−5 mol/L (
Figure 5).
With increasing macrocycle concentrations, colloidal systems are formed with a multimodal particle size distribution. Thus, amphiphilic multithiacalix[4]arene 7 with distinct hydrophobic (tert-butyl groups and aryl fragments) and hydrophilic (primary amino groups) parts of the molecule forms in water dendrimer-like nanoparticles by direct supramolecular self-assembly. The obtained results offer new perspectives for the creation in water of the supramolecular nanostructures by directional self-assembly of pre-constructed building blocks. The next stage of our work will involve extension of the synthetic approach to water-soluble amino multithiacalix[4]arene systems with other macrocyclic and acyclic cores and modification of the amino groups of the synthesized multi-macrocycle by various functional groups.
3. Materials and Methods
3.1. General Experimental Information
All reagents and solvents were used directly as purchased or purified according to the standard procedures. Analytical thin-layer chromatography was carried out using commercial silica gel plates and visualization was effected with short wavelength UV light (254 nm). Column chromatography was performed with silica gel 60 H, slurry packed. NMR spectra were recorded at 400 MHz for
1H, and 100 MHz for
13C with CDCl
3 as solvent. Chemical shifts are reported in delta (δ) units in parts per million (ppm) and splitting patterns are designated as s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet and br, broad. Coupling constants are recorded in Hertz (Hz). The structure of the products was determined by a combination of spectroscopic methods such as IR, 1D and 2D NMR NOESY experiment and MALDI-MS. IR spectra were recorded with Spectrum 400 IR spectrometer (Perkin Elmer, Waltham, MA, USA). Absorbance frequencies are expressed in reciprocal centimeters (cm
–1). MALDI spectra were recorded using an Ultraflex III mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) with 4-nitroaniline as a matrix. Peaks of molecular ions are represented by the most abundant mass. Melting points were determined using the Melting Point Apparatus SMP10 (Cole-Parmer Ltd., Stone, Staffordshire, UK). First grade Millipore
® water was prepared from distilled water on Simplicity 185 (Millipore S.A.S., Molsheim, France). GPC analyses were performed over a GPC column (Agilent PLGel 3 µm Mixed-E, 25 mm) using an Agilent 1200 Liquid Chromatograph (Agilent, Omaha, NE, USA) equipped with a refractometer. Macrocycles
1 and
2 were synthesized according to a procedure reported in the literature [
35,
36].
3.2. Synthesis of 5,11,17,23-Tetra-tert-butyl-25,26,27-tri[3′-(N-phthalimido)propoxy]-28-[tert-butyl(2′-aminoethoxy)carbamate]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate) 3
A mixture of 0.53 g (0.58 mmol) of the compound 2 and 1.50 g (4.60 mmol) of Cs2CO3 in 50 mL of acetone was heated under reflux for 30 min, after which 1.23 g (4.59 mmol) of N-(3-bromopropyl)phthalimide was added. The reaction mixture was heated under reflux for 18 h, after which the solvent was removed, and 20 mL of chloroform, 15 mL of distilled water was added. The mixture was stirred at room temperature for 30 min. The organic layer was separated, washed with water (3 × 15 mL), and dried over anhydrous Na2SO4. The solvent was removed at reduced pressure. Excess of N-(3-bromopropyl)phthalimide was removed by boiling the precipitate with hexane. Compound 3 was obtained from column chromatography (trichlormethane-ethanol 60:1).
