Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcεRI-Dependent Degranulation in Mast Cells
Abstract
1. Introduction
2. Results
2.1. Chemoinformatic and Toxicoinformatic Analysis of Cacalolides from P. decompositum and P. peltatum
2.2. Experimental Validation of Anti-Inflammatory/Anti-Allergic Activity
2.2.1. Cacalolides Inhibit Mast Cell Degranulation Activated by FcεRI Triggering In Vitro
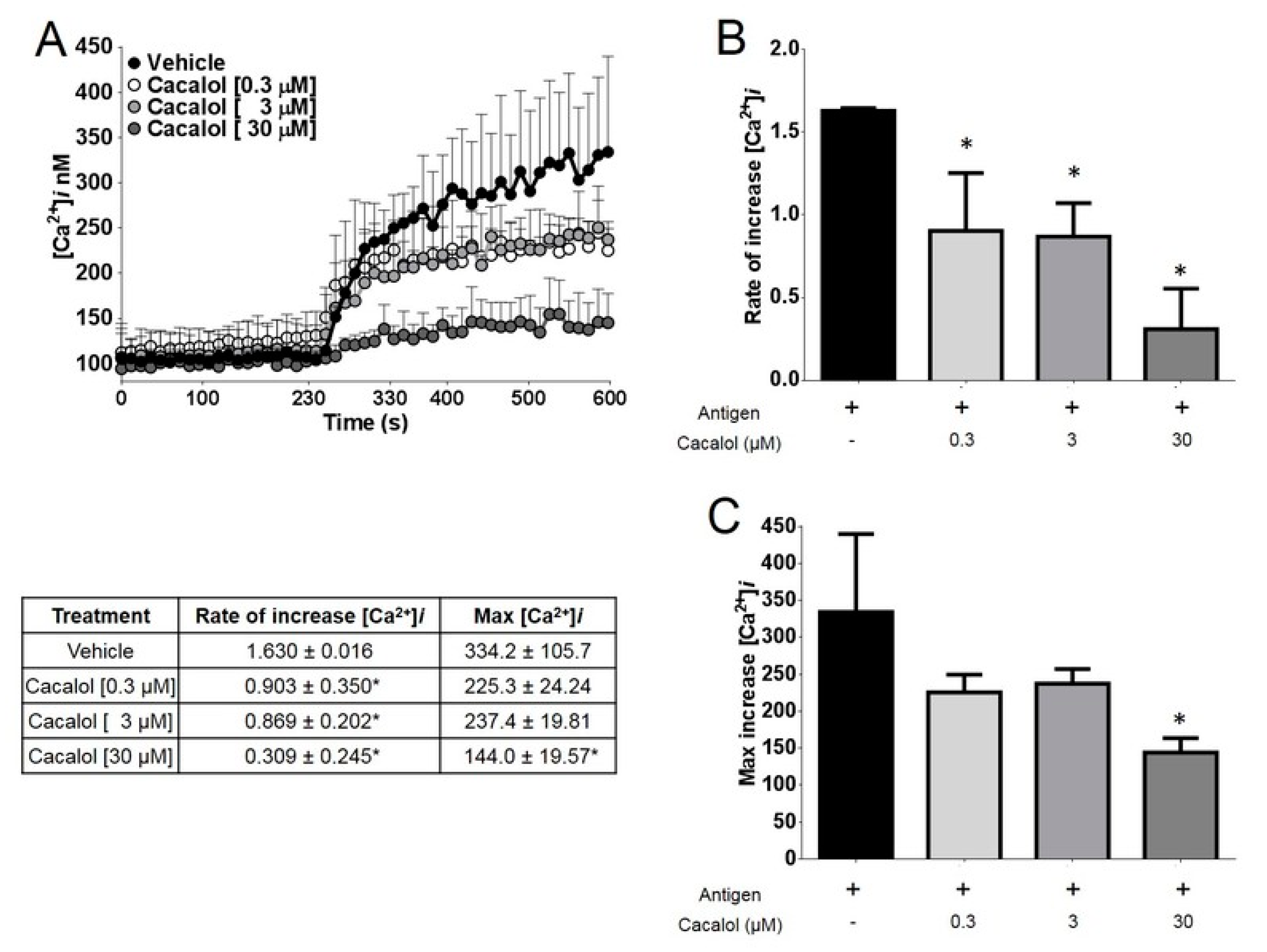
2.2.2. Cacalol Interfered with FcεRI-Induced Intracellular Calcium Mobilization
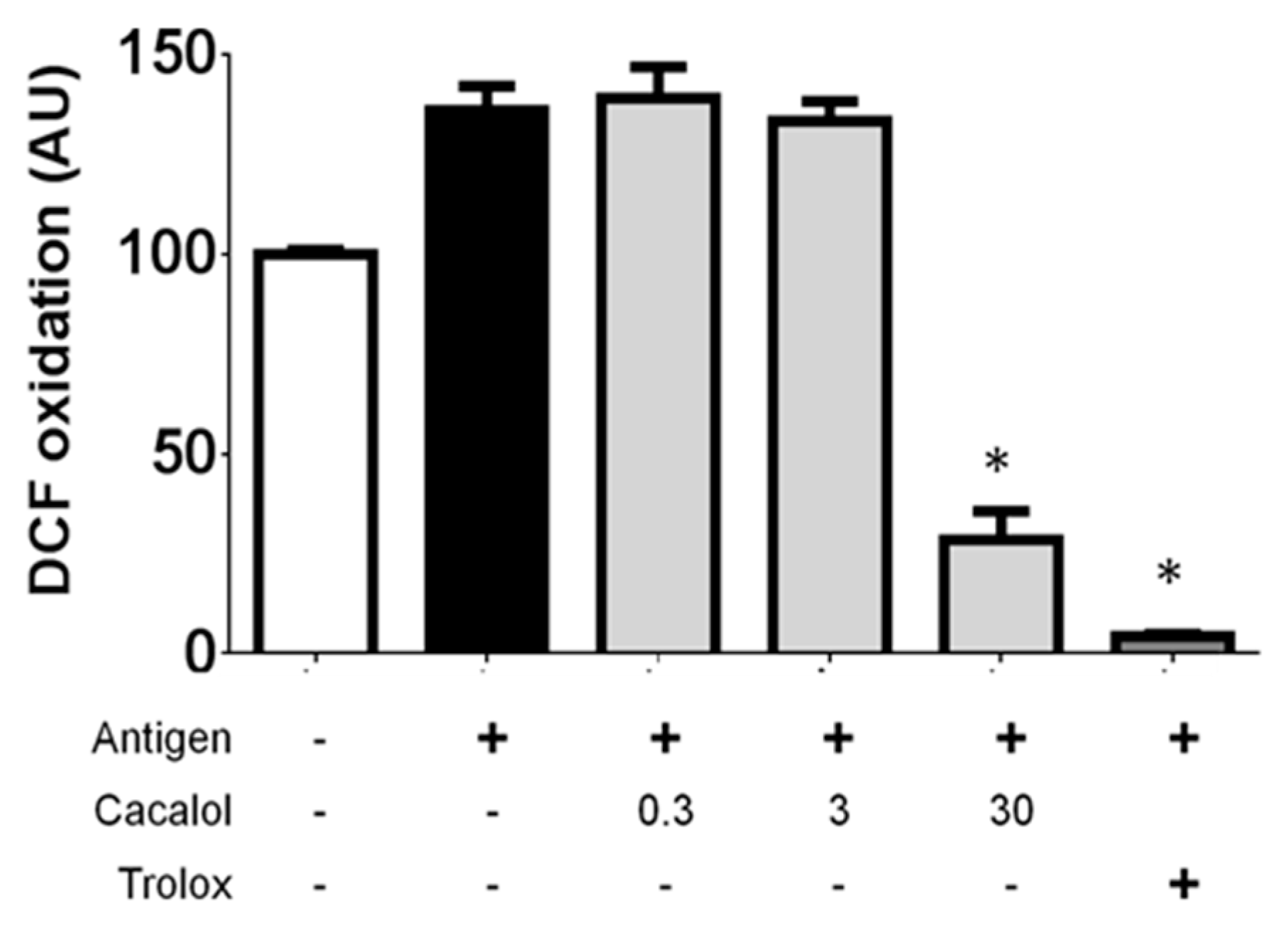
2.2.3. Cacalol Interferes with ROS Production Induced by IgE/Ag Complexes
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation and Purification of Cacalolides
4.3. Chemical Characterization
4.4. Chemoinformatic Analysis
4.5. Properties Calculated Using Molinspiration and PROTOX
4.6. Properties Calculated Using Swiss ADME
4.7. Network Pharmacology
4.8. Animals
4.9. Bone Marrow-Derived Mast Cells Isolation and Culture
4.10. Solubilization of Cacalol, Cacalol Acetate, Cacalone and Maturin Acetate for Experiments
4.11. β-Hexosaminidase Degranulation Assay
4.12. Calcium Mobilization Intracellular Determination ([Ca2+]i)
4.13. ROS Production and Antioxidant Activity
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Beghdadi, W.; Madjene, L.C.; Benhamou, M.; Charles, N.; Gautier, G.; Launay, P.; Blank, U. Mast cells as cellular sensors in inflammation and immunity. Front. Immunol. 2011, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Madera-Salcedo, I.K.; Danelli, L.; Claver, J.; Tiwari, N.; Sánchez-Miranda, E.; Vázquez-Victorio, G.; Ramírez-Valadez, K.A.; Macias-Silva, M.; González-Espinosa, C. Vesicular trafficking and signaling for cytokine and chemokine secretion in mast cells. Front. Immunol. 2014, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Shah, R.; Singh, D. Targeting mast cells: Uncovering prolific therapeutic role in myriad diseases. Int. Immunopharmacol. 2016, 40, 362–384. [Google Scholar] [CrossRef]
