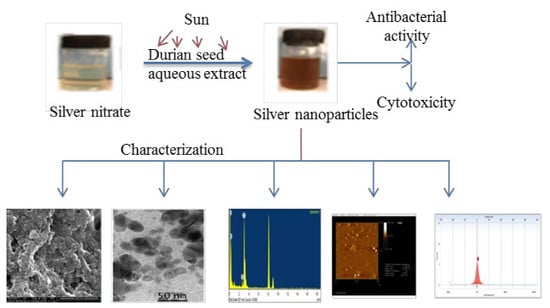
Phyto-Mediated Photo Catalysed Green Synthesis of Silver Nanoparticles Using Durio Zibethinus Seed Extract: Antimicrobial and Cytotoxic Activity and Photocatalytic Applications
Abstract
:1. Introduction
2. Results and Discussion
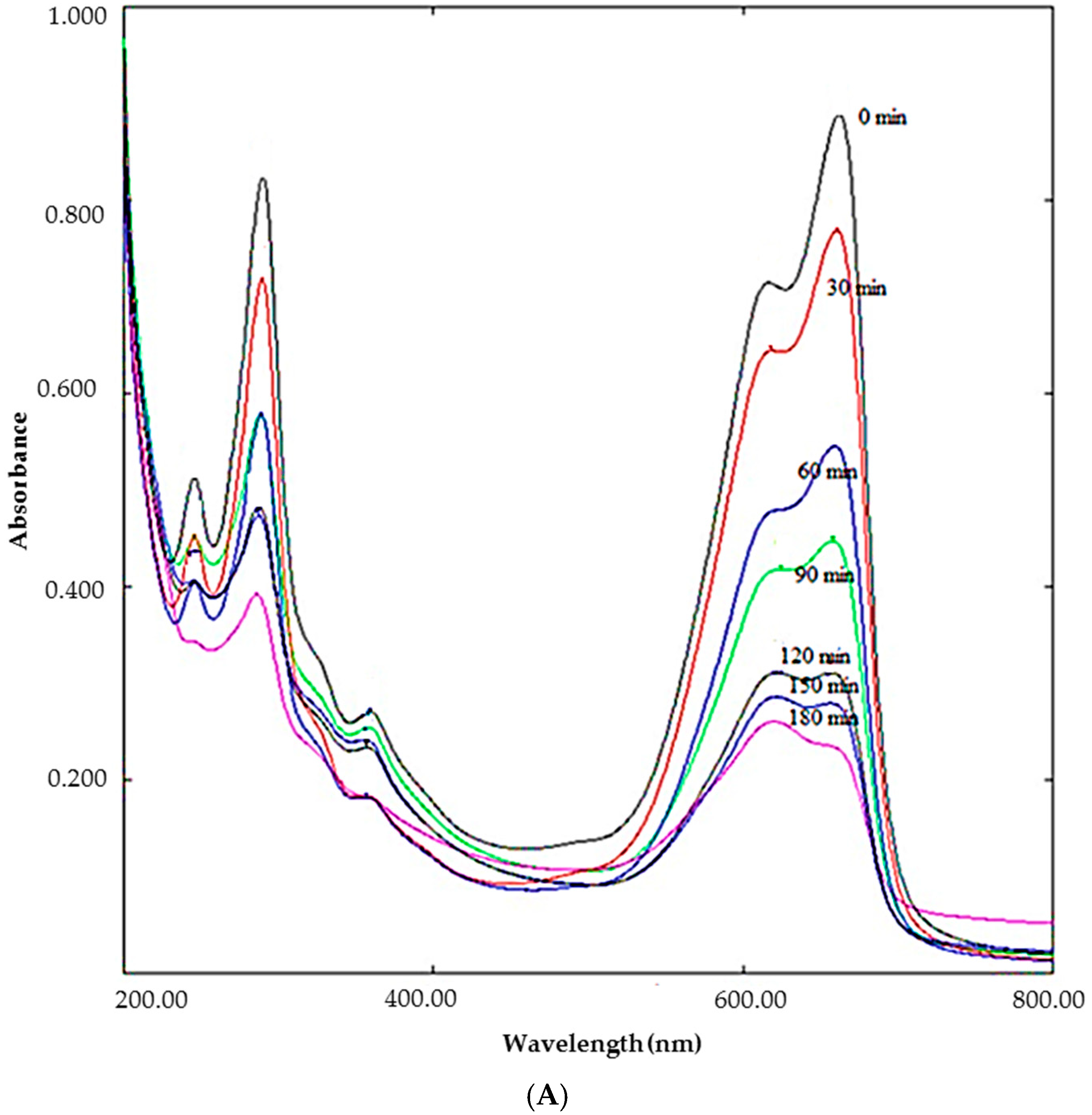
2.1. Synthesis of Silver Nanoparticles: Process Optimization
2.2. Mechanism Involving in DSAgNPs Formation
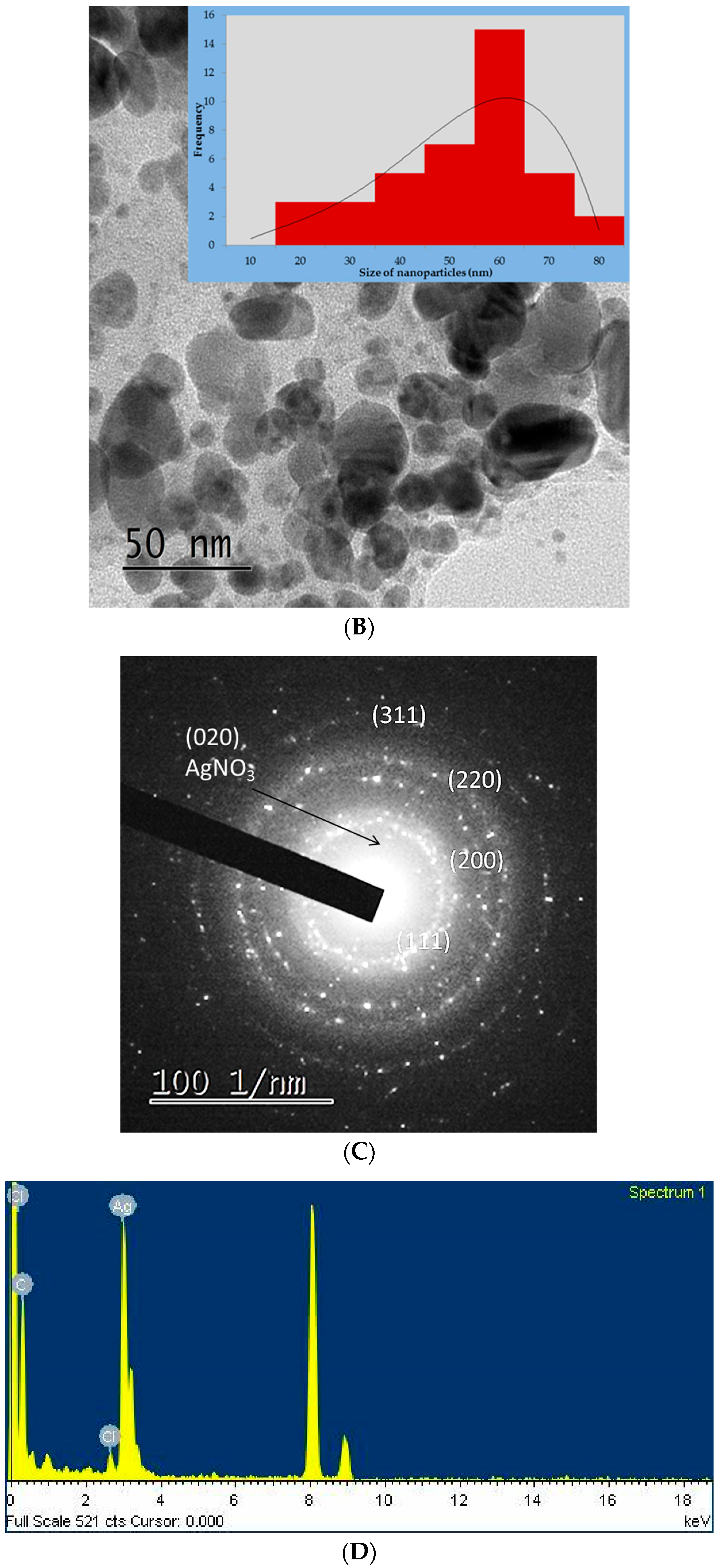
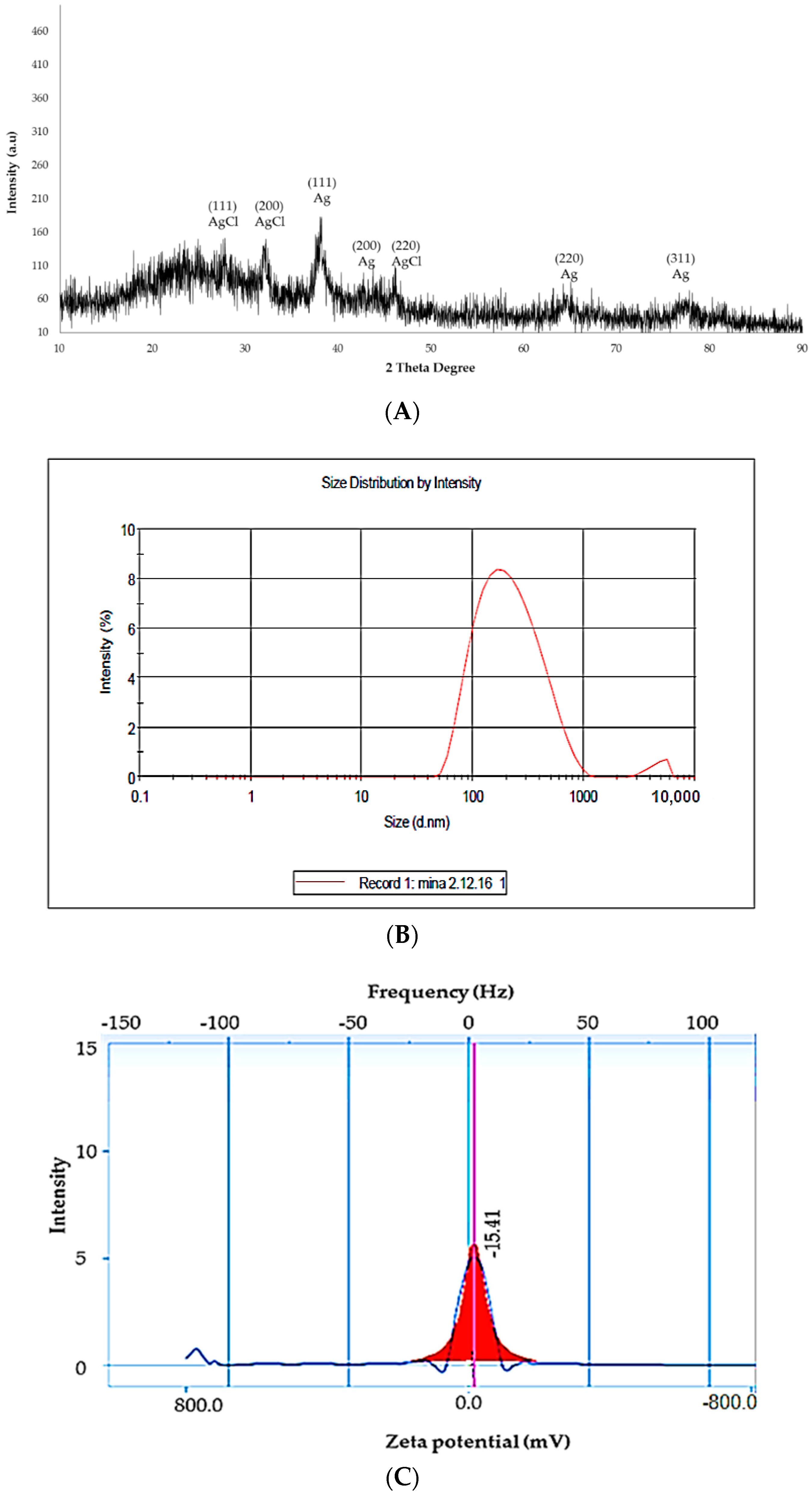
2.3. Characterization of DSAgNPs
2.4. Antimicrobial Activity of DSAgNps
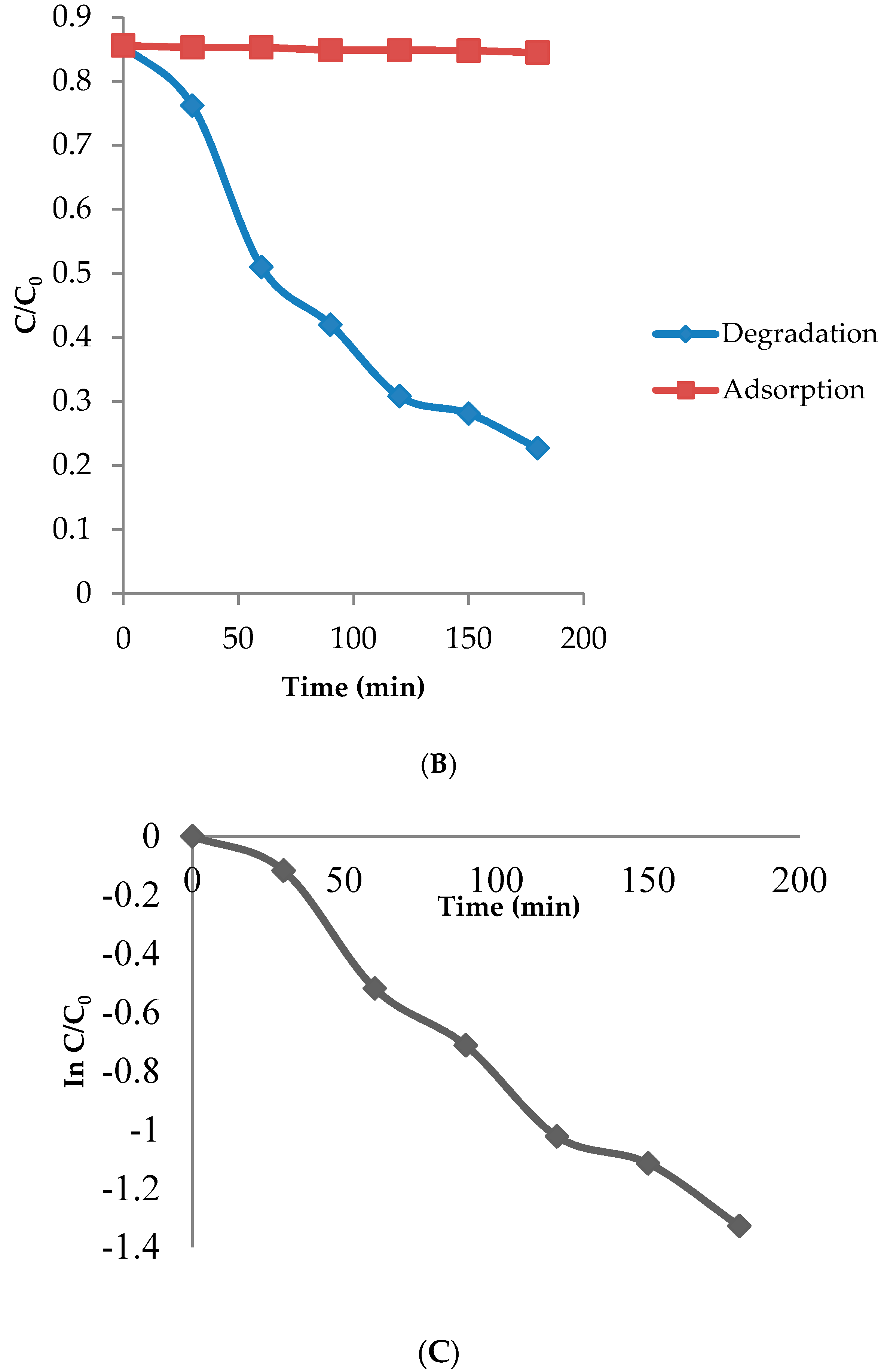
2.5. Photocatalytic Activity
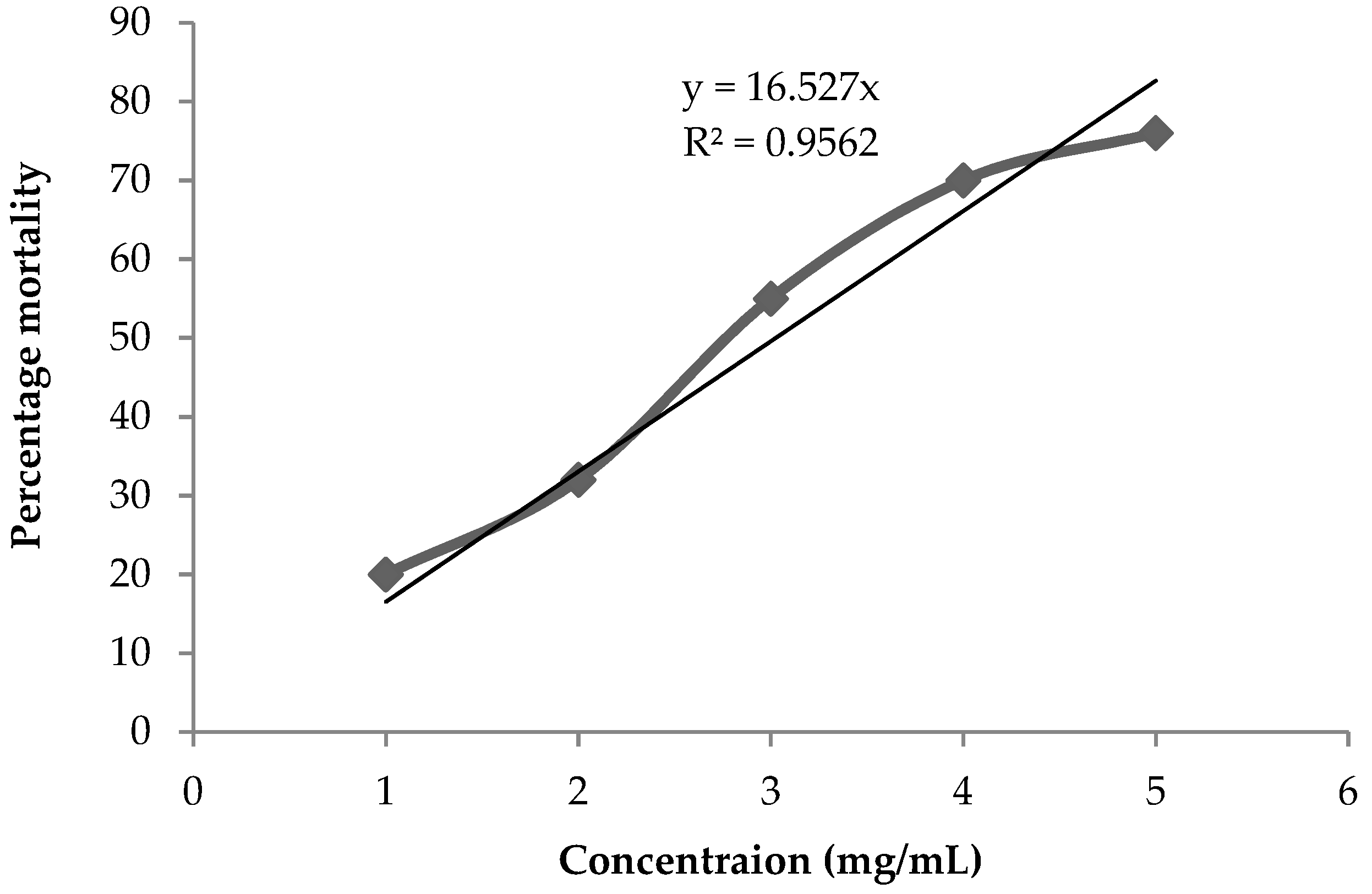
2.6. Cytotoxicity against Artemia salina
3. Materials and Methods
3.1. Materials
3.2. Preparation of Durian Seed Extracts
3.3. Biosynthesis of DSAgNPs
3.4. Optimization of Reaction Parameters
3.5. Characterization of DSAgNPs
3.6. Antimicrobial Activity
3.6.1. Minimum Inhibitory Concentration (MIC)
3.6.2. Minimum Bactericidal Concentration (MBC)
3.6.3. Disc Diffusion Assay
3.7. The Photocatalytic Degradation of Methylene Blue
3.8. Cytotoxicity of DSAgNPs against Artemia salina
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rauwel, P.; Rauwel, E. Emerging trends in nanoparticle synthesis using plant extracts for biomedical applications. Global J. Nanomed. 2017, 1, 555562. [Google Scholar] [CrossRef]
