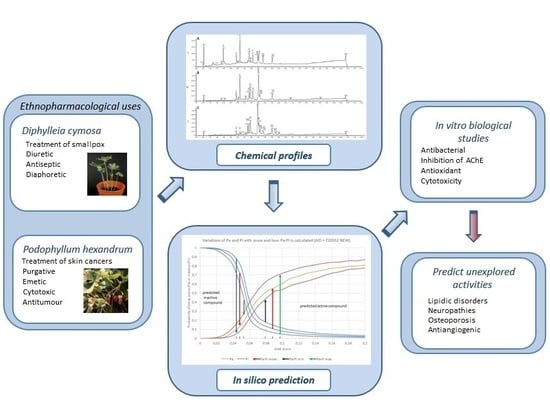
Combined In Vitro Studies and in Silico Target Fishing for the Evaluation of the Biological Activities of Diphylleia cymosa and Podophyllum hexandrum
Abstract
1. Introduction
2. Results
2.1. Chromatographic Profiling by UPLC-DAD-ESI-MS/MS
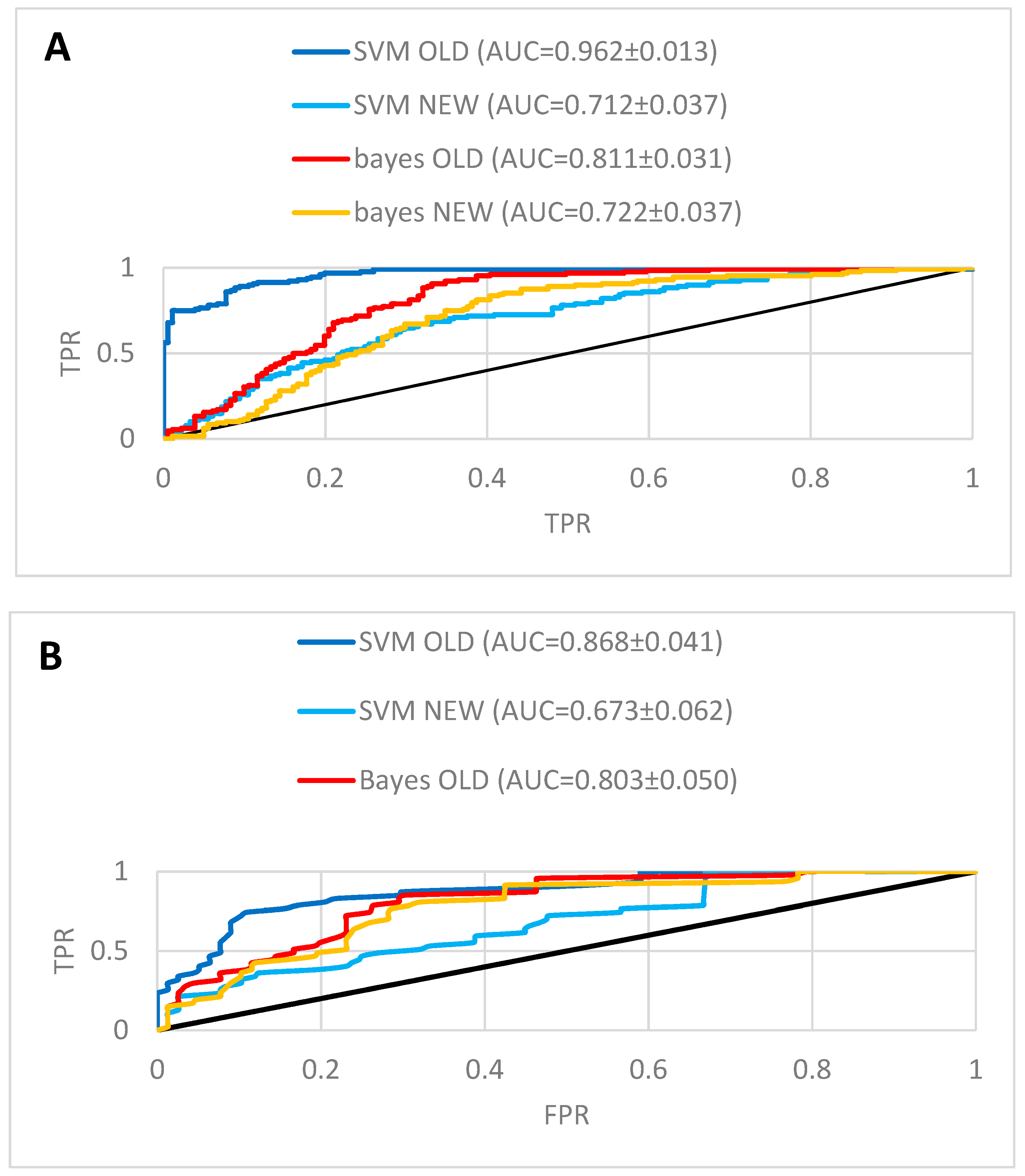
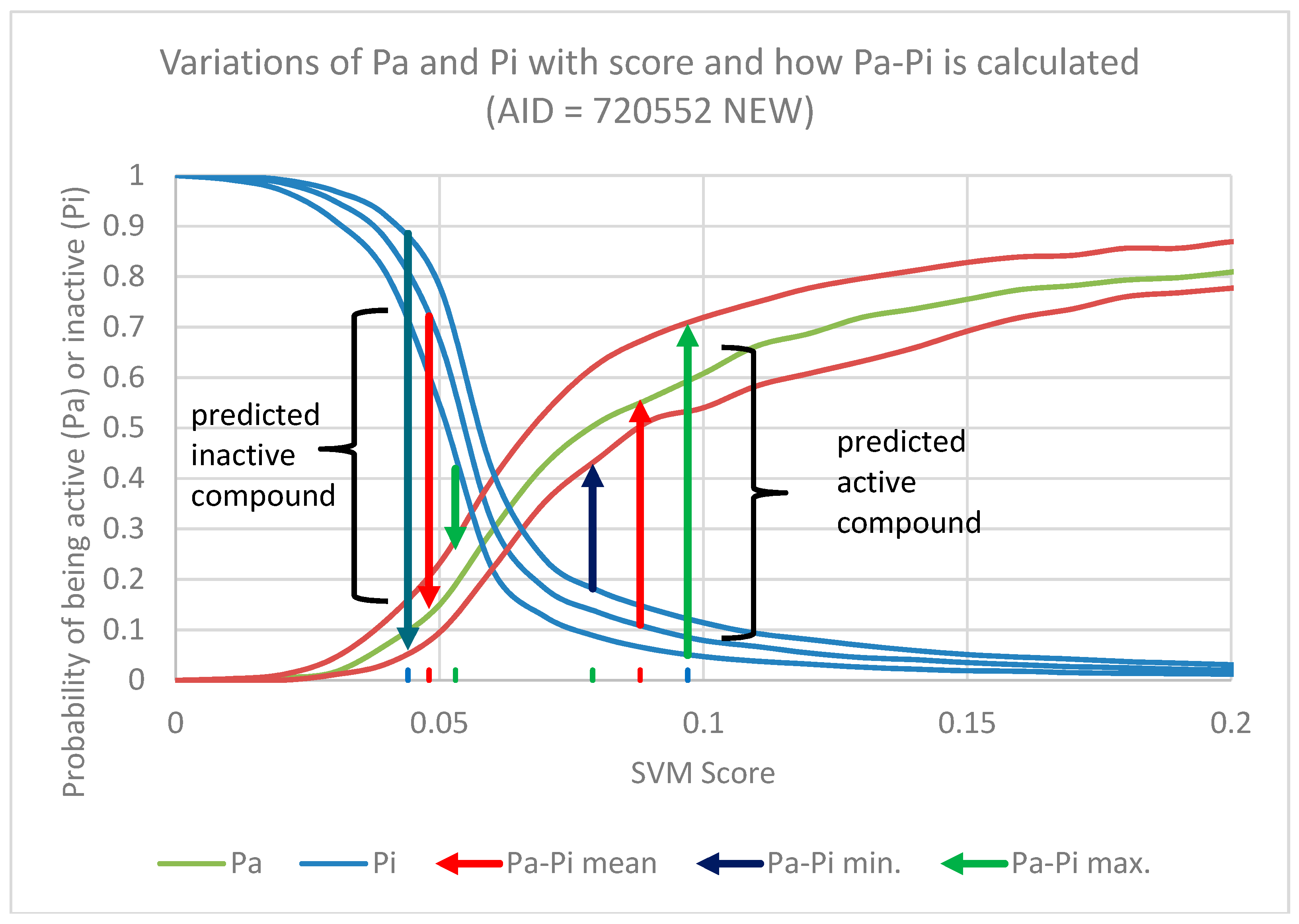
2.2. In Silico Prediction of Biological Activity of Lignans
- (1)
- F-score is a measure of the accuracy of the test, calculated by the harmonic mean of recall or sensitivity [TP/(TP + FN)] and precision [TP/(TP + FP)];
- (2)
- Matthews Correlation Coefficient (MCC) is a balanced measure of the quality of binary classification and is the most informative single score to establish the quality of a binary classifier prediction in a confusion matrix context [38];
- (3)
- Enrichment Factor (EF) is a measure of how many more active compounds we find relative to a random distribution, it is calculated from the proportion of true active compounds selected in relation to the proportion of true active compounds in the entire dataset [39].
2.3. Antibacterial Activity
2.4. Anticholinesterasic Activity
2.5. Antioxidant Activity
2.6. Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
4.3. Chromatographic Characterization of D. cymosa and P. hexandrum Extracts by UPLC-DAD-ESI-MS/MS
4.3.1. Chromatographic Conditions
4.3.2. Mass Spectrometric Conditions
4.4. In Silico Studies
4.4.1. In Silico Prediction of Biological Activity of Lignans
4.4.2. In Silico Prediction of Putative Activity Classes of Lignans
4.5. Evaluation of Antibacterial Activity
4.6. Inhibition of Acetylcholinesterase
4.6.1. Bioautographic Assay
4.6.2. Microplate Assay
4.7. Evaluation of Antioxidant Activity
4.7.1. β-Carotene/Linoleic Acid Co-Oxidation Assay
4.7.2. DPPH Radical Scavenger Activity
4.7.3. Thiobarbituric Acid Reactive Substances (TBARS) Assay
4.8. Evaluation of Cytotoxicity in THP-1 Cells
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The shikimate pathway: Aromatic amino acids and phenylpropanoids. In Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Wiley: Chichester, UK, 2009; pp. 151–156. ISBN 978-0-470-74167-2. [Google Scholar]
- Gordaliza, M.; García, P.A.; del Corral, J.M.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Bohlin, L.; Rosén, B. Podophyllotoxin derivatives: Drug discovery and development. Drug Discov. Today 1996, 1, 343–351. [Google Scholar] [CrossRef]
- Apers, S.; Vlietinck, A.; Pieters, L. Lignans and neolignans as lead compounds. Phytochem. Rev. 2003, 2, 201–217. [Google Scholar] [CrossRef]
- Lv, M.; Xu, H. Recent advances in semisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: An update (2008–2010). Mini-Rev. Med. Chem. 2011, 11, 901–909. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Tian, J.; Qian, K.; Zhao, X.-B.; Morris-Natschke, S.L.; Yang, L.; Nan, X.; Tian, X.; Lee, K.-H. Recent progress on C-4-modified podophyllotoxin analogs as potent antitumor agents. Med. Res. Rev. 2015, 35, 1–62. [Google Scholar] [CrossRef]
- Hui, J.; Zhao, Y.; Zhu, L. Synthesis and in vitro anticancer activities of novel aryl-naphthalene lignans. Med. Chem. Res. 2012, 21, 3994–4001. [Google Scholar] [CrossRef]
- Hemmati, S.; Hassan, S. Justicidin B: A promising bioactive lignan. Molecules 2016, 21, 820. [Google Scholar] [CrossRef]
- Silva, C.G.; De Almeida, V.L.; Campana, P.R.V.; Rocha, M.P. Plant cell cultures as producers of secondary metabolites: Podophyllum lignans as a model. In Transgenesis and Secondary Metabolism, Reference Series in Phytochemistry, 1st ed.; Jha, S., Ed.; Springer International Publishing: Basel, Switzerland, 2017; pp. 67–102. ISBN 978-3-319-28668-6. [Google Scholar]