- Zhang, T.; Finn, D.F.; Barlow, J.W.; Walsh, J.J. Mast cell stabilisers. Eur. J. Pharmacol. 2016, 778, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Arellano, J.I.; Guzmán-Gutiérrez, S.L.; Ibarra-Sánchez, A.; Hernández-Ortega, S.; Nieto-Camacho, A.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Reyes-Chilpa, R.; González-Espinosa, C. Jacareubin inhibits FcεRI-induced extracellular calcium entry and production of reactive oxygen species required for anaphylactic degranulation of mast cells. Biochem. Pharmacol. 2018, 154, 344–356. [Google Scholar] [CrossRef]
- Alarcón-Aguilar, F.J.; Roman-Ramos, R.; Jimenez-Estrada, M.; Reyes-Chilpa, R.; Gonzalez-Paredes, B.; Flores-Saenz, J.L. Effects of three Mexican medicinal plants (Asteraceae) on blood glucose levels in healthy mice and rabbits. J. Ethnopharmacol. 1997, 55, 171–177. [Google Scholar] [CrossRef]
- Alarcón-Aguilar, F.J.; Jimenez-Estrada, M.; Reyes-Chilpa, R.; Gonzalez-Paredes, B.; Contreras, C.C.; Roman-Ramos, R. Hypoglycemic activity of root water decoction, sesquiterpenoids, and one polysaccharide fraction from Psacalium decompositum in mice. J. Ethnopharmacol. 2000, 69, 207–215. [Google Scholar] [CrossRef]
- Romo de Vivar, A.; Pérez-Castorena, A.L.; Arciniegas, A.; Villaseñor, J.L. Secondary metabolites from Mexican species of the tribe Senecioneae (Asteraceae). J. Mex. Chem. Soc. 2007, 51, 160–172. [Google Scholar]
- Jimenez-Estrada, M.; Merino-Aguilar, H.; Lopez-Fernandez, A.; Rojano-Vilchis, N.A.; Roman-Ramos, R.; Alarcon-Aguilar, F.J. Chemical characterization and evaluation of the hypoglycemic effect of fructooligosaccharides from Psacalium decompositum. J. Complement. Integr. Med. 2011, 8, 1413–1423. [Google Scholar] [CrossRef]
- Merino-Aguilar, H.; Arrieta-Baez, D.; Jiménez-Estrada, M.; Magos-Guerrero, G.; Hernández-Bautista, R.J.; del Susunaga-Notario, A.C.; Almanza-Pérez, J.C.; Blancas-Flores, G.; Román-Ramos, R.; Alarcón-Aguilar, F.J. Effect of fructooligosaccharides fraction from Psacalium decompositum on inflammation and dyslipidemia in rats with fructose-induced obesity. Nutrients 2014, 6, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Estrada, M.; Chilpa, R.R.; Apan, T.R.; Lledias, F.; Hansberg, W.; Arrieta, D.; Aguilar, F.A. Anti-inflammatory activity of cacalol and cacalone sesquiterpenes isolated from Psacalium decompositum. J. Ethnopharmacol. 