- Saifuddin, N.; Wong, C.W.; Nur Yasumira, A.A. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanyan, R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Ramirez, I.M.; Bashir, S.; Luo, Z.; Liu, J.L. Green synthesis and characterization of polymer-stabilized silver nanoparticles. Colloids and Surf. B Biointerfaces 2009, 73, 185–191. [Google Scholar] [CrossRef]
- Otunola, G.A.; Afolayan, A.J.; Ajayi, E.O.; Odeyemi, S.W. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiber officinale, and Capsicum frutescents. Pharmacogn. Mag. 2017, 13, 201–208. [Google Scholar] [CrossRef]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Ravichandran, V.; Shalini, S.; Vasanthi, S.; Shaa, S.A.A.; Harish, R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mat. Lett. 2016, 180, 264–267. [Google Scholar] [CrossRef]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.V.; Parkinson, Y.W.; Choi, J.L.; Speshock, S.M.; Hussain, M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Gurunathan, S.; Lee, K.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
- Shanker, K.; Mohan, G.K.; Ashwaq Hussain, Md.; Jayarambabu, N.; Pravallika, P.L. Green biosynthesis, characterization, in vitro antidiabetic activity, and investigational acute toxicity studies of some herbal-mediated silver nanoparticles on animal models. Pharmcogn. Mag. 2017, 13, 188–192. [Google Scholar]