5,11,17,23-Tetra-tert-butyl-25,26,27-[3′-(N-phthalimido)propoxy]-28-[tert-butyl(2′-aminoethoxy)carbamate]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate) 3 was obtained as a white solid at a yield of 73% (0.61 g). Mp 194–195 °С. IR (KBr/cm−1): 1710 (C(O)-NH), 3440 (-NH); 1H-NMR, CDCl3-d1, δ (ppm): 1.20 (s, 9H, (CH3)3C), 1.22 (s, 27H, (CH3)3C), 1.43 (s, 9H, (CH3)3C-О), 1.77 (m, 6H, CH2-CH2-CH2), 2.98 (m, 2H, CH2-CH2-NH-), 2.98 (m, 2H, CH2-CH2-NH-), 3.62 (t, 2H, J = 7.0 Hz, -CH2-CH2-Phth,), 3.69 (t, 4H, J = 6.7 Hz -CH2-CH2-Phth,), 3.98 (m, 4H, -CH2-CH2-Phth), 4.02 (m, 2H, CH2-CH2-NH-), 5.51 (s, 1H, -NH), 7.36 (br.s, 2H, Ar-H), 7.37 (br.s., 2H, Ar-H), 7.39 (m, 2H, Ar-H), 7.41 (m, 2H, Ar-H), 7.73 (m, 6H, Ar’-Phth), 7.85 (m, 6H, Ar’-Phth). 13C-NMR, δ (ppm): 28.46, 28.79, 28.85, 30.98, 31.15, 31.21, 34.10, 34.14, 34.19, 35.19, 35.24, 67.67, 67.90, 69.91, 123.22, 127.74, 128.12, 128.30, 128.44, 129.49, 129.63, 130.00, 130.05, 132.07, 132.11, 133.88, 145.85, 145.92, 155.88, 157.03, 157.31, 157.50, 168.09, 168.12. MALDI–TOF MS (4-nitroaniline) analysis shows a signal at m/z = 1426.9 corresponding to [М + H]+ (calc. mass for M (C80H88N4O12S4): 1425.8) Elemental analysis for (C80H88N4O12S4). Calculated (%): C, 67.39, H, 6.22, N, 3.93, S, 8.99. Found (%): C, 67.77 %, H, 6.28%, N, 3.88%, S, 9.17%.
3.3. Synthesis of 5,11,17,23-Tetra-tert-butyl-25,26,27-tri[3′-(N-phthalimido)propoxy]-28-[2′-aminoethoxy]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate) 4
In a round bottom flask of 100 mL 0.50 g (0.35 mmol) of macrocycle 3, 1.43 mL (18.8 mmol) of trifluoroacetic acid, 20 mL of dichloromethane and 5 mL of water were added. The reaction mixture was stirred for 24 h at 30 °C. The solvent was evaporated, and the residue was dissolved in 20 mL chloroform. The organic layer was washed with 5% NaHCO3 and water (3 × 20 mL), diluted, and evaporated to dryness.
5,11,17,23-Tetra-tert-butyl-25,26,27-[3′-(N-phthalimido)propoxy]-28-[2′-aminoethoxy]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate) 4 was obtained as a white solid at a yield of 95% (0.44 g). Mp 125–126 °С. IR (KBr/cm−1): 1707 (C(O)-NH); 1H-NMR, CDCl3-d1, δ (ppm): 1.21 (s, 9H, (CH3)3C), 1.23 (s, 27H, (CH3)3C), 1.61 (m, 4H, CH2-CH2-CH2), 1.7 1 (m, 2H, CH2-CH2-CH2), 2.39 (m, 2H, CH2-CH2-NH-), 3.62 (m, 6H, -CH2-CH2-Phth), 3.89 (m, 2H, CH2-CH2-NH-), 3.91-3.97 (m, 6H, -CH2-CH2- CH2-Phth), 7.31 (d, 2H, J = 2.4 Hz, Ar-H), 7.34 (d, 2H, J = 2.4 Hz, Ar-H), 7.34 (s., 2H, Ar-H), 7.37 (s, 2H, Ar-H), 7.68–7.84 (m, 12H, Ar’-Phth). 13C-NMR, δ (ppm): 168.78, 168.11, 157.14, 156.65, 154.41, 147.59, 146.97, 146.71, 134.46, 133.96, 133.83, 132.05, 131.53, 129.77, 129.70, 128.54, 128.34, 127.88, 127.79, 127.51, 126.67, 123.67, 123.27, 68.46, 67.39, 63.86, 39.69, 36.51, 35.13, 34.37, 34.26, 31.14, 31.03, 29.70, 29.07, 28.61, 28.23. MALDI–TOF MS (4-nitroaniline) analysis shows a signal at m/z = 1325.4 corresponding to [М + H]+ (calc. mass for M (C75H80N4O10S4): 1324.5) Elemental analysis for (C75H80N4O10S4). Calculated (%): C, 67.95, H, 6.08, N, 4.23, S, 9.67. Found (%): C, 68.14%, H, 6.34%, N, 4.57%, S, 10.15%.