2006, 105, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Rojano-Vilchis, N.A.; Jimenez-Estrada, M.; Nieto-Camacho, A.; Torres-Avilez, A.; Bye, R.A.; Chavez-Avila, V.M.; Canales-Martinez, M.; Martinez-Elizalde, K.S.; Rodriguez-Monroy, M.A. Isolation and anti-inflammatory effects of maturin acetate from the roots of Psacalium peltatum (Asteraceae). J. Med. Plants Res. 2013, 7, 1600–1607. [Google Scholar] [CrossRef]
- Del Carmen Juárez-Vázquez, M.; Alonso-Castro, A.J.; Rojano-Vilchis, N.; Jiménez-Estrada, M.; García-Carrancá, A. Maturin acetate from Psacalium peltatum (Kunth) Cass. (Asteraceae) induces immunostimulatory effects in vitro and in vivo. Toxicol. In Vitro 2013, 27, 1001–1006. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, L.; Yin, Z.; Lin, J. Improving chemical similarity ensemble approach in target prediction. J. Cheminform. 2016, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein. J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Furuta, E.; Shindo, K.; Watabe, M.; Xing, F.; Pandey, P.R.; Mo, Y.Y. Cacalol, a natural sesquiterpene, induces apoptosis in breast cancer cells by modulating Akt-SREBP-FAS signaling pathway. Breast Cancer. Res. Treat. 2011, 128, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Estrada, M.; Reyes-Chilpa, R.; Navarro-Ocaña, A.; Baez, D.A. Reactivity of several reactive oxygen species (ROS) with the sesquiterpene cacalol. Nat. Prod. Comm. 2008, 3, 479–482. [Google Scholar]
- Feske, S. ORAI1 and STIM1 deficiency in human and mice: Roles of store-operated Ca2+ entry in the immune system and beyond. Immunol. Rev. 2009, 231, 189–209. [Google Scholar] [CrossRef]