- Arancibia-Avila, P.; Toledo, F.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.G. Antioxidant properties of durian fruit as influenced by ripening. LWT-Food Sci. Technol. 2008, 41, 2118–2125. [Google Scholar] [CrossRef]
- Toledo, F.; Aranciba-Avila, P.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.G. Screening of the antioxidant and nutritional properties, phenolic contents and proteins of five durian cultivars. Int. J. Food Sci. Nutr. 2008, 59, 415–427. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Haruenkit, R.; Poovarodom, S.; Jastrzebski, Z.; Drzewiecki, J. Durian (Durio zibethinus Murr.) cultivars as nutritional supplementation to rat’s diets. Food Chem. Toxicol. 2008, 46, 581–589. [Google Scholar] [CrossRef]
- Chansiripornchai, P.; Pongsamart, S. Treatment of infected openwounds on two dogs using a film dressing of polysaccharide extracted from the hulls of durian (Durio zibethinus Murr.): Case report. Thai J. Vet. Med. 2008, 38, 55–61. [Google Scholar]
- Jaswir, I.; Man, Y.B.C.; Selamat, J.; Ahmad, F.; Sugisawa, H. Retention of volatile components of durian fruit leather during processing and storage. J. Food Process. Preserv. 2008, 32, 740–750. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Hokputsa, S.; Gerddit, W.; Pongsamart, S.; Inngjerdingen, K.; Heinze, T.; Koschella, A. Water-soluble polysaccharides with pharmaceutical importance from durian rinds (Durio Zibethinus Murr.): Isolation, fractionation, characterisation and bioactivity. Carbohydr. Polym. 2004, 56, 471–481. [Google Scholar] [CrossRef]
- Hameed, S.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S.; Athar, M. Toxicity of Fusarium solani strains on brine shrimp (Artemia salina). Zool. Res. 2009, 30, 468–472. [Google Scholar]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; Mclaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Patova, O.A.; Selivanov, N.U.; Selivanov, O.G. The antioxidant properties of pectin fractions isolated from vegetables using a simulated gastric fluid. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X. Characterization of pectic polysaccharides extracted from apple pomace by hot-compressed water. Carbohydr. Polym. 2014, 102, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kamboj, S.; Khurana, R.; Singh, G.; Rana, V. Physicochemical and functional performance of pectin extracted by QbD approach from Tamarindus indica L. pulp. Carbohydr. Polym. 2015, 134, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Maria-Ferreira, D.; Da Silva, L.M.; Mendes, D.A.G.B.; de Almeida Cabrini, D.; Nascimento, A.M.; Iacomini, M. Rhamnogalacturonan from Acmella oleracea (L.) R.K. Jansen: Gastroprotective and ulcer healing properties in rats. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Rastogi, L.; Arunachalam, J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys. 2011, 129, 558–563. [Google Scholar] [CrossRef]
- Nadezhda, V.I.; Natalya, N.T.; Lydmila, A.E.; Vasilyi, A.B. The study of the reaction of pectin-Ag(0) nanocomposites formation. Int. J. Carbohydr. Chem. 2012. [Google Scholar] [CrossRef]
- Awwad, A.m.; Salem, N.M.; Ibrahim, Q.M.; Abdeen, A.O. Phytochemical fabrication and characterization of silver/ silver chloride nanoparticles using Albizia julibrissin flowers extract. Adv. Mater. Lett. 2015, 6, 726–730. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kalaiselvam, S.; Jayavel, R. Green synthesis of silver nanoparticles using Beta vulgaris: Role of process conditions on size distribution and surface structure. Mater. Chem. Phys. 2013, 140, 135–147. [Google Scholar] [CrossRef]
- Singh, G.; Babele, P.K.; Shahi, S.K.; Sinha, R.P.; Tyagi, M.B.; Kumar, A. Green synthesis of silver nanoparticles using cell extracts of Anabaena doliolum and screening of its antibacterial and antitumor activity. J. Microbiol. Biotechnol. 2014, 24, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Park, Y. A new paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014, 30, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kvytek, L.; Prucek, R.; Kolar, M.; Vecerova, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2008, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Patil Shriniwas, P.; Kumbhar Subhash, T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.M.; Velmurugan, P.; Park, Y.J.; Park, Y.J.; Bang, K.S.; Oh, B.T. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 162, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Satapathy, P.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Sorgeloos, P. Availability of reference Artemia cysts. Mar. Ecol. Prog. Ser. 1980, 3, 363–364. [Google Scholar] [CrossRef]
- Gajbhiye, S.N.; Hirota, R. Toxicity of heavy metals to brine shrimp Artemia. J. Indian Fish Assoc. 1990, 20, 43–50. [Google Scholar]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 2006, 144, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, V.; Katramados, I.; Newman, J.D. Monitoring the effect of metal ions on the mobility of Artemia salina nauplii. Biosensors 2011, 1, 36–45. [Google Scholar] [CrossRef]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicol. Environ. Safety 1981, 5, 382–387. [Google Scholar] [CrossRef]