- Li, M.; Ge, L.; Kang, T.; Suna, P.; Xing, H.; Yang, D.; Zhang, J.; Paré, P.W. High-elevation cultivation increases anti-cancer podophyllotoxin accumulation in Podophyllum hexandrum. Ind. Crops Prod. 2018, 121, 338–344. [Google Scholar] [CrossRef]
- Broomhead, A.J.; Dewick, P.M. Tumour-inhibitory aryltetralin lignans in Podophyllum versipelle, Diphylleia cymosa and Diphylleia grayi. Phytochemistry 1990, 29, 3831–3837. [Google Scholar] [CrossRef]
- Shaw, J.M.H. Review of other herbaceous Berberidaceae. Part II. The genus Podophyllum. Part III. In The Genus Epimedium and Other Herbaceous Berberidaceae Including the Genus Podophyllum; Green, P.S., Mathew, B., Eds.; Kew Publishing: London, UK, 2002; pp. 211–314. ISBN 1-84246-039-0. [Google Scholar]
- Meacham, C.A. Phylogeny of the Berberidaceae with an evaluation of classifications. Syst. Bot. 1980, 5, 149–172. [Google Scholar] [CrossRef]
- Shaw, J.M.H. A Taxonomic Revision of Podophyllum L. (Berberidaceae). Master’s Thesis, University of Nottingham, Nottingham, UK, 1996. [Google Scholar]
- Wang, W.; Lu, A.-M.; Ren, Y.; Endress, M.E.; Chena, Z.-D. Phylogeny and classification of Ranunculales: Evidence from four molecular loci and morphological data. Perspect. Plant Ecol. Syst. 2009, 11, 81–110. [Google Scholar] [CrossRef]
- Ying, T.S. On Dysosma and Sinopodophyllum Ying gen. nov. of the Berberidaceae. Acta Phytotaxon. Sin. 1979, 17, 15–22. [Google Scholar]
- Chaurasia, O.P.; Ballah, B.; Tayade, A.; Kumar, R.; Kumar, G.P.; Singh, S.B. Podophyllum L.: An endangered and anticancerous medicinal—An overview. Indian J. Tradit. Knowl. 2012, 11, 234–241. [Google Scholar]
- Lloyd, J.U.; Lloyd, C.G. Diphylleia cymosa. Drugs Med. North Am. 1887, 2, 120–121. [Google Scholar]
- Moerman, D.E. Medicinal Plants of Native America. In Technical Reports, No. 19; University of Michigan Museum of Antropology: Ann Arbor, MI, USA, 1986; Volume 1, p. 354. [Google Scholar]
- Foster, E. Phytogeographic and botanical considerations of medicinal plants in Eastern Asia and Eastern North America. In Herbs, Spices, and Medicinal Plants: Recents, Advances in Botany, Horticulture, and Pharmacology; Cracker, L.E., Sim, J.E., Eds.; Food Product Press: New York, NY, USA, 1989; Volume 4, pp. 115–144. ISBN 1-56022-857-1. [Google Scholar]
- Broomhead, A.J.; Rahman, M.M.A.; Dewick, P.M.; Jackson, D.E.; Lucas, J.A. Biosynthesis of Podophyllum lignans 5: Matairesinol as precussor of Podophyllum lignans. Phytochemistry 1991, 30, 1489–1492. [Google Scholar] [CrossRef]
- Silva, C.G. Tissue Culture and Phytochemical Studies of Podophyllum, Diphylleia and Passiflora Species. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2000. [Google Scholar]
- Botta, B.; Delle Monache, G.; Misiti, D.; Vitali, A.; Zappia, G. Aryltetralin lignans: Chemistry, pharmacology and biotransformations. Curr. Med. Chem. 2001, 8, 1363–1381. [Google Scholar] [CrossRef]
- Barbour, E.R. Exploring the Implications of Climate Change for the Range of an Endemic Plant Species: Threats and Conservation Options. Honors Project. Smith College: Northampton, MA, USA, 2014. Available online: https://scholarworks.smith.edu/theses/28/ (accessed on 1 December 2018).
- Choudhary, D.K.; Kaul, B.L.; Khan, S. Cultivation and conservation of Podophyllum hexandrum—An overview. J. Med. Aromat. Plant Sci. 1998, 20, 1071–1073. [Google Scholar]
- Nadeem, M.; Palni, L.M.S.; Purohit, A.N.; Pandey, H.; Nandi, S.K. Propagation and conservation of Podophyllum hexandrum Royle: An important medicinal herb. Biol. Conserv. 2000, 92, 121–129. [Google Scholar] [CrossRef]