- Saul, S.; Gibhardt, C.S.; Schmidt, B.; Lis, A.; Pasieka, B.; Conrad, D.; Jung, P.; Gaupp, R.; Wonnenberg, B.; Diler, E.; et al. A calcium-redox feedback loop controls human monocyte immune responses: The role of ORAICa2+ channels. Sci. Signal. 2016, 9, ra26. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vidales, V.; Granados-Oliveros, G.; Nieto-Camacho, A.; Reyes-Solís, M.; Jiménez-Estrada, M. Cacalol and cacalol acetate as photoproducers of singlet oxygen and as free radical scavengers, evaluated by EPR spectroscopy and TBARS. RSC Adv. 2014, 4, 1371–1377. [Google Scholar] [CrossRef]
- Inman, W.D.; Luo, J.; Jolad, S.D.; King, S.R.; Cooper, R. Antihyperglycemic sesquiterpenes from Psacalium decompositum. J. Nat. Prod. 1999, 62, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N.; Gon, Y.; Nunomura, S.; Endo, D.; Yamashita, K.; Matsumoto, K.; Hashimoto, S.; Ra, C. Inhibitory effects of parthenolide on antigeninduced microtubule formation and degranulation in mast cells. Int. Immunopharmacol. 2008, 8, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Oyama, M.; Takimoto, N.; Kato, C.; Nozawa, Y.; Akao, Y.; Iinuma, M. Inhibitory effects of sesquiterpene lactones isolated from Eupatorium chinense L. on IgE-mediated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Bioorg. Med. Chem. 2009, 17, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.; Oropeza, M.; Torres-Sosa, C.; Jimenez-Estrada, M.; Reyes-Chilpa, R. Sesquiterpenoids from antidiabetic Psacalium decompositum block ATP sensitive potassium channels. J. Ethnopharmacol. 2009, 123, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Anaya, A.L.; Hernández-Bautista, B.E.; Torres-Barragán, A.; León-Cantero, J.; Jiménez-Estrada, M. Phytotoxicity of cacalol and some derivatives obtained from the roots of Psacalium decompositum (A. Gray) H. Rob. & Brettell (Asteraceae), matarique or maturin. J. Chem. Ecol. 1996, 22, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kedrowski, B.L.; Hoppe, R.W. A concise synthesis of (+/−)-cacalol. J. Org. Chem. 2008, 73, 5177–5179. [Google Scholar] [CrossRef] [PubMed]