- Arulvasu, C.; Jennifer, S.M.; Prabhu, D.; Chandhirasekar, D. Toxicity effect of silver nanoparticles in brine shrimp Artemia. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Avinash, B.; Supraja, N.; Santhi Priya, Ch.; Prasad, T.N.V.K.V.; Alpha Raj, M. Synthesis characterization and evaluation of the antimicrobial activity of neem leaf extract-mediated silver nanoparticles. Int. J. Pure Appl. Biosci. 2017, 5, 776–786. [Google Scholar]
- Phull, A.-R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliate. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Prakash, S.; Ahila, N.K.; Sri Ramkumar, V.; Ravindran, J.; Kannapiran, E. Antimicrofouling properties of chosen marine plants: An eco-friendly approach to restrain marine microfoulers. Biocatal. Agric. Biotechnol. 2015, 4, 114–121. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Bacteria | Zone of Inhibition (mm) | |
|---|---|---|
| Gentamycin * | DSAgNPs ** | |
| S. typhi | 25 ± 1.32 | 7 ± 0.20 |
| S. typhimurium | 24 ± 2.91 | 11 ± 0.51 |
| E. coli | 20 ± 1.06 | 7 ± 0.22 |
| S. aureus | 23 ± 1.12 | 8 ± 0.13 |
| S. haemolyticus | 23 ± 1.82 | 11 ± 0.55 |
| B. subtilis | 25 ± 1.36 | 8 ± 0.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumitha, S.; Vasanthi, S.; Shalini, S.; Chinni, S.V.; Gopinath, S.C.B.; Anbu, P.; Bahari, M.B.; Harish, R.; Kathiresan, S.; Ravichandran, V. Phyto-Mediated Photo Catalysed Green Synthesis of Silver Nanoparticles Using Durio Zibethinus Seed Extract: Antimicrobial and Cytotoxic Activity and Photocatalytic Applications. Molecules 2018, 23, 3311. https://doi.org/10.3390/molecules23123311
