The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials
Abstract
1. Introduction
2. Neurodegenerative Disease
2.1. A Burden on Health Organizations without Current Cure
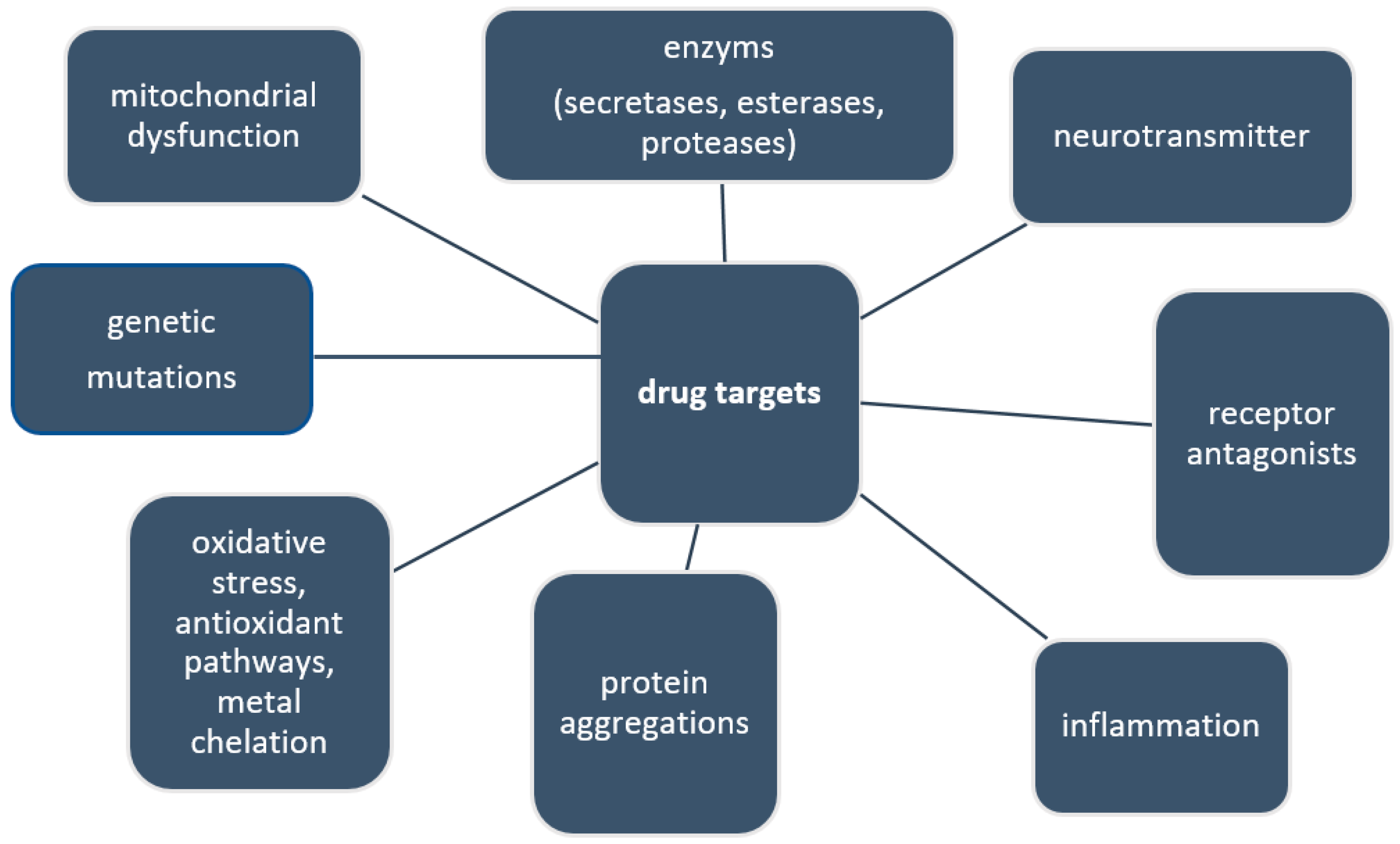
2.2. Current and Future Drug Targets
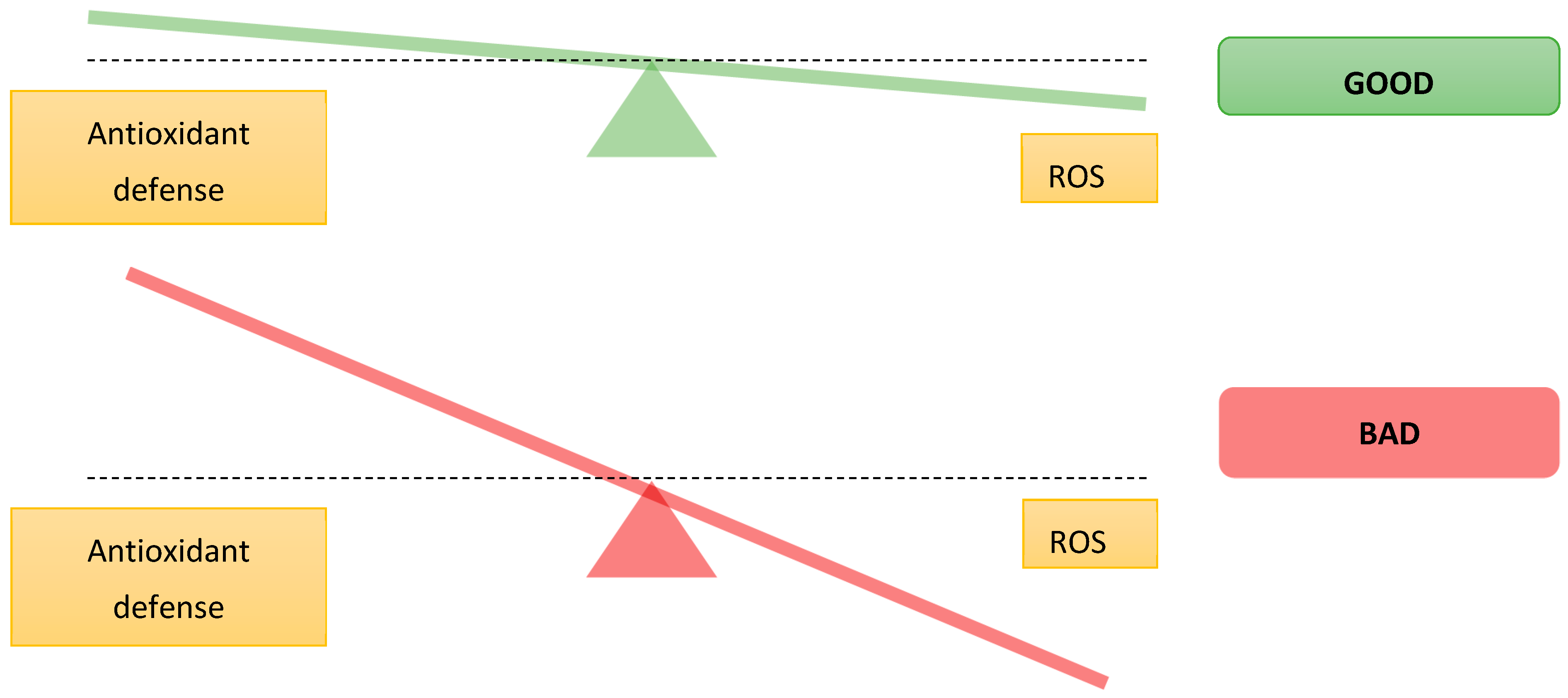
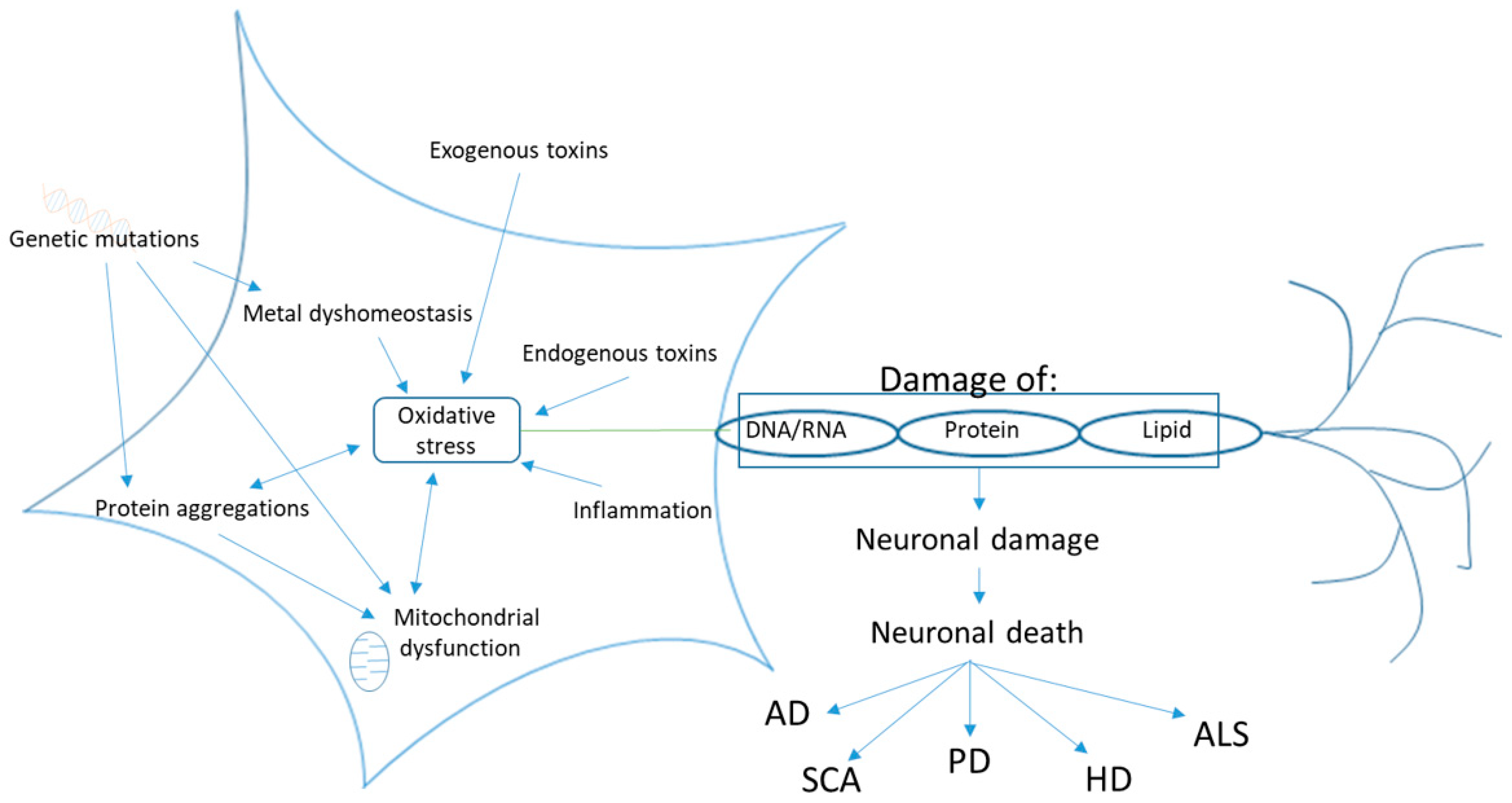
2.3. Oxidative Stress
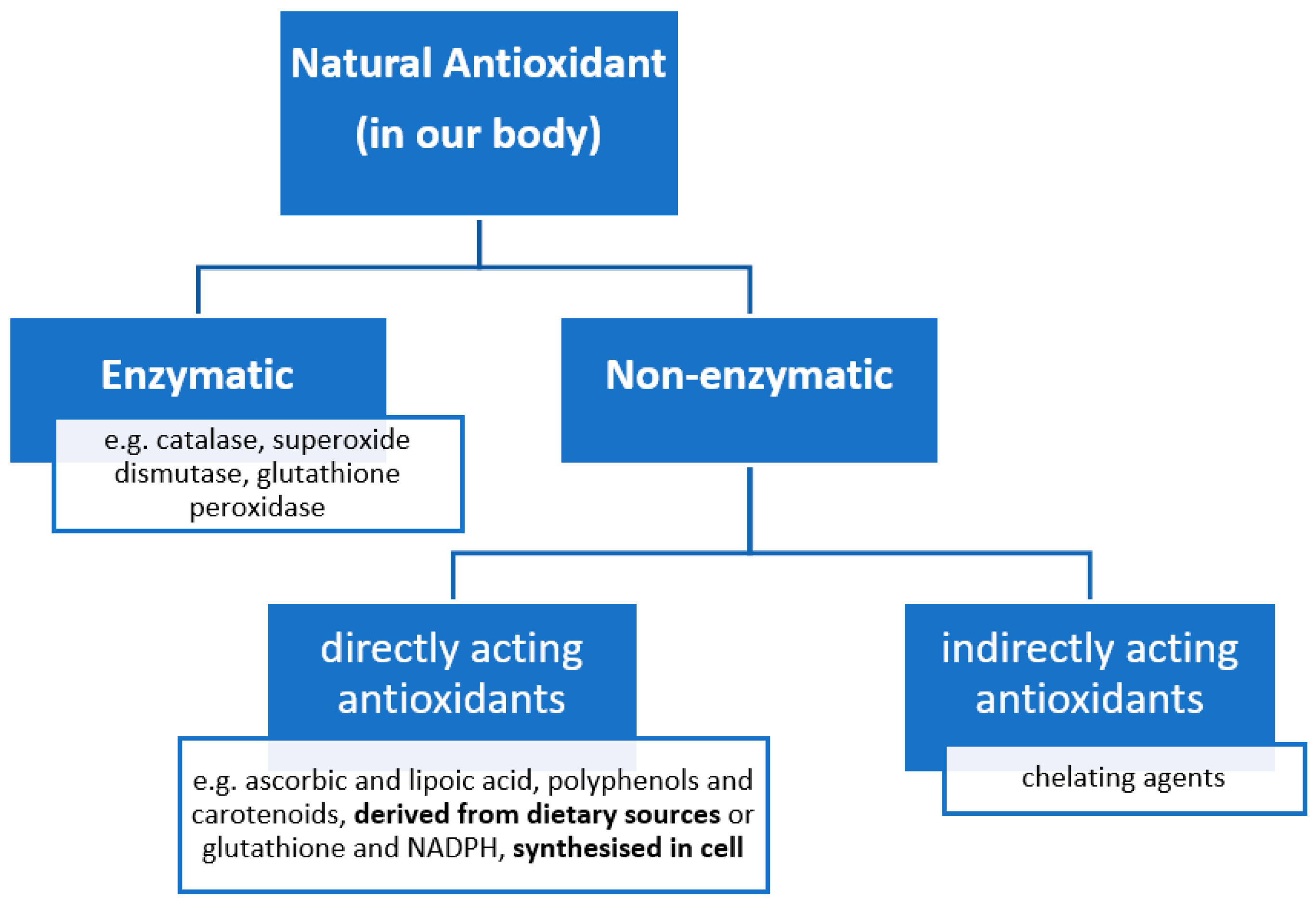
2.4. Antioxidants to Counteract Oxidative Stress
2.5. Natural Product Drug Discovery
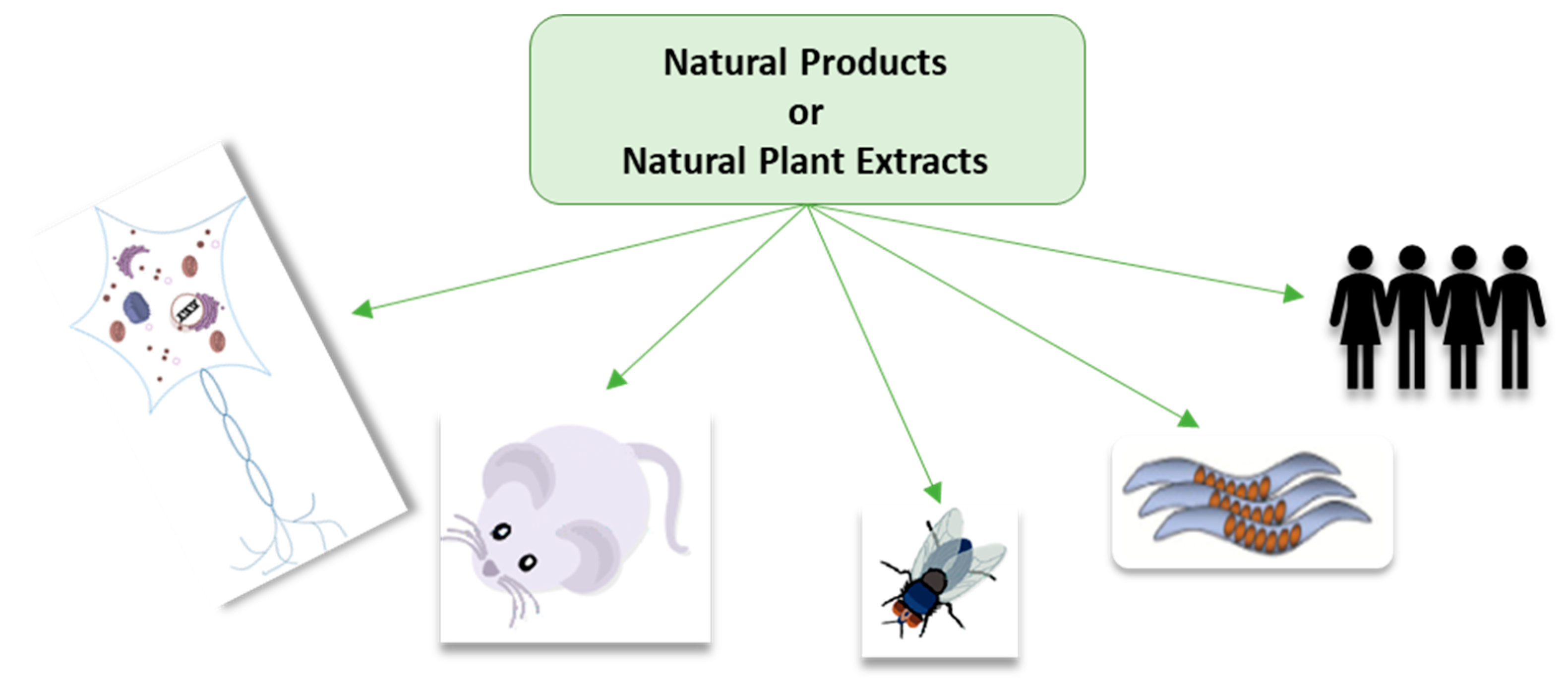
3. In Vitro and In Vivo Antioxidant Activity of Plant Natural Products and Extracts