- Yuste, F.; Diaz, E.; Walls, F.; Jankowski, K. The structure of cacalone. J. Org. Chem. 1976, 41, 4103–4106. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucl. Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A Boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, J.; Shi, L.; Mikailov, M.; Zhu, H.; Wang, K.; He, L.; Yang, L. DRAR-CPI: A server for identifying drug repositioning potential and adverse drug reactions via the chemical protein interactome. Nucl. Acids Res. 2011, 39, W492–W498. [Google Scholar] [CrossRef]
- Mattingly, C.J.; Colby, G.T.; Forrest, J.N.; Boyer, J.L. The Comparative toxicogenomics database (CTD). Environ. Health Perspect. 2003, 111, 793–795. [Google Scholar] [CrossRef]
- Madera-Salcedo, I.K.; Cruz, S.L.; Gonzalez-Espinosa, C. Morphine prevents lipopolysaccharide-induced TNF secretion in mast cells blocking IB kinase activation and SNAP-23 phosphorylation: Correlation with the formation of a β-Arrestin/TRAF6 complex. J. Immunol. 2013, 191, 3400–3409. [Google Scholar] [CrossRef]
- Manetz, T.S.; Gonzalez-Espinosa, C.; Arudchandran, R.; Xirasagar, S.; Tybulewicz, V.; Rivera, J. Vav1 regulates phospholipase cgamma activation and calcium responses in mast cells. Mol. Cell. Biol. 2001, 21, 3763–3774. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Arudchandran, R.; Manetz, T.S.; Zhang, W.; Sommers, C.L.; Love, P.E.; Rivera, J.; Samelson, L.E. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 2000, 12, 525–535. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]
- Davies, M.J.; Forni, L.G.; Willson, R.L. Vitamin E analogue Trolox CEsr and pulse-radiolysis studies of free-radical reactions. Biochem. J. 1988, 255, 513–522. [Google Scholar]
- Bentayeb, K.; Rubio, C.; Nerín, C. Study of the antioxidant mechanisms of Trolox and eugenol with 2,2′-azobis (2-amidinepropane) dihydrochloride using ultra-high performance liquid chromatography coupled with tandem mass spectrometry. Analyst 2012, 137, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Tsukane, M.; Koike, M.; Nakamura, C.; Ohguchi, K.; Ito, M.; Akao, Y.; Koshimizu, S.; Nozawa, Y.; Wakimoto, T.; et al. Inhibitory effects of whisky congeners on IgEmediated degranulation in rat basophilic leukemiaRBL2H3 cells and passive cutaneous anaphylaxis reaction in mice. J. Agric. Food. Chem. 2010, 58, 7149–7157. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
