Exogenous Melatonin Mitigates Acid Rain Stress to Tomato Plants through Modulation of Leaf Ultrastructure, Photosynthesis and Antioxidant Potential
Abstract
:1. Introduction
2. Results
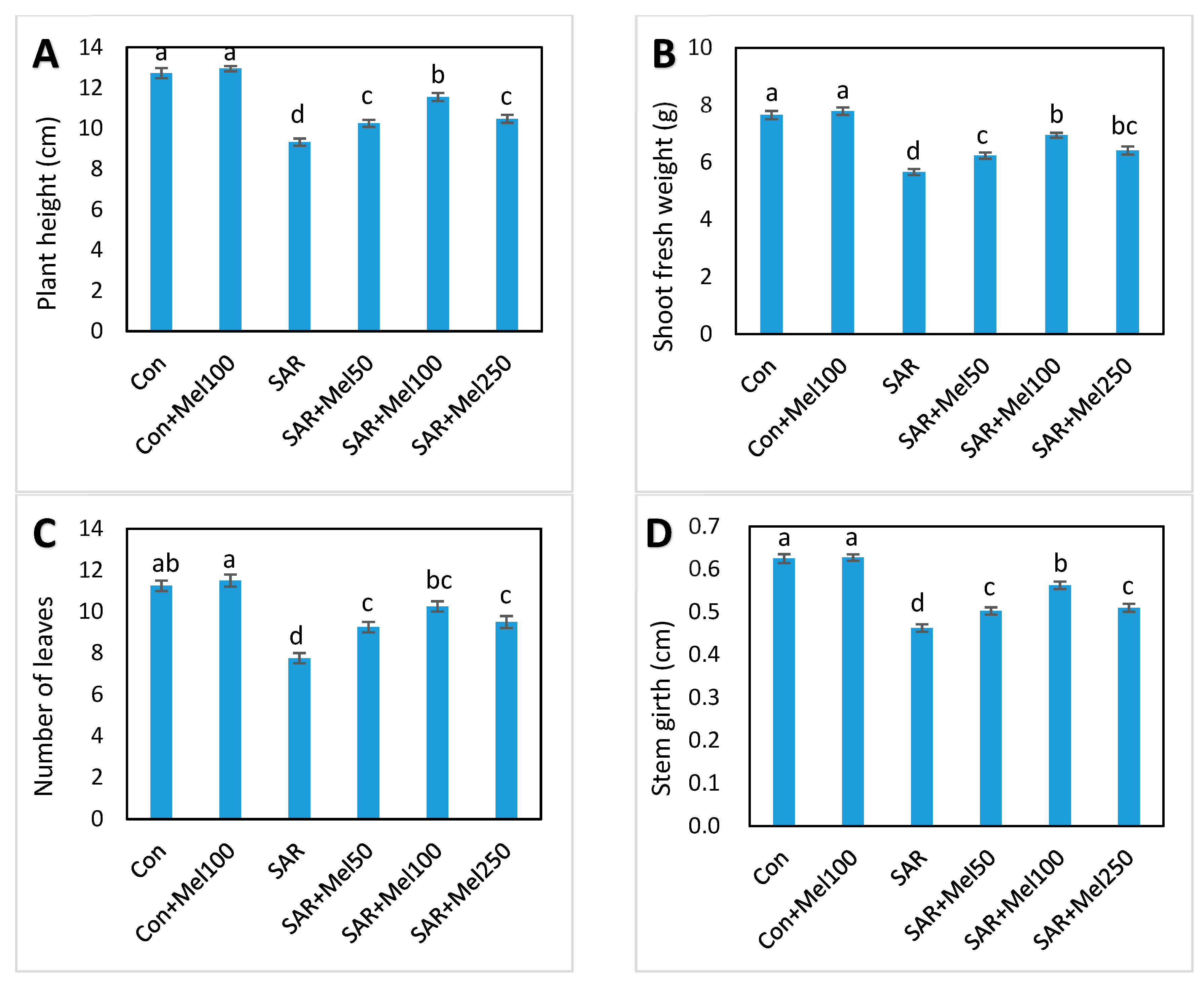
2.1. Effects of Melatonin on Growth of Tomato Plant under SAR Stress
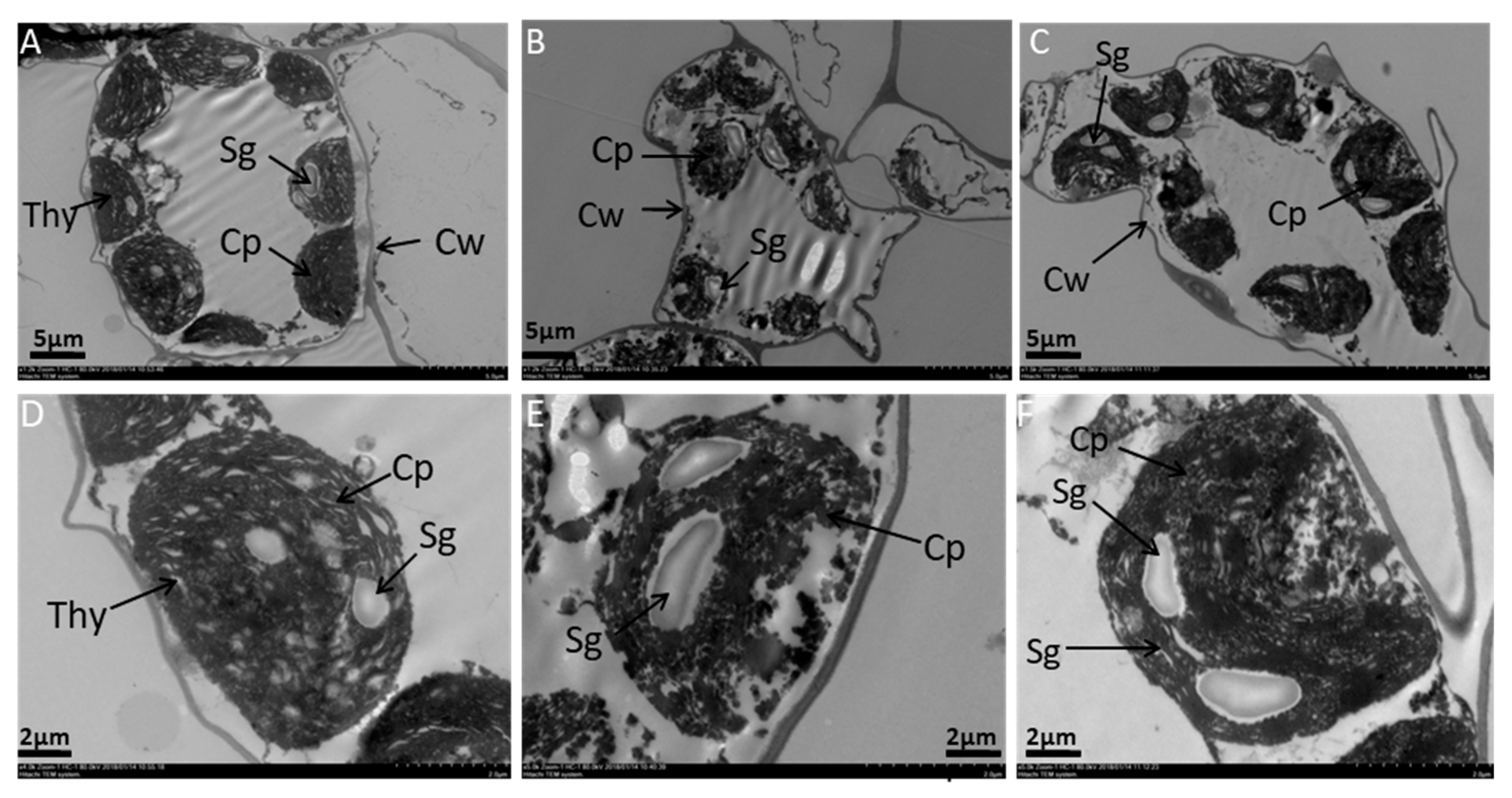
2.2. Effects of Melatonin on Ultrastructure Alterations in Mesophyll Cells and Chloroplast of Tomato Leaf under SAR Stress
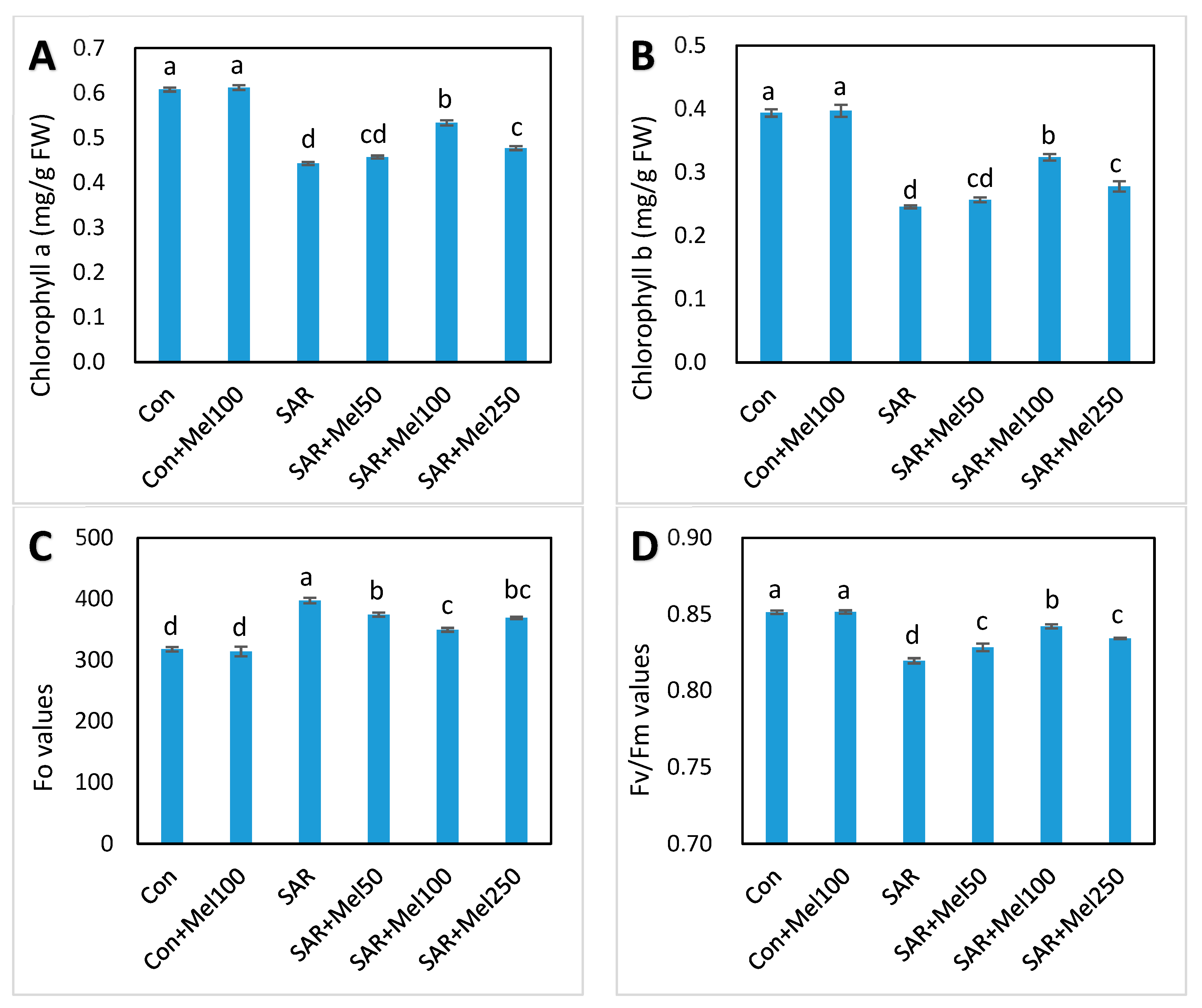
2.3. Effects of Melatonin on Photosynthesis in Tomato Plant under SAR Stress
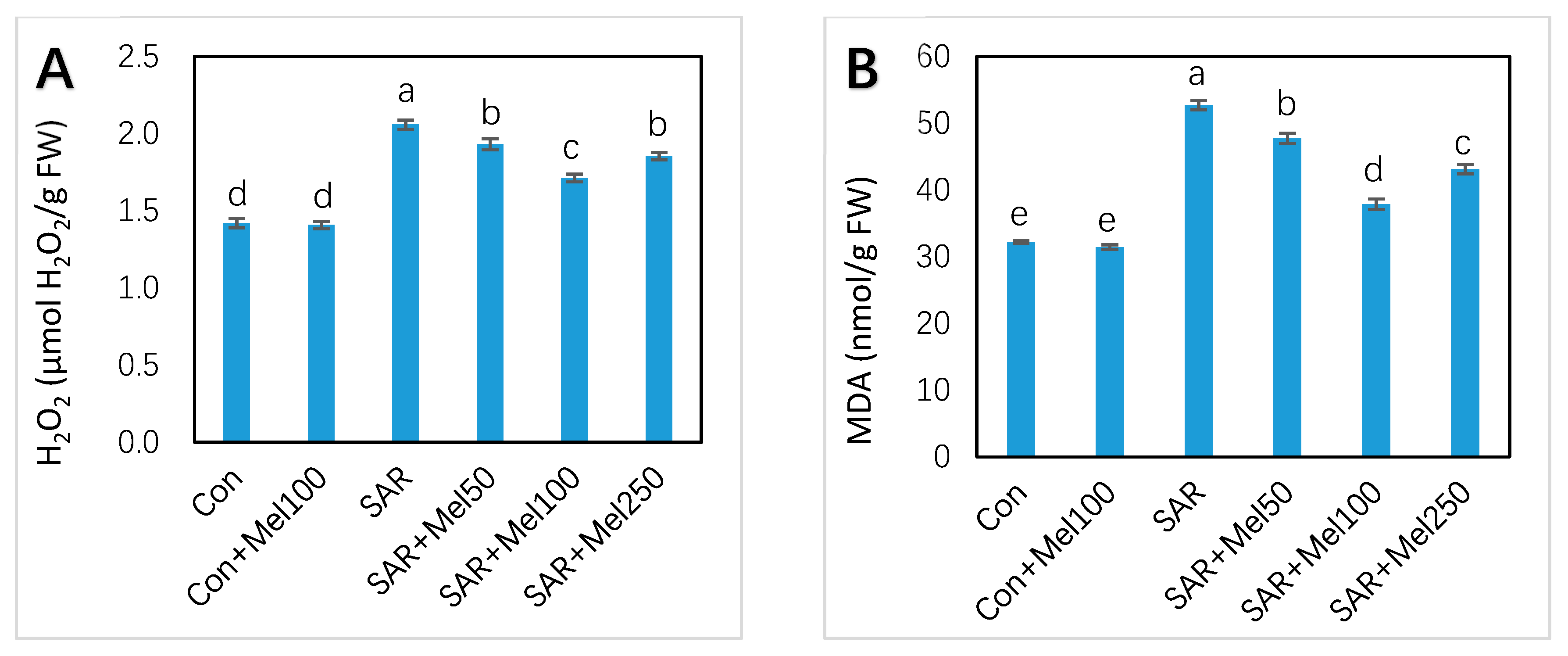
2.4. Effects of Melatonin on H2O2 and MDA Content under SAR Stress
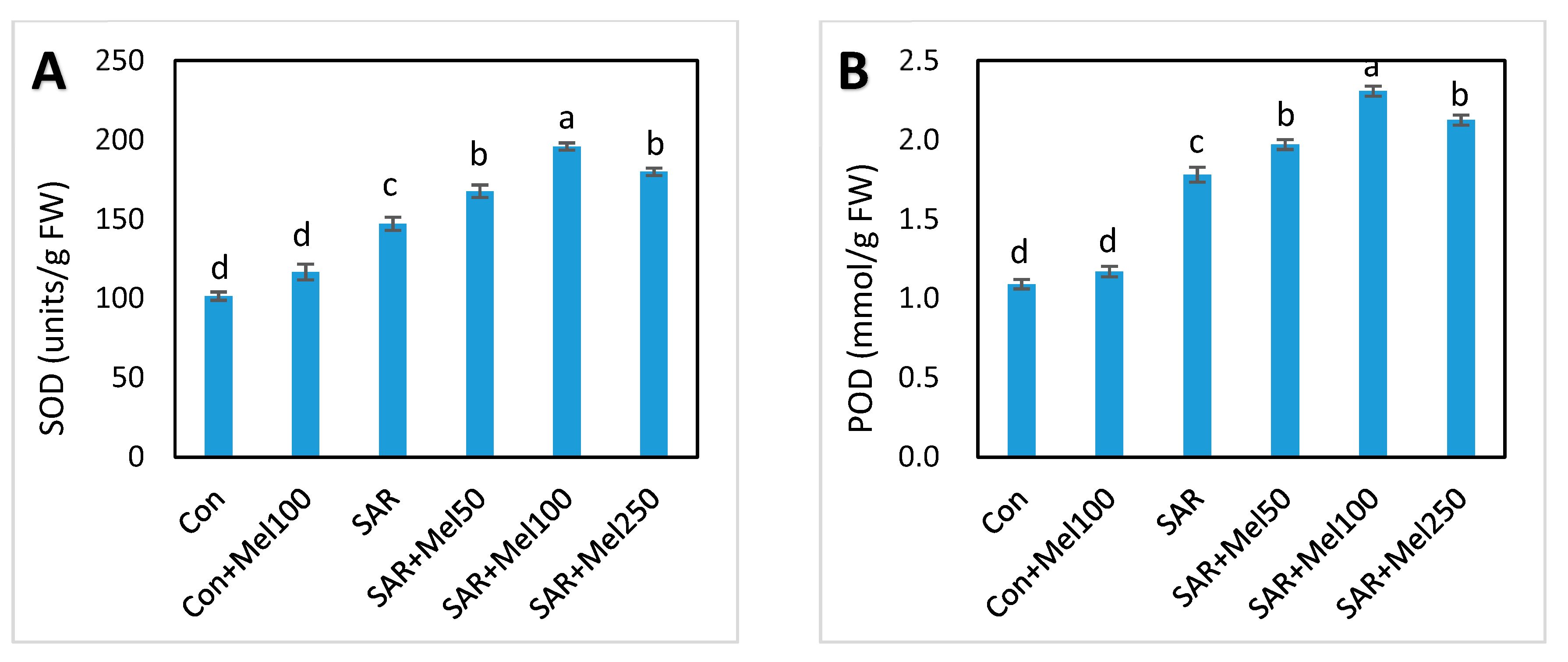
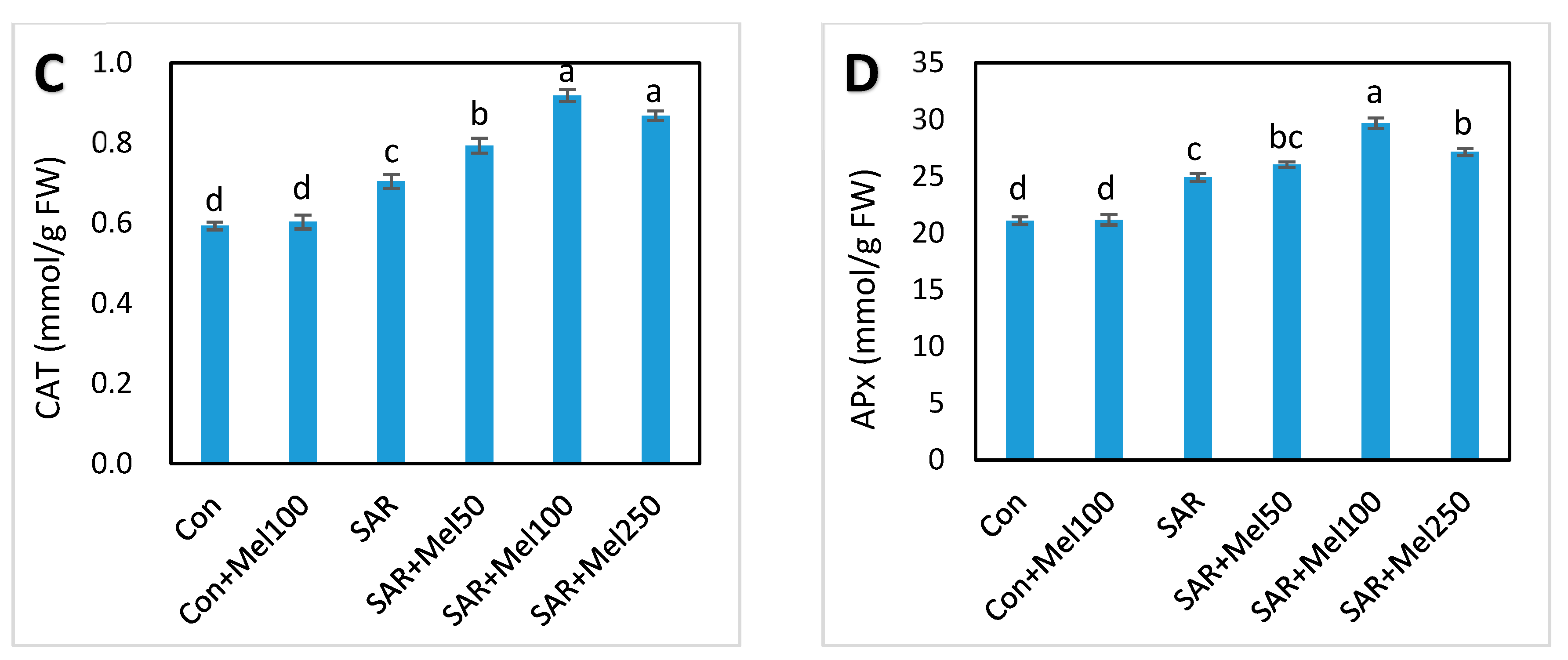
2.5. Effect of Melatonin on Enzymatic Antioxidant Compounds under SAR Stress
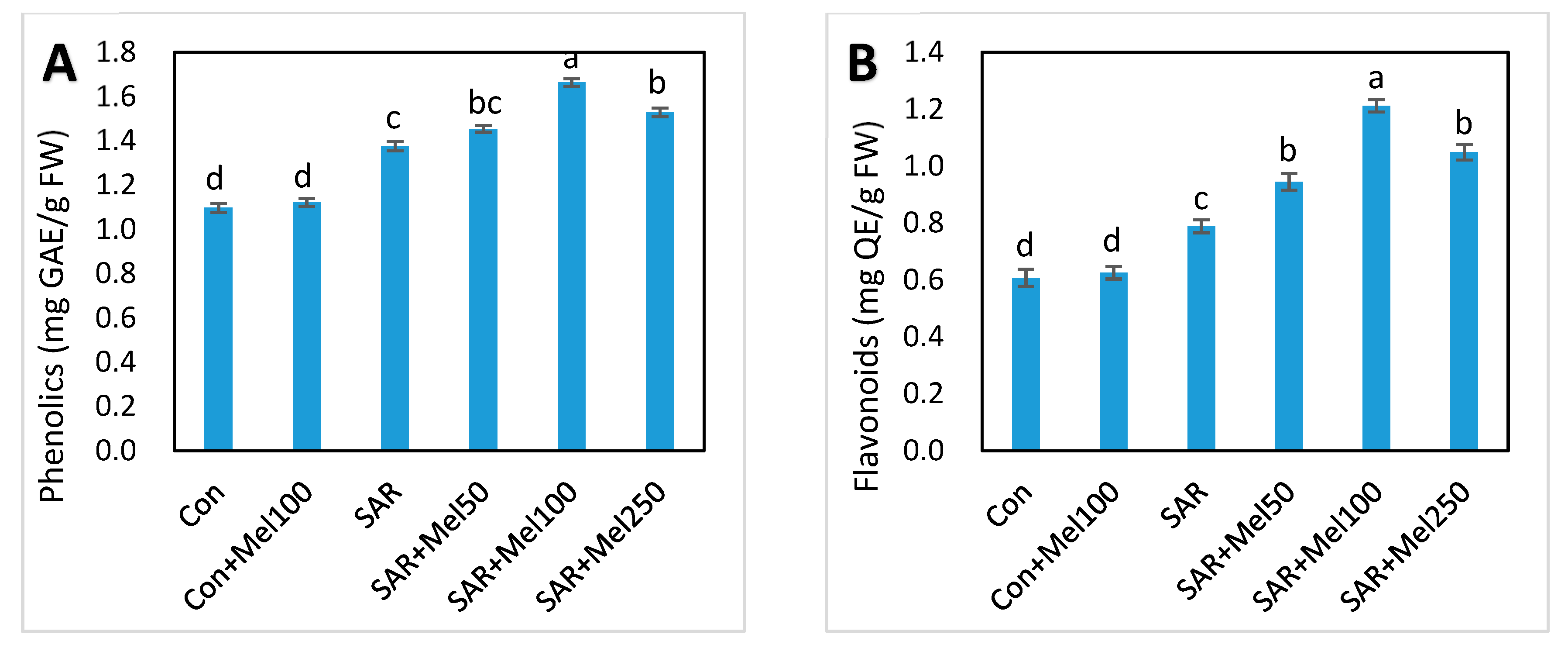
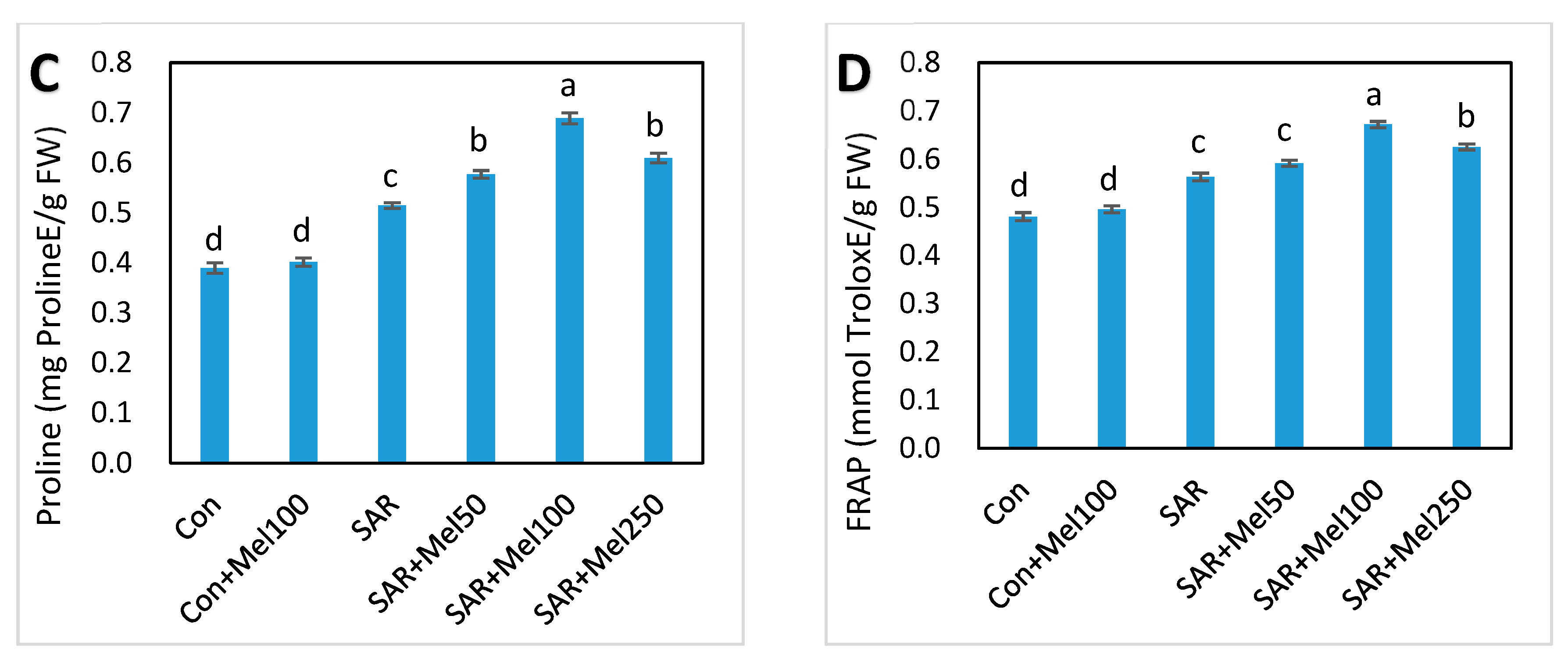
2.6. Effects of Melatonin on Nonenzymatic and Total Antioxidant Activity under SAR Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurement of Plant Growth
