Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production
Abstract
1. Introduction
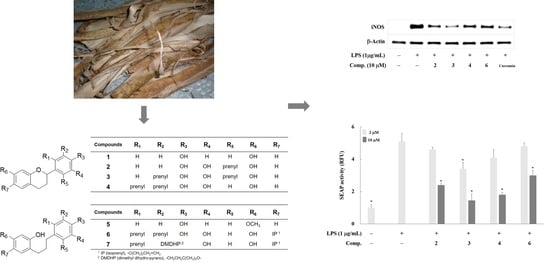
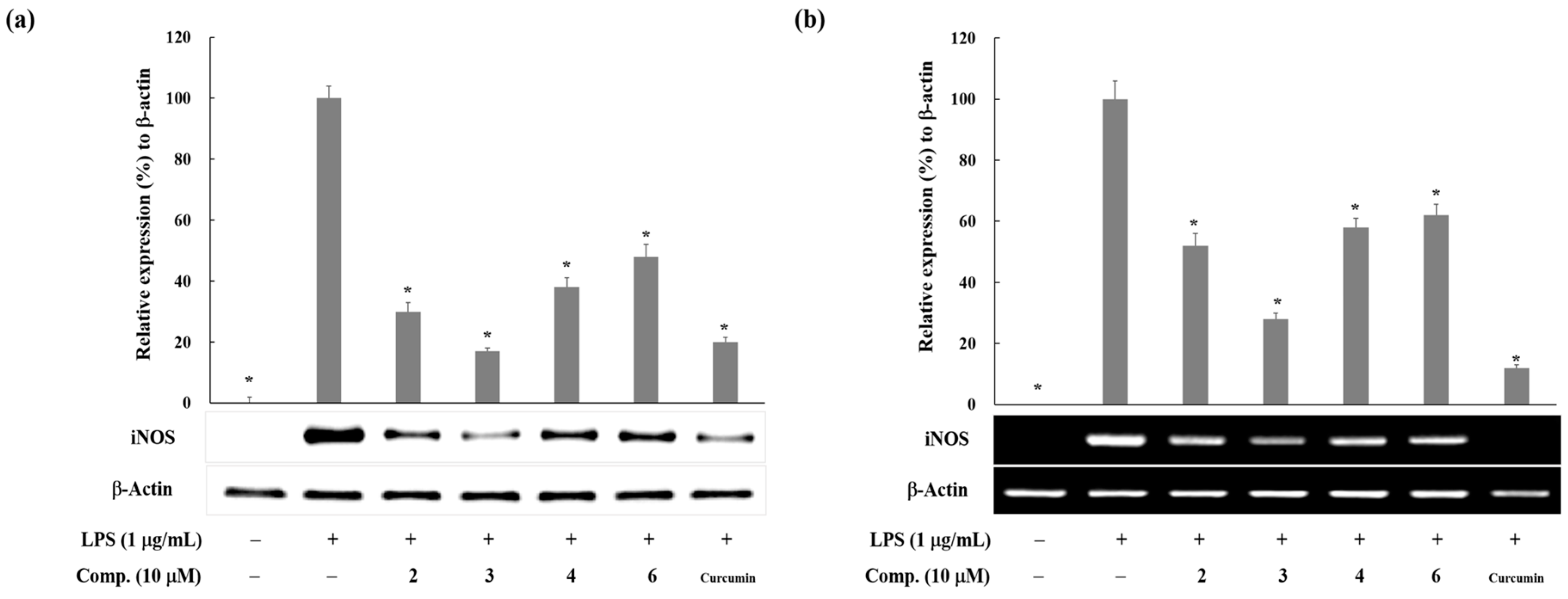
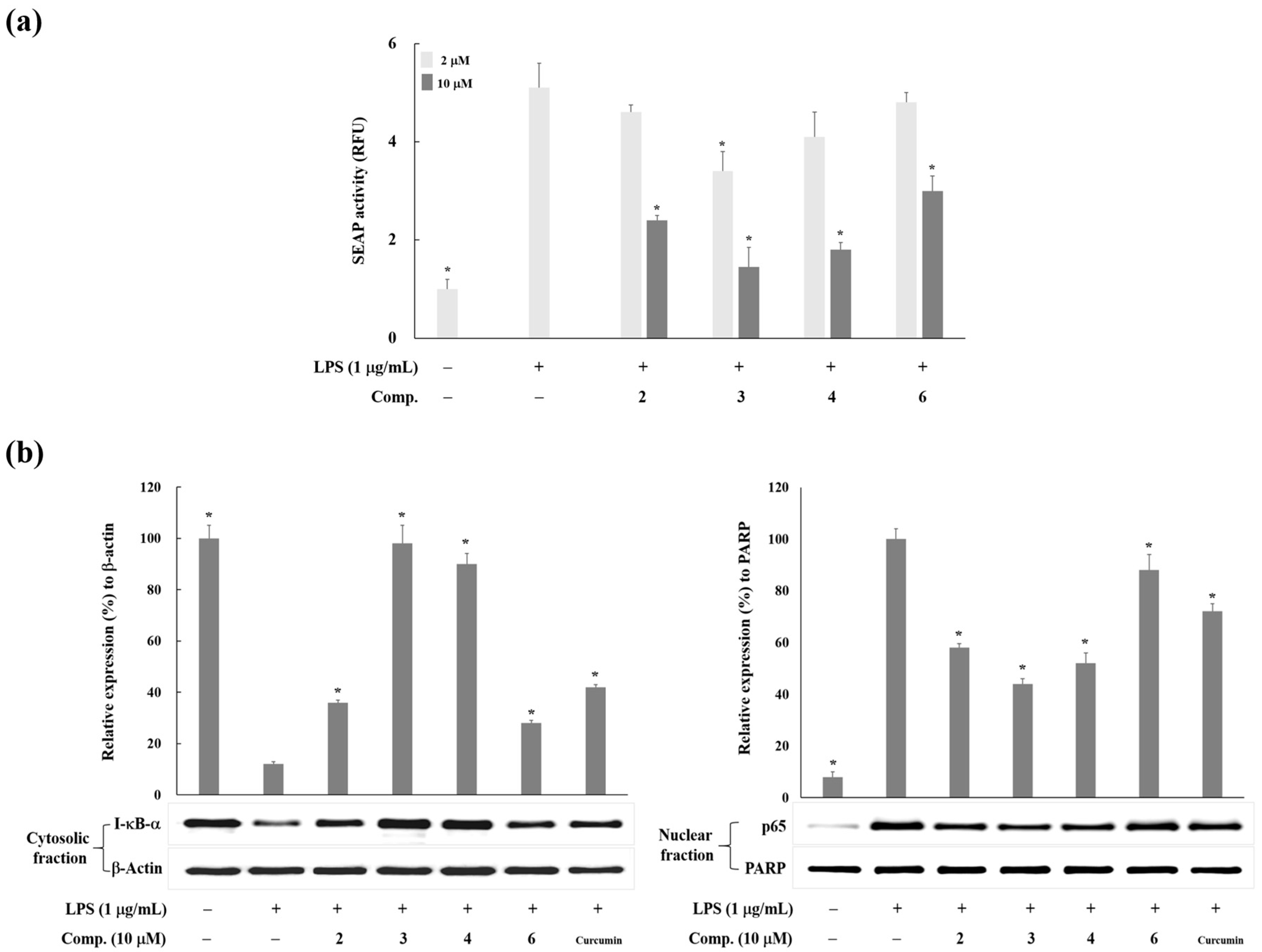
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material, Extraction, Isolation, and Sample Preparation
3.2. Cell Culture
3.3. Measurement of Nitric Oxide Production and Cell Viability
3.4. Western Blot Analysis
3.5. Reverse Transcription and Polymerase Chain Reaction (RT-PCR) Analysis
3.6. Measurement of NF-κB Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.K.; Ha, H.; Lee, H.Y.; Park, S.J.; Jeong, S.L.; Choi, Y.J.; Shin, H.K. Inhibitory effects of heartwood extracts of Broussonetia kazinoki Sieb on the development of atopic dermatitis in NC/Nga mice. Biosci. Biotechnol. Biochem. 2010, 74, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lim, J.; Lee, D.Y.; Ryu, J.H.; Lim, J.S. Kazinol C from Broussonetia kazinoki activates AMP-activated protein kinase to induce antitumorigenic effects in HT-29 colon cancer cells. Oncol. Rep. 2015, 33, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ryu, J.H.; Ahn, H.; Lee, H.J. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia 2003, 74, 350–354. [Google Scholar] [CrossRef]
- Baek, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Ha, T.J.; Rengasamy, R.; Yang, M.S.; Park, K.H. Tyrosinase inhibitory effects of 1,3-diphenylpropanes from Broussonetia kazinoki. Bioorg. Med. Chem. 2009, 17, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, D.H.; Lee, H.J.; Lee, Y.; Ryu, K.H.; Jung, B.I.; Song, Y.S.; Ryu, J.H. New estrogenic compounds isolated from Broussonetia kazinoki. Bioorg. Med. Chem. Lett. 2010, 20, 3764–3767. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Lee, C.G.; Lee, D.Y.; Li, H.; Jeon, R.; Ryu, J.H.; Kim, S.G. Enhanced antioxidant effect of prenylated polyphenols as Fyn inhibitor. Free Radic. Biol. Med. 2012, 53, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Han, S.; Hua, L.; Ahn, Y.H.; Cho, H.; Lee, C.J.; Lee, H.; Cho, Y.Y.; Ryu, J.H.; Jeon, R.; et al. Kazinol-E is a specific inhibitor of ERK that suppresses the enrichment of a breast cancer stem-like cell population. Biochem. Biophys. Res. Commun. 2016, 470, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Li, H.; Jeong, J.H.; Noh, M.; Ryu, J.H. Kazinol B from Broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. Fitoterapia 2016, 112, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, S.J.; Yoo, M.; Go, G.Y.; Lee, D.Y.; Kim, Y.K.; Seo, D.W.; Kang, J.S.; Ryu, J.H.; Bae, G.U. Kazinol-P from Broussonetia kazinoki enhances skeletal muscle differentiation via p38MAPK and MyoD. Biochem. Biophys. Res. Commun. 2015, 456, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Porasuphatana, S.; Tsai, P.; Rosen, G.M. The generation of free radicals by nitric oxide synthase. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 134, 281–289. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Dusting, G.J. Nitric oxide in coronary artery disease: Roles in atherosclerosis, myocardial reperfusion and heart failure. EXS 1996, 76, 33–55. [Google Scholar] [PubMed]