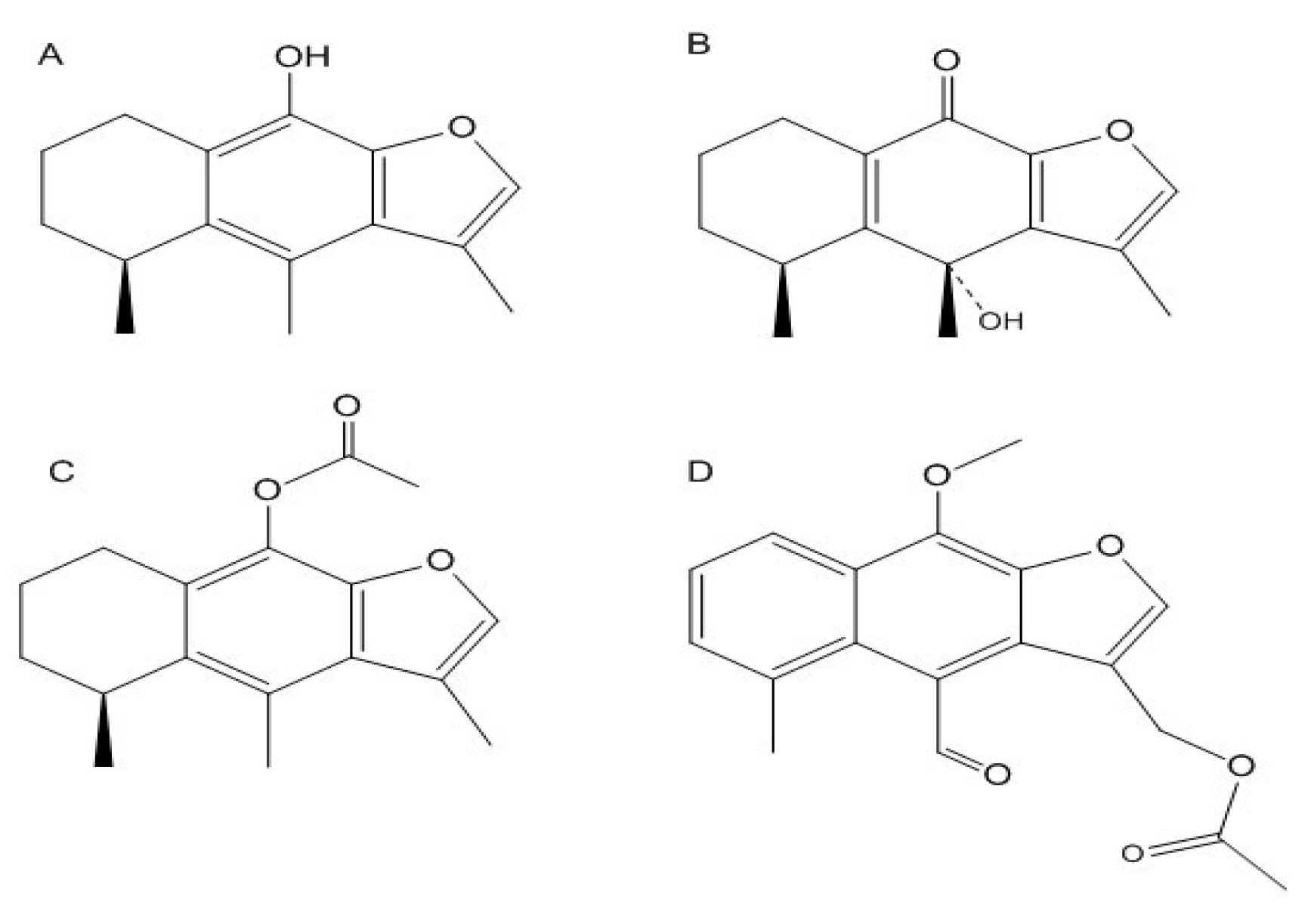

| Cacalol | Cacalone | Cacalol Acetate | Maturin Acetate | ||
|---|---|---|---|---|---|
| Physicochemical Properties | Log P | 3.65 | 2.56 | 3.42 | 3.20 |
| Log S | −5.56 | −4.04 | −4.34 | −5.09 | |
| TPSA | 33.37 A | 50.44 A | 39.44 A | 65.74 A | |
| MW | 230.30 g/mol | 246.30 g/mol | 272.34 g/mol | 312.34 g/mol | |
| RB | 0 | 0 | 2 | 5 | |
| BD | 1 | 1 | 0 | 0 | |
| BA | 2 | 3 | 3 | 5 | |
| Molar refractivity | 70.64 | 68.83 | 78.26 | 86.20 | |
| Pharmacokinetic Properties | GI absorption | High | High | High | High |
| BBB permeable | Yes | Yes | Yes | Yes | |
| P-gp substrate | Yes | Yes | No | No | |
| CYP1A2 inhibitor | Yes | No | No | Yes | |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes | |
| CYP2C9 inhibitor | No | No | No | Yes | |
| CYP2D6 Inhibitor | No | No | No | No | |
| CYP3A4 inhibitor | No | No | No | Yes | |
| Log Kp (Skin permeation) | −4.62 cm/s | −6.14 cm/s | −5.79 cm/s | −5.99 cm/s | |
| Medicinal Chemistry Properties | Lipinski | Yes | Yes | Yes | Yes |
| Ghose | Yes | Yes | Yes | Yes | |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | |
| Lead-likeness | No, 2 Violations | No, 1 violation | Yes | Yes | |
| Synthetic accessibility | 2.75 | 4.32 | 4.72 | 3.77 | |
| Toxicoinformatic Properties | Toxicity Class | 5 | 4 | 5 | 5 |
| Mutagenic | None | None | None | None | |
| Tumorigenic | None | None | None | None | |
| Irritant | None | None | High | None | |
| Reproductive effects | None | None | None | None | |
| Possible Toxic Target | None | None | None | Amine oxidase prostaglandin G/H synthase 1 | |
| Toxic Fragments | None | None | None | None |
| Compound | Pathway or GO Term | Count | p-Value | Data Base |
|---|---|---|---|---|