- Majumder, A.; Jha, S. Characterization of podophyllotoxin yielding cell lines of Podophyllum hexandrum. Caryologia 2009, 62, 220–235. [Google Scholar]
- Majumder, A.; Jha, S. Biotechnological approaches for the production of potential anticancer leads podophyllotoxin and paclitaxel: An overview. J. Biol. Sci. 2009, 1, 46–69. [Google Scholar]
- Nadeem, M.; Ram, M.; Alam, P.; Ahmad, M.M.; Mohammad, A.; Al-Qurainy, F.; Khan, S.; Abdin, M.Z. Fusarium solani, P1, a new endophytic podophyllotoxin-producing fungus from roots of Podophyllum hexandrum. Afr. J. Microbiol. Res. 2012, 6, 2493–2499. [Google Scholar] [CrossRef]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-Ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryltetralin lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Online identification of chlorogenic acids, sesquiterpene lactones, and flavonoids in the Brazilian arnica Lychnophora ericoides Mart. (Asteraceae) leaves by HPLC-DAD-MS and HPLC-DAD-MS/MS and a validated HPLC-DAD method for their simultaneous analysis. J. Agric. Food Chem. 2008, 56, 1193–1204. [Google Scholar] [CrossRef]
- Suman, T.; Elangomathavan, R.; Kasipandi, M.; chakkaravarthi, K.; Tamilven, D.; Parimelazhagan, T. Diphyllin: An effective anticandidal agent isolated from Cleistanthus collinus leaf extract. J. Basic Appl. Sci. 2018, 5, 130–137. [Google Scholar] [CrossRef]
- Santos, F.M.; Lopes, J.C.D. Prediction of AMES mutagenicity of small molecules through pharmacophore fingerprints and SVM Modeling. In Proceedings of the 50th International Conference on Medicinal Chemistry, Rouen, France, 2–4 July 2014. [Google Scholar] [CrossRef]
- Rocha, M.P.; Campana, P.R.V.; Scoaris, D.O.; Almeida, V.L.; Lopes, J.C.D.; Silva, A.F.; Pieters, L.; Silva, C.G. Biological activities of extracts from Aspidosperma subincanum Mart. and in silico prediction for inhibition of acetylcholinesterase. Phytother. Res. 2018, 32, 2021–2033. [Google Scholar] [CrossRef]
- Briñez-Ortega, E.; Almeida, V.L.; Lopes, J.C.D.; Castellanos, A.E.B. Partial inclusion of bis (1,10-phenanthroline) silver (I) salicylate in β-cyclodextrin: Spectroscopic characterization, in vitro and in silico antimicrobial evaluation. An. Acad. Bras. Ciênc. 2018. submitted for publication. [Google Scholar]
- Chicco, D. Ten quick tips for machine learning in computational biology. BioData Min. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Lopes, J.C.D.; Dos Santos, F.M.; Martins-José, A.; Augustyns, K.; De Winter, H. The power metric: A new statistically robust enrichment-type metric for virtual screening applications with early recovery capability. J. Cheminform. 2017, 9, 7. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Yu, X.; Che, Z.; Xu, H. Recent advances in the chemistry and biology of podophyllotoxins. Chem. Eur. J. 2017, 23, 4467–4526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Yang, L.; Tian, X. Podophyllotoxin: Current perspectives. Curr. Bioact. Compd. 2007, 3, 37–66. [Google Scholar]
- Song, J.H.; Sun, D.X.; Chen, B.; Ji, D.H.; Pu, J.; Xu, J.; Tian, F.D.; Guo, L. Inhibition of CYP3A4 and CYP2C9 by podophyllotoxin: Implication for clinical drug–drug interactions. J. Biosci. 2011, 36, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Singh, S.K.; Kumar, B.S.; Doble, M. Synthesis, antifungal activity evaluation and QSAR studies on podophyllotoxin derivatives. Cent. Eur. J. Chem. 2007, 5, 880–897. [Google Scholar]
- Medicinal Uses for Podophyllotoxins. Available online: https://patents.google.com/patent/US4788216 (accessed on 1 December 2018).