- Prado, C.M.; Yano, L.; Rocha, G.; Starling, C.M.; Capelozzi, V.L.; Leick-Maldonado, E.A.; Martins Mde, A.; Tiberio, I.F. Effects of inducible nitric oxide synthase inhibition in bronchial vascular remodeling-induced by chronic allergic pulmonary inflammation. Exp. Lung Res. 2011, 37, 259–268. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.L.; Kwon, G.; Hill, J.R.; Marshall, C.A.; Corbett, J.A. Cytokines and nitric oxide in islet inflammation and diabetes. Proc. Soc. Exp. Biol. Med. 1996, 211, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Storch-Hagenlocher, B.; Biessmann, A.; Wildemann, B. Inducible nitric oxide synthase and argininosuccinate synthetase: Co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with beta-amyloid 1–42 in vitro. Neurosci. Lett. 2002, 322, 121–125. [Google Scholar] [CrossRef]
- Puglisi, M.A.; Cenciarelli, C.; Tesori, V.; Cappellari, M.; Martini, M.; Di Francesco, A.M.; Giorda, E.; Carsetti, R.; Ricci-Vitiani, L.; Gasbarrini, A. High nitric oxide production, secondary to inducible nitric oxide synthase expression, is essential for regulation of the tumour-initiating properties of colon cancer stem cells. J. Pathol. 2015, 236, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.B.; Chang, F.R.; Wei, L.M.; Wu, Y.C. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis and their cytotoxicity. J. Nat. Prod. 2003, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, J.; Hano, Y.; Nomura, T. Compounds of Broussonetia papyrifera (L.) Vent. 2. Structures of two isoprenylated flavans, Kazinol A and B. Heterocycles 1985, 23, 2835–2842. [Google Scholar]
- Ikuta, J.; Hano, Y.; Nomura, T.; Kawakami, Y.; Sato, T. Compounds of Broussonetia kazinoki SIEB. I. Structures of two new isoprenylated flavans and five new isoprenylated 1,3-diphenylpropane derivatives. Chem. Pharm. Bull. 1986, 34, 1968–1979. [Google Scholar]