| Cacalol | Innate immune system (hsa-168249) | 24 | 2.43 × 10−20 | Reactome |
| VEGFA-VEGFR2 pathway (hsa-4420097) | 12 | 4.42 × 10−12 | Reactome | |
| Fc epsilon receptor signaling (hsa-2454202) | 12 | 4.42 × 10−12 | Reactome | |
| PI3K-Akt pathway (hsa04151) | 12 | 4.91 × 10−12 | KEGG 1 | |
| MAPK signaling pathway (hsa04010) | 11 | 7.89 × 10−12 | KEGG 1 | |
| Protein kinase activity (GO:0004672) | 26 | 1.58 × 10−31 | GO-MF 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Arellano, J.I.; Gómez-Verjan, J.C.; Rojano-Vilchis, N.A.; Mendoza-Cruz, M.; Jiménez-Estrada, M.; López-Valdés, H.E.; Martínez-Coria, H.; Gutiérrez-Juárez, R.; González-Espinosa, C.; Reyes-Chilpa, R.; et al. Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcεRI-Dependent Degranulation in Mast Cells. Molecules 2018, 23, 3367. https://doi.org/10.3390/molecules23123367
Castillo-Arellano JI, Gómez-Verjan JC, Rojano-Vilchis NA, Mendoza-Cruz M, Jiménez-Estrada M, López-Valdés HE, Martínez-Coria H, Gutiérrez-Juárez R, González-Espinosa C, Reyes-Chilpa R, et al. Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcεRI-Dependent Degranulation in Mast Cells. Molecules. 2018; 23(12):3367. https://doi.org/10.3390/molecules23123367
Chicago/Turabian StyleCastillo-Arellano, Jorge Iván, Juan Carlos Gómez-Verjan, Nadia A. Rojano-Vilchis, Myrna Mendoza-Cruz, Manuel Jiménez-Estrada, Héctor E. López-Valdés, Hilda Martínez-Coria, Roger Gutiérrez-Juárez, Claudia González-Espinosa, Ricardo Reyes-Chilpa, and et al. 2018. "Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcεRI-Dependent Degranulation in Mast Cells" Molecules 23, no. 12: 3367. https://doi.org/10.3390/molecules23123367
APA StyleCastillo-Arellano, J. I., Gómez-Verjan, J. C., Rojano-Vilchis, N. A., Mendoza-Cruz, M., Jiménez-Estrada, M., López-Valdés, H. E., Martínez-Coria, H., Gutiérrez-Juárez, R., González-Espinosa, C., Reyes-Chilpa, R., & Arrieta-Cruz, I. (2018). Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcεRI-Dependent Degranulation in Mast Cells. Molecules, 23(12), 3367. https://doi.org/10.3390/molecules23123367