4.3. Observation of Leaf Ultrastructure
4.4. Determination of Photosynthesis Activities
4.5. Assay of Hydrogen Peroxide (H2O2) and Lipid Peroxide (MDA) Content
4.6. Estimation of Enzymatic Antioxidant Compounds
4.7. Determination of Nonenzymatic Antioxidant Compounds
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.; Wang, W.-H.; Liu, T.-W.; Wu, F.-H.; Zheng, H.-L. Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol. Biochem. 2013, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.F.; Vuuren, D.P.V.; Derwent, R.G.; Posch, M. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut. 2002, 141, 349–382. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Gao, F. Biogeochemical effects of forest vegetation on acid precipitation-related water chemistry: A case study in southwest china. J. Environ. Monit. 2010, 12, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Kita, I.; Sato, T.; Kase, Y.; Mitropoulos, P. Neutral rains at athens, greece: A natural safeguard against acidification of rains. Sci. Total Environ. 2004, 327, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, P.; Hara, H.; Houle, D.; Novak, M. Acid Rain—Deposition to Recovery; Springer: Dordrecht, The Netherlands, 2007; pp. 241–247. [Google Scholar]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Gholamhoseini, M.; Joghan, A.K.; Majdi, M.; Kashkooli, A.B. The role of calcium in improving photosynthesis and related physiological and biochemical attributes of spring wheat subjected to simulated acid rain. Physiol. Mol. Biol. Plants 2013, 19, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, W. Antioxidant response of soybean seedlings to joint stress of lanthanum and acid rain. Environ. Sci. Pollut. Res. 2013, 20, 8182–8191. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna-Santos, B.F.; Silva, L.C.; Azevedo, A.A.; Aguiar, R. Effects of simulated acid rain on leaf anatomy and micromorphology of Genipa americana L. (Rubiaceae). Braz. Arch. Biol. Technol. 