- Bae, G.; Yu, J.R.; Lee, J.; Chang, J.; Seo, E.K. Identification of nyasol and structurally related compounds as the active principles from Anemarrhena asphodeloides against respiratory syncytial virus (RSV). Chem. Biodivers. 2007, 4, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.H. Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.Y.; Ahn, K.S.; Lee, J.; Kim, Y.S. Kojic acid, a potential inhibitor of NF-kappaB activation in transfectant human HaCaT and SCC-13 cells. Arch. Pharm. Res. 2001, 24, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Ramos, M.J. NF-kappaB in human disease: Current inhibitors and prospects for de novo structure based design of inhibitors. Curr. Med. Chem. 2005, 12, 357–374. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
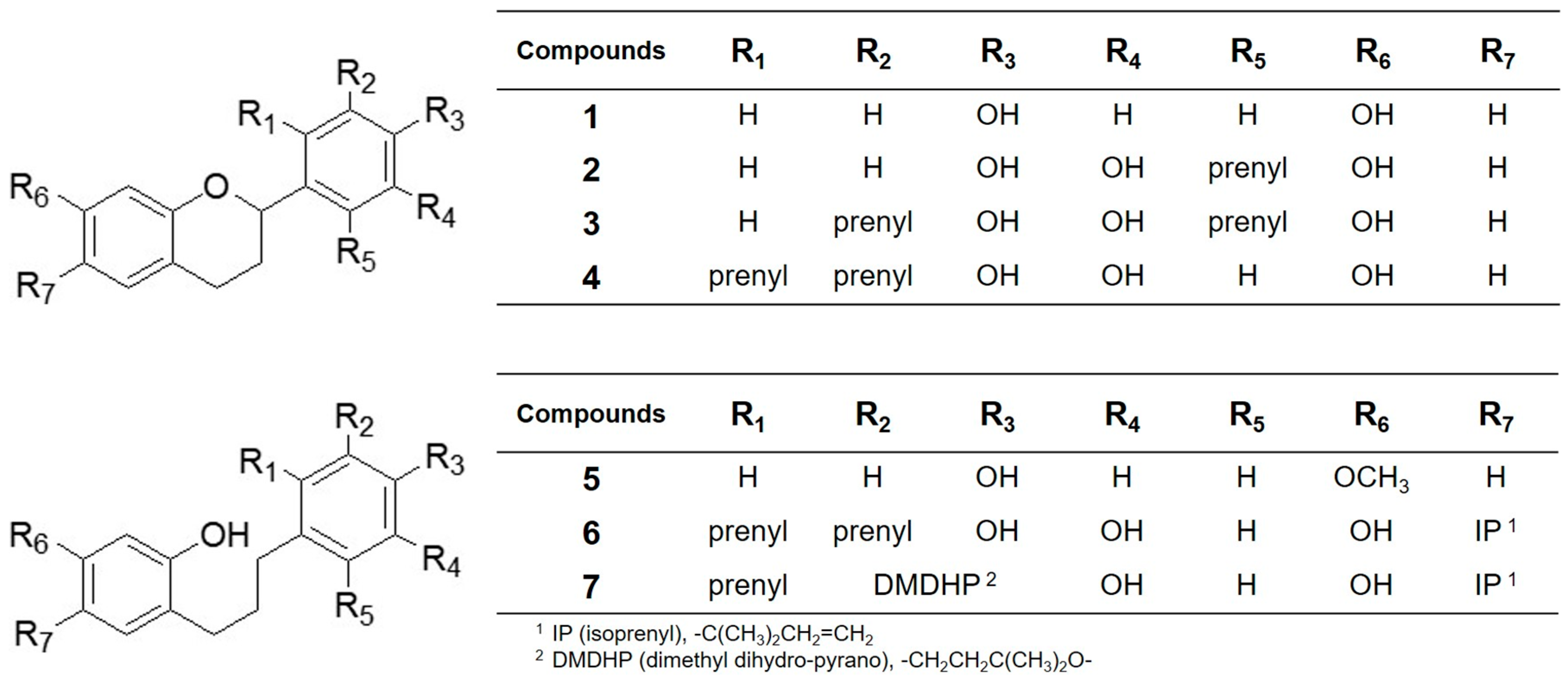
| Compounds | IC50 (µM) 1 |
|---|---|
| 1 | >20 |
| 2 | 5.2 ± 0.2 |
| 3 | 4.2 ± 0.2 |
| 4 | 5.3 ± 0.4 |
| 5 | 16.9 ± 1.1 |
| 6 | 5.0 ± 0.4 |
| 7 | 8.0 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.Y.; Lee, H.J.; Ryu, J.-H. Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production. Molecules 2018, 23, 639. https://doi.org/10.3390/molecules23030639
Lee DY, Lee HJ, Ryu J-H. Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production. Molecules. 2018; 23(3):639. https://doi.org/10.3390/molecules23030639
Chicago/Turabian StyleLee, Da Yeon, Hwa Jin Lee, and Jae-Ha Ryu. 2018. "Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production" Molecules 23, no. 3: 639. https://doi.org/10.3390/molecules23030639
APA StyleLee, D. Y., Lee, H. J., & Ryu, J.-H. (2018). Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production. Molecules, 23(3), 639. https://doi.org/10.3390/molecules23030639