3.4. Synthesis of 5,11,17,23-Tetra-tert-butyl-25,26,27-tri[3′-(N-phthalimido)propoxy]-28-[2′-bromoacetamidethoxy]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate) 5
In a round bottom flask of 100 mL equipped with a dropping funnel and ace bath, 0.50 g (0.38 mmol) of compound 4 and 0.26 mL (6.2 mmol) of N,N-diisopropylethylamine in 50 mL of dry dichloromethane were added. A solution of bromoacetyl bromide 0.083 g (0.42 mmol) in 10 mL of dry dichloromethane was placed in the dropping funnel and added to the reaction mixture. After dropping of bromoacetyl bromide solution, the reaction mixture was stirred for 2 h at room temperature, and water was added. The organic layer was separated and washed three times with 15 mL of water. Dichloromethane was evaporated and macrocycle 5 was obtained with column chromatography (eluent dichloromethane:methanol 100:1).
5,11,17,23-Tetra-tert-butyl-25,26,27-[3′-(N-phthalimido)propoxy]-28-[2′-bromoacetamidethoxy]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate) 5 was obtained as a slightly yellow solid at a yield of 76% (0.41 g). M.p. 180–181 °С. IR (KBr/cm−1): 1708 (C(O)-NH), 3384 (-NH); 1H-NMR, CDCl3-d1, δ (ppm): 1.16 (s, 18H, (CH3)3C), 1.19 (s, 9H, (CH3)3C), 1.20 (s, 9H, (CH3)3C), 1.26 (s, 9H, (CH3)3C), 1.75 (m, 6H, CH2 -CH2-CH2), 3.20 (q, 2H, J = 5.6 Hz, CH2-CH2-NH-), 3.54 (t, 2H, J = 7.3 Hz -CH2-CH2-Phth), 3.67 (t, 4H, J = 6.7 Hz,-CH2-CH2-Phth,), 3.79 (s, 2H, -CH2-NH-), 3.97 (m, 2H, O-CH2-CH2-CH2), 3.99 (m, 4H, O-CH2-CH2-CH2) 4.07 (m, 2H, CH2-CH2-NH-), 7.33 (d, 2H, J = 2.4 Hz, AB system Ar-H), 7.37 (br.s, 4H, Ar-H), 7.38 (br.s, 2H, Ar-H), 7.45 (br.t, 1H, NH), 7.68-7.78 (m, 12H, Ar’-H). 13C-NMR, δ (ppm): 188.13, 173.25, 168.14, 168.07, 165.60, 157.47, 157.38, 156.67, 145.93, 145.89, 145.63, 133.95, 133.91, 132.09, 132.02, 129.95, 129.79, 129.57, 129.38, 128.50, 128.23, 127.92, 127.35, 123.24, 69.20, 67.91, 67.72, 40.10, 35.54, 35.17, 34.46, 34.18, 32.24, 32.19, 31.13, 30.18, 29.72, 29.01, 28.88, 28.84. MALDI–TOF MS (4-nitroaniline) analysis shows a signal at m/z = 1469.7 corresponding to [М + Na]+ and m/z(av.) = 1485.7 corresponding to [М + K]+ (calc. mass for M(av.) (C77H81BrN4O11S4): 1446.6) Elemental analysis for (C77H81BrN4O11S4). Calculated (%): C, 63.93; H, 5.64; N, 3.87; S, 8.86. Found (%):C, 64.08, H, 5.71, N, 3.98, S, 9.01.
3.5. Synthesis of Multithiacalix[4]arene 6
In a round-bottomed flask, 0.50 g (0.35 mmol) of macrocycle 1, 0.11 g (0.088 mmol) of macrocycle 5 and 40 mL of acetonitrile were added. The reaction mixture was stirred for 24 h at 25 °C. The precipitate was filtered off and washed with acetonitrile. The residue was dried in a vacuum desiccator.