- Lee, C.T.L.; Lin, V.C.K.; Zhang, S.X.; Zhu, X.K.; VanVliet, D.; Hu, H.; Beers, S.A.; Wang, Z.Q.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-AIDS agents. 29 Anti-HIV activity of modified podophyllotoxin derivatives. Bioorg. Med. Chem. Lett. 1997, 7, 2897–2902. [Google Scholar] [CrossRef]
- Zhu, X.K.; Guan, J.; Xiao, Z.Y.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents. Part 61: Anti-HIV activity of new podophyllotoxin derivatives. Bioorg. Med. Chem. 2004, 12, 4267–4273. [Google Scholar] [CrossRef]
- Hammonds, T.R.; Denyer, S.P.; Jackson, D.E.; Irving, W.L. Studies to show that with podophyllotoxin the early replicative stages of herpes simplex virus type 1 depend upon functional cytoplasmic microtubules. J. Med. Microbiol. 1996, 45, 167–172. [Google Scholar] [CrossRef]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Gomez-Zurita, M.A.; Luz de La Puente, M.; Betancur-Galvis, L.A.; Sierra, J.; Feliciano, A.S. Synthesis, cytotoxicity and antiviral activity of podophyllotoxin analogues modified in the E-ring. Eur. J. Med. Chem. 2003, 38, 899–911. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takahashi, T.; Sawada, Y.; Iida, M.; Matsuda, T.; Kojima, H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol. Pharm. Bull. 2009, 32, 195–202. [Google Scholar] [CrossRef]
- Iwasaki, T.; Kondo, K.; Nishitani, T.; Kuroda, T.; Hirakoso, K.; Ohtani, A.; Takashima, K. Synthesis and hypolipidemic activity of diesters of arylnaphthalene lignan and their heteroaromatic analogs. Chem. Pharm. Bull. 1995, 43, 1701–1705. [Google Scholar] [CrossRef]
- Yang, S.H.; Li, W.; Sumien, N.; Forster, M.; Simpkins, J.W.; Liu, R. Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots. Prog. Neurobiol. 2017, 157, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Sun, Q.; Wang, X.J.; Zhang, S.G.; An, S.S.; Cheng, J.; Gao, R.; Xiao, H. Pharmacological effect of deoxypodophyllotoxin: A medicinal agent of plant origin, on mammalian neurons. NeuroToxicology 2010, 31, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, K.; Hedin, P.J.; Jonsson, L.; Lerndal, T.; Lien, J.; Weitoft, T.; Axelson, M. Endocrine effects of the podophyllotoxin derivative drug CPH 82 (Reumacon w) in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2000, 29, 89–94. [Google Scholar] [PubMed]
- Pugh, N.; Khan, I.A.; Moraes, R.M.; Pasco, D.S. Podophyllotoxin lignans enhance IL-1β but suppress TNF-α mRNA expression in LPS-treated monocytes. Immunopharmacol. Immunotoxicol. 2001, 23, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.B.; You, Y.J.; Ahn, B.Z. Deoxypodophyllotoxin; the cytotoxic and antiangiogenic component from Pulsatilla koreana. Planta Med. 2002, 68, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C.; Correia, B.L.; Stinghen, A.E.M.; Santos, A.M.C. Acetylcholinesterase inhibitory activity of uleine from Himantanthus lancifolius. Z. Naturforsch. 2010, 65c, 440–444. [Google Scholar] [CrossRef]
- Trevisan, M.T.S.; Macedo, F.V.V.; Meent, M.V.; Rhee, I.K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from plants to treat Alzheimer’s disease. Quim. Nova 2003, 26, 301–304. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, E0197563. [Google Scholar] [CrossRef]
- Vellosa, J.C.R.; Regasini, L.O.; Khalil, N.M.; Bolzani, V.S.; Khalil, O.A.K.; Manente, F.A.; Pasquini Neto, H.; Oliveira, O.M.M.F. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclet. Quim. 2011, 36, 7–20. [Google Scholar] [CrossRef]
- Dar, R.A.; Brahman, P.K.; Khurana, N.; Wagay, J.A.; Lone, Z.A.; Ganaie, M.A.; Pitre, K.S. Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arab. J. Chem. 2017, 10, S1119–S1128. [Google Scholar] [CrossRef]
- Sharma, E.; Arora, B.S. Identification of aryltetralin lignans from Podophyllum hexandrum using hyphenated techniques. Int. J. Pharm. Sci. Drug Res. 2015, 7, 83–88. [Google Scholar]
- Sharma, E.; Kumar, A. Identification and quantification of podophyllotoxin from Podophyllum hexandrum by ESI-LC/MS/MS. Int. J. Appl. Phis. Biochem. Res. 2015, 5, 1–8. [Google Scholar]
- Avula, B.; Wang, Y.H.; Moraes, R.M.; Khan, I.A. Rapid analysis of lignans from leaves of Podophyllum peltatum L. samples using UPLC-UV-MS. Biomed. Chromatogr. 2011, 25, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, N.; He, W.; Li, R.; Shi, D.; Pang, L.; Dong, N.; Xu, H.; Ji, H. Simultaneous determination of three major lignans in rat plasma by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Diphylleia sinensis extract. Biomed. Chromatogr. 2014, 28, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Sultan, P.; Shawl, A.S.; Jan, A.; Hamid, B.; Irshad, H. Germplasm conservation and quantitative estimation of podophyllotoxin and related glycosides of Podophyllum hexandrum. Crop Sci. 2014, 12, 267–274. [Google Scholar]
- Nanjundaswamy, N.; Satishi, S.; Rai, K.M.L.; Shashikanth, S.; Raveesha, K.A. Antibacterial activity of synthetic precursors of podophyllotoxin. Int. J. Biomed. Sci. 2007, 3, 112–115. [Google Scholar] [PubMed]
- Ahmad, T.; Salam, M.D. Antimicrobial activity of methanolic and aqueous extracts of Rheum emodi and Podophyllum hexandrum. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 182–185. [Google Scholar]
- Umesha, B.; Basavaraju, Y.B.; Mahendra, C.; Shivakumar, S.B.; Rao, K.P.; Krishna, M.H. Synthesis and biological activity of novel nitrogen containing analogues of podophyllotoxin. Indo Am. J. Pharm. Res. 2014, 4, 905–914. [Google Scholar]
- Miller, S.I.; Mekalanos, J.J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 1990, 172, 2485–2490. [Google Scholar] [CrossRef]
- Shi, C.; Geders, T.W.; Park, S.W.; Wilson, D.J.; Boshoff, H.I.; Abayomi, O.; Barry, C.E., III; Schnappinger, D.; Finzel, B.C.; Aldrich, C.C. Mechanism-based inactivation by aromatization of the transaminase BioA involved in biotin biosynthesis in Mycobaterium tuberculosis. J. Am. Chem. Soc. 2011, 133, 18194–18201. [Google Scholar] [CrossRef]