2006, 49, 313–321. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Polishchuk, A.V.; Vodka, M.V.; Belyavskaya, N.A.; Khomochkin, A.P.; Zolotareva, E.K. The effect of acid rain on ultrastructure and functional parameters of photosynthetic apparatus in pea leaves. Cell Tissue Biol. 2016, 10, 250–257. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Chen, M.; Wang, L.; Liang, C.; Zhou, Q.; Huang, X. Interactive effects of cadmium and acid rain on photosynthetic light reaction in soybean seedlings. Ecotoxicol. Environ. Saf. 2012, 79, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, G.; Yang, C.; Ou, Z.; Peng, C. Responses of chlorophyll fluorescence and xanthophyll cycle in leaves of Schima superba Gardn. & Champ. and Pinus massoniana Lamb. to simulated acid rain at Dinghushan Biosphere Reserve, China. Acta Physiol. Plant. 2007, 29, 33–38. [Google Scholar]
- Kováčik, J.; Klejdus, B.; Bačkor, M.; Stork, F.; Hedbavny, J. Physiological responses of root-less epiphytic plants to acid rain. Ecotoxicology 2011, 20, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S.; Demirtas, A. Effects of cement flue dust from a cement factory on stress parameters and diversity of aquatic plants. Toxicol. Ind. Health 2010, 26, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2011, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Solís-Muñoz, P.; Solís-Herruzo, J.A.; Fernández-Moreira, D.; Gómez-Izquierdo, E.; García-Consuegra, I.; Muñoz-Yagüe, T.; García Ruiz, I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J. Pineal Res. 2011, 51, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, Z.; Wang, S.; Gong, B.; Wen, D.; Wang, X.; Wei, M.; Shi, Q. Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Sci. Hortic. 2015, 181, 18–25. [Google Scholar] [CrossRef]
- Gong, X.; Shi, S.; Dou, F.; Song, Y.; Ma, F. Exogenous melatonin alleviates alkaline stress in Malus hupehensis rehd. By regulating the biosynthesis of polyamines. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-zno stress. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Liang, C.; Wang, L.; Hu, G.; Zhou, Q. Combined effects of lanthanumion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 2011, 84, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-Z.; Deng, X.-P.; Xu, B.-C.; Gao, Z.-J.; Ding, W.-L. Photosynthetic activity and efficiency of Bothriochloa ischaemum and Lespedeza davurica in mixtures across growth periods under water stress. Acta Physiol. Plant. 2014, 36, 1033–1044. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Liao, C.; Fan, C.; Zhou, Q.; Huang, X. Combined effects of lead and acid rain on photosynthesis in soybean seedlings. Biol. Trace Elem. Res. 2014, 161, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Wang, W.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, Y.; Wang, L.; Zhou, Q.; Huang, X.; Sun, Z. Combined effects of lanthanum (iii) and acid rain on antioxidant enzyme system in soybean roots. PLoS ONE 2015, 10, e0134546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.C.; Magalhães, P.C.; de Castro, E.M.; Carneiro, N.P.; Padilha, F.A.; Júnior, C.C.G. Aba application to maize hybrids contrasting for drought tolerance: Changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul. 2014, 73, 205–217. [Google Scholar] [CrossRef]
- Posmyk, M.; Kontek, R.; Janas, K. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol. Environ. Saf. 2009, 72, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chang, Y.-H.; Shao, Y.-Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in taiwan. Food Chem. 2006, 98, 529–538. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Portland Press Limited: London, UK, 1983. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M. Antioxidant activities of Sechium edule (jacq.) swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D. Exogenous Melatonin Mitigates Acid Rain Stress to Tomato Plants through Modulation of Leaf Ultrastructure, Photosynthesis and Antioxidant Potential. Molecules 2018, 23, 388. https://doi.org/10.3390/molecules23020388
Debnath B, Hussain M, Irshad M, Mitra S, Li M, Liu S, Qiu D. Exogenous Melatonin Mitigates Acid Rain Stress to Tomato Plants through Modulation of Leaf Ultrastructure, Photosynthesis and Antioxidant Potential. Molecules. 2018; 23(2):388. https://doi.org/10.3390/molecules23020388
Chicago/Turabian StyleDebnath, Biswojit, Mubasher Hussain, Muhammad Irshad, Sangeeta Mitra, Min Li, Shuang Liu, and Dongliang Qiu. 2018. "Exogenous Melatonin Mitigates Acid Rain Stress to Tomato Plants through Modulation of Leaf Ultrastructure, Photosynthesis and Antioxidant Potential" Molecules 23, no. 2: 388. https://doi.org/10.3390/molecules23020388
APA StyleDebnath, B., Hussain, M., Irshad, M., Mitra, S., Li, M., Liu, S., & Qiu, D. (2018). Exogenous Melatonin Mitigates Acid Rain Stress to Tomato Plants through Modulation of Leaf Ultrastructure, Photosynthesis and Antioxidant Potential. Molecules, 23(2), 388. https://doi.org/10.3390/molecules23020388