Multithiacalix[4]arene 6 was obtained as a slightly yellow solid at a yield of 97% (0.60 g). M.p. 154–155 °С. IR (KBr/cm−1): 1709 (C(O)-NH), 3204 (-NH); 1H-NMR, CDCl3-d1, δ (ppm): 1.09 (s, 36H, (CH3)3C), 1.16 (s, 36H, (CH3)3C), 1.18 (s, 108H, (CH3)3C), 1.69 (m, 24H, CH2-CH2-CH2), 3.39 (br.s., 24H, CH3-N-), 3.46 (m, 8H, -NH-CH2-CH2-CH2-N+), 3.62(m, 24H, -CH2-CH2-Phth,), 3.94 (m, 24H, O-CH2-CH2-CH2-Phth-), 3.97 (m, 8H, NH-CH2-CH2-CH2-N+), 3.99 (m, 8H, O-CH2-CH2-NH-), 4.42 (s, 8H, O-CH2-C(O)-NH-), 4.95 (s, 8H, -NH-CH2-N+), 7.29 (br.s., 8H, Ar-H core), 7.27–7.48 (m, 32H, Ar-H), 7.64–7.89 (m, 48H, Ar-H Phth), 8.37 (br.s, 4H, NH), 8.67 (br.s, 4H, NH). 13C-NMR, δ (ppm): 169.12, 168.26, 168.21, 162.81, 157.36, 156.34, 147.83, 146.22, 146.06, 145.90, 135.00, 134.15, 133.98, 132.21, 132.14, 129.32, 129.17, 128.64, 128.43, 128.40, 128.31, 128.20, 123.37, 123.32, 74.50, 67.68, 65.39, 64.87, 62.96, 51.88, 38.79, 36.38, 35.38, 35.26, 34.43, 34.38, 34.29, 34.25, 31.42, 31.42, 31.26, 31.26, 31.22, 31.22, 28.95, 28.91, 23.25. MALDI–TOF MS (4-nitroaniline) analysis shows a signal at m/z = 7074.2 corresponding to [М + H]+ (calc. mass for M(C376H428Br4N24O52S20): 7073.3) Elemental analysis for (C376H428Br4N24O52S20). Calculated (%): C, 63.75; H, 6.20; N, 4.75; S, 9.05. Found (%): C, 63.98, H, 6.35, N, 4.91, S, 9.17.
3.6. Synthesis of Multithiacalix[4]arene 7
In a 100-mL round-bottomed flask, 0.50 g (0.071 mmol) of multithiacalix[4]arene 6, 0.69 mL (14.2 mmol) of hydrazine hydrate, 20 mL of ethanol and 20 mL of THF were added. The reaction mixture was stirred for 24 h at 25 °C. The solvent was evaporated, and the residue was dissolved in 20 mL chloroform. The organic layer was washed with water (3 × 20 mL), diluted, and evaporated to dryness.
Multithiacalix[4]arene 7 was obtained as a slightly yellow solid at a yield of 71% (0.28 g). M.p. 141–142 °С. IR (KBr/cm−1): 1683, 1709 (C(O)-NH), 3350 (-NH); 1H-NMR, CDCl3-d1, δ (ppm): 1.07 (s, 36H, (CH3)3C), 1.18 (s, 76H, (CH3)3C), 1.18–1.27 (m, 24H, O-CH2-CH2-CH2-Phth-), 1.27 (s, 76H, (CH3)3C), 2.17 (m, 8H, -NH-CH2-CH2-CH2-N+), 2.38–2.43 (m, 24H, -O-CH2-CH2-CH2-Phth), 2.87 (m, 8H, -O-CH2-CH2-NH), 3.35 (br.s., 24H, -N+-(CH3)2), 3.43 (br.s., 8H, NH-CH2-CH2-CH2-N+), 3.90 (m, 24H, -CH2-CH2-Phth), 3.97 (m, 8H, O-CH2-CH2-NH-), 4.29 (br.s, 8H, O-CH2-C(O)-NH-), 4.92 (br.s, 8H, -NH-CH2-N+), 7.31 (m., 32H, Ar-H), 7.35 (br.s., 8H, Ar-H core), 8.84 (br.s, 4H, NH), 9.57 (br.s, 4H, NH). 13C-NMR, δ (ppm): 157.36, 146.43, 146.10, 128.80, 128.57, 128.50, 128.31, 128.02, 127.43, 127.24, 67.65, 51.43, 51.18, 38.81, 38.61, 34.35, 32.74, 32.10, 31.26, 29.71, 23.18. MALDI–TOF MS (4-nitroaniline) analysis shows a signal at m/z = 5514.2 corresponding to [М + H]+ (calc. mass for M (C280H404Br4N24O28S20): 5512.3) Elemental analysis for (C280H404Br4N24O28S20). Calculated (%):C, 60.89; H, 7.52; N, 6.09; S, 11.61 Found (%): C, 61.12; H, 7.64; N, 6.17; S, 11.76.