Sumitha S, Vasanthi S, Shalini S, Chinni SV, Gopinath SCB, Anbu P, Bahari MB, Harish R, Kathiresan S, Ravichandran V. Phyto-Mediated Photo Catalysed Green Synthesis of Silver Nanoparticles Using Durio Zibethinus Seed Extract: Antimicrobial and Cytotoxic Activity and Photocatalytic Applications. Molecules. 2018; 23(12):3311. https://doi.org/10.3390/molecules23123311
Chicago/Turabian StyleSumitha, Samuggam, Sethu Vasanthi, Sivadasan Shalini, Suresh V. Chinni, Subash C.B. Gopinath, Periasamy Anbu, Mohammed Baidi Bahari, Rajak Harish, Sathasivam Kathiresan, and Veerasamy Ravichandran. 2018. "Phyto-Mediated Photo Catalysed Green Synthesis of Silver Nanoparticles Using Durio Zibethinus Seed Extract: Antimicrobial and Cytotoxic Activity and Photocatalytic Applications" Molecules 23, no. 12: 3311. https://doi.org/10.3390/molecules23123311
APA StyleSumitha, S., Vasanthi, S., Shalini, S., Chinni, S. V., Gopinath, S. C. B., Anbu, P., Bahari, M. B., Harish, R., Kathiresan, S., & Ravichandran, V. (2018). Phyto-Mediated Photo Catalysed Green Synthesis of Silver Nanoparticles Using Durio Zibethinus Seed Extract: Antimicrobial and Cytotoxic Activity and Photocatalytic Applications. Molecules, 23(12), 3311. https://doi.org/10.3390/molecules23123311