- Reuter, K.; Steinbach, A.; Helms, V. Interfering with Bacterial Quorum Sensing. Perspect. Med. Chem. 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Bolós, M.; Llorens-Martín, M.; Jurado-Arjona, J.; Hernández, F.; Rábano, A.; Avila, J. Direct evidence of internalization of Tau by microglia in vitro and in vivo. J. Alzheimers Dis. 2016, 50, 77–87. [Google Scholar] [CrossRef]
- Gordaliza, M.; Faircloth, G.T.; Castro, M.A.; Miguel del Corral, J.M.; Ljpez-Vazquez, M.L.; San Feliciano, A. Immunosuppressive cyclolignans. J. Med. Chem. 1996, 39, 2865–2868. [Google Scholar] [CrossRef]
- Gordaliza, M.; Castro, M.A.; Miguel del Corral, J.M.; López-Vazquez, M.L.; San Feliciano, A.; Faircloth, G.T. In vico immunosuppressive activity of some cyclolignans. Bioorg. Med. Chem. Lett. 1997, 72, 2781–2786. [Google Scholar] [CrossRef]
- Sharma, N.; Akhade, A.S.; Qadri, A. Sphingosine-1-phosphate suppresses TLR-induced CXCL8 secretion from human T cells. J. Leukoc. Biol. 2013, 93, 521–528. [Google Scholar] [CrossRef]
- Kaplan, I.W. Condylomata acuminata. New Orleans Med. Surg. J. 1942, 94, 388–390. [Google Scholar]
- Iwasaki, T.; Kondo, K.; Nishitani, T.; Kuroda, T.; Hirakoso, K.; Ohtani, A.; Takashima, K. Arylnaphtalene lignans as novel series of hypolipidemic agents raising high-density lipoprotein level. Chem. Pharm. Bull. 1995, 43, 1701–1705. [Google Scholar] [CrossRef]
- Granneman, J.G.; Moore, H.P.H.; Mottillo, E.P.; Zhu, Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem. 2009, 284, 3049–3057. [Google Scholar] [CrossRef]
- Barbosa-Filho, B.M.J.; Medeiros, K.C.P.; Diniz, M.F.F.M.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Junior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Braz. J. Pharmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef]
- Hung, T.T.; Na, M.; Min, B.S.; Ngoc, T.M.; Lee, I.; Zhang, X.; Bae, K. Acetylcholinesterase inhibitory effect of lignans isolated from Schizandra chinensis. Arch. Pharm. Res. 2007, 30, 685–690. [Google Scholar] [CrossRef]
- Itoh, K.; Ishige, A.; Hosoya, E. Cerebral Function Improving Drug. Patent-PCT Int Appl-89 08. 21 September 1989. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=EP&NR=0400148A1&KC=A1&FT=D&ND=3&date=19901205&DB=EPODOC&locale=en_EP (accessed on 1 December 2018).
- El-Hassan, A.; El-Sayed, M.; Hamed, A.I.; Rhee, I.K.; Ahmed, A.A.; Zeller, K.P.; Verpoorte, R. Bioactive constituents of Leptadenia arborea. Fitoterapia 2003, 74, 184–187. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H.; Zulkifli, R.M. Anticholinesterase and anti-inflammatory constituents from Beilschmiedia pulverulenta. Nat. Prod. Sci. 2016, 22, 225–230. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Li, W.; Wang, C.; Li, H.; Ju, W.; Chen, J.; Sun, J. Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenanthera from different locations. Chem. Biodivers. 2018, 15, e1800030. [Google Scholar] [CrossRef]
- Horizonte, B. 3D-Pharma: Uma Ferramenta Para Triagem Virtual Baseada em Fingerprints de Farmacóforos. Available online: http://www.bibliotecadigital.ufmg.br/dspace/handle/1843/BUBD-9DKHDA (accessed on 1 December 2018).
- Shemetulskis, N.E.; Weininger, D.; Blankley, C.J.; Yang, J.J.; Humblet, C. Stigmata: An algorithm to determine structural commonalities in diverse datasets. J. Chem. Inf. Comput. Sci. 1996, 36, 862–871. [Google Scholar] [CrossRef]
- Bolton, E.E.; Kim, S.; Bryant, S.H. PubChem3D: Conformer generation. J. Cheminform. 2011, 3, 4. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Nicholls, A. Conformer Generation with OMEGA: Learning from the data set and the analysis of failures. J. Chem. Inf. Model. 2012, 52, 2919–2936. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer generation with OMEGA: Algorithm and validation using high quality structures from the protein databank and Cambridge structural database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef]
- Santos, F.M.; De Winter, H.; Augustyns, K.; Lopes, J.C.D. Use of Extensive Cross-Validation and Bootstrap Application (ExCVBA) for Molecular Modeling of Some Pharmacokinetics Properties. Available online: https://www.researchgate.net/profile/Julio_Lopes2/publication/282644866_2015_-_Poster_OpenTox-_Use_of_Extensive_Cross-Validation_and_Bootstrap_Application_ExCVBA_for_Molecular_Modeling_of_Some_Pharmacokinetics_Properties/data/561518bd08aec622441191cc/2015-Poster-OpenTox-Use-of-Extensive-Cross-Validation-and-Bootstrap-Application-ExCVBA-for-Molecular-Modeling-of-Some-Pharmacokinetics-Properties.pdf (accessed on 1 December 2018).
- Nicholls, A. What do we know?: Simple statistical techniques that help. Methods Mol. Biol. 2011, 672, 531–581. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Nicholls, A. Confidence limits, error bars and method comparison in molecular modeling. Part 1: The calculation of confidence intervals. J. Comput. Aided Mol. Des. 2014, 28, 887–918. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A Library for Support Vector Machines. ACM Transactions on Intelligent Systems and Technology, 2:27:1-27:27. 2011. Available online: http://www.csie.ntu.edu.tw/~cjlin/libsvm (accessed on 1 December 2018).
- De Winter, H.; Lopes, J.C.D. Reply to the comment made by Šicho, Vorśilák and Svozil on ‘The Power metric: A new statistically robust enrichment-type metric for virtual screening applications with early recovery capability’. J. Cheminform. 2018, 10, 14. [Google Scholar] [CrossRef]
- CPAN 2017. The Comprehensive Perl Archive Network. Available online: http://search.cpan.org/perldoc? Algorithm%3A%3ANaiveBayes (accessed on 19 November 2018).
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Norinder, U.; Carlsson, L.; Boyer, S.; Eklund, M. Introducing conformal prediction in predictive modeling. A transparent and flexible alternative to applicability domain determination. J. Chem. Inf. Model. 2014, 54, 1596–1603. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; ISBN 1-56238-783-9. [Google Scholar]
- Fukuda, M.; Ohkoshi, E.; Makino, M.; Fujimoto, Y. Studies on the constituents of the leaves of Baccharis dracunculifolia (Asteraceae) and their cytotoxic activity. Chem. Pharm. Bull. 2006, 54, 1465–1468. [Google Scholar] [CrossRef]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautography method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Junior, V.A.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Santos, R.J.; Genovese, M.I.; Lajolo, F.M. Avaliação da atividade antioxidante utilizando o sistema β-caroteno/ácido linoleico e método de sequestro de radicais DPPH. Cienc. Tecnol. Aliment. 2006, 26, 446–452. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.; Coube, C.S.; Leitão, S.G. Screnning of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
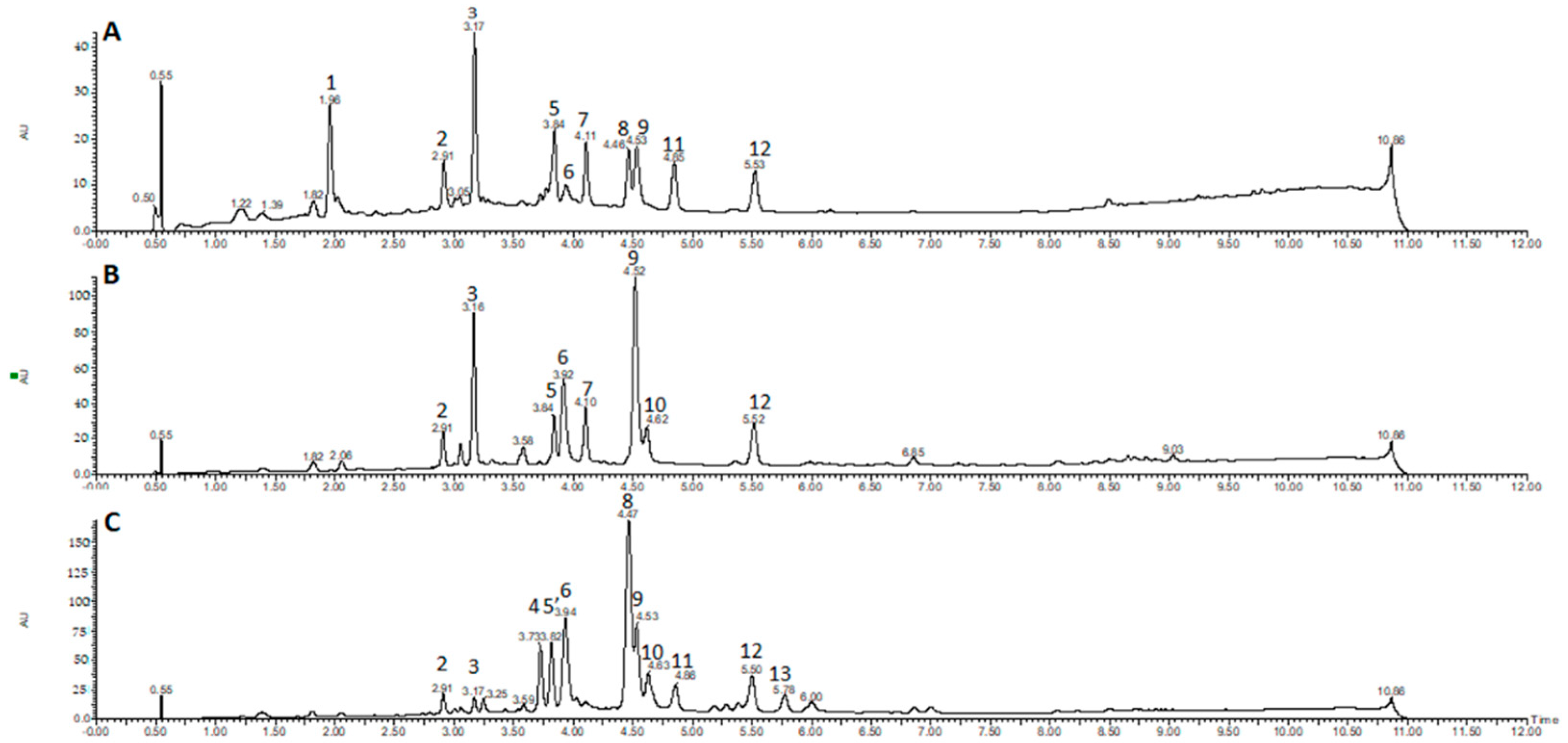
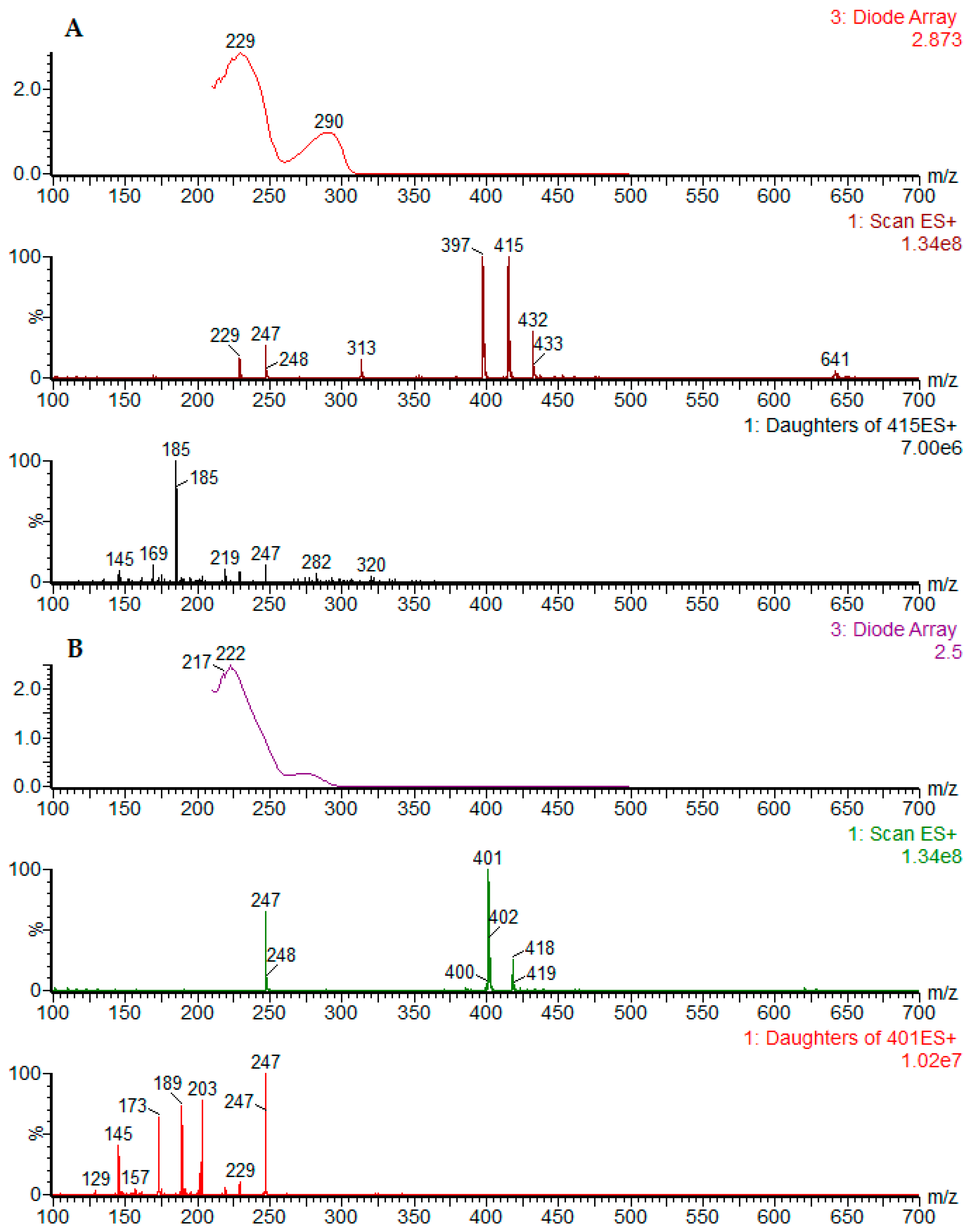
| Peak No. | Sample | RT (min) | Identity | [M − H]− Parent Ion | [M + H]+ Parent Ion | MS2 Fragments Negative Mode (Daughter Ion) | MS2 Fragments Positive Mode (Daughter Ion) | UV (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | D. cymosa leaves | 1.96 | caffeoylquinic acid | 353 | 355 | 191 [M − H − caffeoyl] 179 [M − H − quinic] | - | 217, 246, 295, 326 |
| 2 | D. cymosa leaves D. cymosa roots P. hexandrum rhizomes and roots | 2.91 | quercetin hexoside | 463 | 465 | - | 303 [M + H − hexose] | 255, 352 |
| 3 | D. cymosa leaves D. cymosa roots P. hexandrum rhizomes and roots | 3.17 | kaempferol hexoside | 447 | 449 | 285 [M − H − hexose] | 287 pM + H − hexose] | 265, 348 |
| 4 | P. hexandrum rhizomes and roots | 3.73 | podophyllotoxin hexoside | 575621 [M + formiate]− | 577 | 413 [M − H − hexose] | - | 290 |
| 5 | D. cymosa leaves D. cymosa roots | 3.84 | diphyllin hexoside | - | 543 | - | 381 [M + H − hexose] 363, 333, 319 | 261 |
| 5′ | P. hexandrum rhizomes and roots | 3.84 | 4′-demethylPTOX | 399 | 401 | - | - | 287 |
| 6 | D. cymosa leaves D. cymosa roots P. hexandrum rhizomes and roots | 3.92 | α-peltatin | 399 | 401 | - | 247 [M + H − C9H12O3] | 275 |
| 7 | D. cymosa leaves D. cymosa roots | 4.11 | diphyllin hexoside | - | 543 | - | 381 [M + H − hexose] 363, 333, 319 | 261 |
| 8 | D. cymosa leaves P. hexandrum rhizomes and roots | 4.46 | podophyllotoxin | 459 [M + formiate]− | 415 | 247 [M + H − C9H12O3] 185 (247-H2O-CO2) | 290 | |
| 9 | D. cymosa leaves D. cymosa roots P. hexandrum rhizomes and roots | 4.53 | kaempferol | 285 | - | - | 265, 366 | |
| 10 | D. cymosa roots P. hexandrum rhizomes and roots | 4.62 | β-peltatin | 413 | 415 | - | 247 [M + H − C9H12O3] 185 (247-H2O-CO2) | 275 |
| 11 | P. hexandrum rhizomes and roots | 4.86 | isopicropodophyllone | 411 | 413 | - | - | 290 |
| 12 | D. cymosa leaves D. cymosa roots P. hexandrum rhizomes and roots | 5.52 | diphyllin | 379 | 381 | 319, 291, 275 | 363, 333, 319 | 261 |
| 13 | P. hexandrum rhizomes and roots | 5.78 | deoxypodophyllotoxin | 444 [M + formiate]− | - | - | 291 |
| Method | Dataset | Full Experimental | Activity Classes | ||
|---|---|---|---|---|---|
| AUC | Number of Data Points | AUC | Number of Data Points | ||
| Naïve Bayes | NEW a | 0.730 ± 0.037 | 292 | 0.767 ± 0.054 | 125 |
| Naïve Bayes | OLD b | 0.816 ± 0.031 | 292 | 0.803 ± 0.050 | 125 |
| SVM | NEW a | 0.710 ± 0.037 | 305 | 0.673 ± 0.062 | 125 |
| SVM | OLD b | 0.962 ± 0.014 | 305 | 0.868 ± 0.041 | 125 |
| Method | Dataset | TP | FP | TN | FN | F-Score | MCC | EF |
|---|---|---|---|---|---|---|---|---|
| Naïve Bayes | NEW a | 30 | 17 | 151 | 94 | 0.35 | 0.19 | 1.54 |
| SVM | NEW a | 46 | 21 | 159 | 79 | 0.48 | 0.30 | 1.66 |
| Both | NEW a | 60 | 33 | 148 | 68 | 0.54 | 0.31 | 1.56 |
| Naïve Bayes | OLD b | 51 | 17 | 151 | 73 | 0.53 | 0.36 | 1.81 |
| SVM | OLD b | 106 | 18 | 162 | 19 | 0.85 | 0.75 | 2.06 |
| Both | OLD b | 112 | 33 | 148 | 16 | 0.82 | 0.68 | 1.87 |
| Studies | Etnopharmacological Uses, Predicted and Observed Biological Activities | Literature | |
|---|---|---|---|
| Ethnopharmacological uses | P. hexandrum: Treatment of diarrhoea and liver problems, to promote conception, eye treatment, chronic constipation, hepatic stimulant, antitumour, purgative, cholagogue and purgative | [11,24] | |
| D. cymosa: Diuretic, antiseptic, diaphoretic and for the treatment of smallpox | [21,24] | ||
| Biological activities reported in the literature | Antitumour, insecticidal, antimalarial, fungicidal, antiviral, anti-inflammatory, neurotoxic, immunosuppressive, antirheumatic, antispasmogenic and hypolipidemic properties | [41,42] | |
| Predicted biological activities using both machine learning methods | ADMET | Cytochrome P450 3A4 (30/753), human intestinal absorption (48/753) | [43] |
| Antibacterial | S. typhimurium (3/753), M. tuberculosis (4/753), P. aeruginosa (18/753), S. aureus (23/753) | [42] | |
| Antifungal | C. albicans (12/753) | [44] | |
| Antiparasitic | Plasmodium falciparum (5/753), Trypanosoma (15/753), Caenorhabditis elegans (16/753), G. lamblia (44/753) | [45] | |
| Antitumour | Ape1 Endonuclease (6/753), Agonist of p53 (7/753), GLI family zinc finger 1 (11/753), TOR pathway (13/753), RecQ-Like Dna Helicase 1 (RECQ1) (14/753), Microphthalmia-associated transcription factor (17/753), Hsf1 protein (22/753), serine/threonine-protein kinase 33 (25/753), miR-21 (27/753), sentrin-specific protease 8 (31/753), Acute myelogenous leukemia (35/753), Steroid receptor coactivator 3 (40/753), dual specificity protein phosphatase 3 (43/753), leukemia (45/753) | [41,42] | |
| Antivirus | HIV-1 (9/753), herpes (42/753) | [46,47,48,49] | |
| Cytotoxicity and genotoxicity | Lymphoblastoid (2/753), ATAD5 (10/753), isogenic chicken DT40 (19/753), HEK293 (32/753), MAGI-CCR5 (50/753) | [41,42] | |
| Endocrine disorders | Muscleblind-like protein 1 (26/753), estrogen receptor alpha agonist (34/753), estrogen receptor alpha antagonist (38/753), androgen receptor antagonist (46/753), | [50] | |
| Lipid disorders | Regulator of G-protein signaling 8 (28/753), 1-acylglycerol- 3-phosphate O-acyltransferase ABHD5 (41/753) | [51,52] | |
| Neuropathies | Sphingosine 1-phosphate receptor 1 (20/753), regulator of G-protein signaling 4 (33/753), peripheral myelin protein 22 (36/753), Mitochondria permeability (47/753), DNA damage-inducible transcript 3 protein (49/753) | [53] | |
| Others | Angiogenesis (21/753), osteoporosis (29/753) Anti-Inflammatory (1/753) | [5,40,41,42,54,55,56] | |
| Samples | S. aureus Mean ± SD | B. cereus Mean ± SD | E. coli Mean ± SD | EHEC E. coli Mean ± SD | P. aeruginosa Mean ± SD | Salmonella Typhi Mean ± SD |
|---|---|---|---|---|---|---|
| D. cymosa (leaves) | 24.76 ± 5.8 | 0 | 34.30 ± 2.33 | 0 | 23.18 ± 9.94 | 24.88 ± 3.93 |
| D. cymosa (roots) | 67.56 ± 0.8 | 100.68 ± 0.22 | 44.68 ± 8.80 | 16.61 ± 2.13 | 20.41 ± 8.82 | 41.33 ± 8.32 |
| P. hexandrum (rhizomes and roots) | 58.43 ± 2.7 | 100.66 ± 0.28 | 49.28 ± 6.97 | 18.53 ± 5.21 | 22.51 ± 15.91 | 42.52 ± 6.40 |
| Podophyllotoxin | 53.34 ± 8.8 | 0 | 51.57 ± 9.08 | 33.00 ± 7.76 | 30.19 ± 9.39 | 31.62 ± 7.54 |
| Samples | Antioxidant Activity (IC50-µg/mL) | Inhibition of AChE | Cytotoxicity (CC50) | |||
|---|---|---|---|---|---|---|
| β-Carotene/linoleic Acid | DPPH Radical Sequestration | TBARS Assay | TLC | Microplate (%I) | ||
| D. cymosa (leaves) | 19.48 ± 5.90 | 133.94 ± 25.60 | >50 | + | 64.22 ± 4.87 | 368.0 ± 13.8 |
| D. cymosa (roots) | 20.76 ± 1.76 | 43.77 ± 6.69 | 10.20 ± 1.46 | - | 40.86 ± 3.70 | 100.0 ± 5.3 |
| P. hexandrum (rhizomes and roots) | 30.70 ± 2.12 | 24.66 ± 4.45 | 13.66 ± 1.35 | ++++ | 47.04 ± 3.17 | 338.9 ± 15.1 |
| Podophyllotoxin | >200 | >200 | >50 | - | 32.73 ± 5.38 | 400 ± 10.3 |
| Quercetin | 0.3 ± 0.1 | NA | NA | NA | NA | NA |
| Pyrogallol | NA | 1.14 ± 0.15 | NA | NA | NA | NA |
| Propylgalate | NA | NA | <20 | NA | NA | NA |
| Physostigmine | NA | NA | NA | ++++ | 89.81 ± 1.16 | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, M.P.; Campana, P.R.V.; Scoaris, D.d.O.; Almeida, V.L.d.; Lopes, J.C.D.; Shaw, J.M.H.; Silva, C.G. Combined In Vitro Studies and in Silico Target Fishing for the Evaluation of the Biological Activities of Diphylleia cymosa and Podophyllum hexandrum. Molecules 2018, 23, 3303. https://doi.org/10.3390/molecules23123303
Rocha MP, Campana PRV, Scoaris DdO, Almeida VLd, Lopes JCD, Shaw JMH, Silva CG. Combined In Vitro Studies and in Silico Target Fishing for the Evaluation of the Biological Activities of Diphylleia cymosa and Podophyllum hexandrum. Molecules. 2018; 23(12):3303. https://doi.org/10.3390/molecules23123303
Chicago/Turabian StyleRocha, Marina Pereira, Priscilla Rodrigues Valadares Campana, Denise de Oliveira Scoaris, Vera Lucia de Almeida, Julio Cesar Dias Lopes, Julian Mark Hugh Shaw, and Claudia Gontijo Silva. 2018. "Combined In Vitro Studies and in Silico Target Fishing for the Evaluation of the Biological Activities of Diphylleia cymosa and Podophyllum hexandrum" Molecules 23, no. 12: 3303. https://doi.org/10.3390/molecules23123303
APA StyleRocha, M. P., Campana, P. R. V., Scoaris, D. d. O., Almeida, V. L. d., Lopes, J. C. D., Shaw, J. M. H., & Silva, C. G. (2018). Combined In Vitro Studies and in Silico Target Fishing for the Evaluation of the Biological Activities of Diphylleia cymosa and Podophyllum hexandrum. Molecules, 23(12), 3303. https://doi.org/10.3390/molecules23123303