3.1. In Vitro Cell-Based Research
3.2. In Vivo Drosophila Models
3.3. In Vivo C. elegans Models
3.4. In Vivo Rodent Models
4. Clinical Trials: Positive vs. Negative Outcomes
5. Ginkgo Biloba Extract EGb 761®: A Plant Extract Story with Varying Clinical Trial Outcomes
5.1. In Vitro Activity of Ginkgo Biloba
5.2. In Vivo Activity of Ginko Biloba
5.3. Clinical Trials of Ginko Biloba
6. Conclusion/Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamin |
| AAPH | 2,2′-Azobis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADAS-Cog | AD Assessment Scale cognitive subscale |
| ADL | activities of daily living |
| AEP | asparagine endopeptidase |
| ALS | Amyotrophic lateral sclerosis/MND motor neurone disease |
| APP | amyloid precursor protein |
| APP/PS1 | AD mouse model |
| Aβ | amyloid beta |
| α-syn | alpha-synuclein |
| CAT | catalase |
| CCl4 | carbon tetrachloride |
| CHP | cumene hydroperoxide |
| CRISP | clustered regularly interspaced short palindromic repeats |
| DPPH | 2,2-diphenylpicrylhydrazyl |
| ERK | extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| FTLD | frontotemporal lobar degeneration |
| GAE | gallic acid equivalence |
| GCLM | Glutamate-cysteine ligase regulatory subunit |
| GFP | green fluorescence protein |
| GSH | glutathione |
| HCH | hexachlorocyclohexane |
| HD | Huntington’s disease |
| HO-1 | heme oxygenase-1 |
| HPLC | high performance liquid chromatography |
| L-DOPA | Levodopa |
| LRRK2 | leucine-rich repeat kinase-2 |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MJD | Machado-Joseph disease |
| SCA3 | Spinocerebellar ataxia type 3 |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MS | mass spectrometry |
| NCCIH | National Centre for Complementary and Integrative Health |
| NGF | nerve growth factor |
| NMDA | N-Methyl-D-aspartate |
| NMR | nuclear magnetic resonance (spectroscopy) |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| PD | Parkinsn’s disease |
| PI3K/Akt/GSK3 | Phosphoinositide 3-kinase/RAC-alpha serine/threonine-protein kinase/Glycogen synthase kinase 3 |
| PKC | protein kinase C |
| RNAi | RNA interference |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SCA | Spinocerebellar ataxia |
| SIRT1 | sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae) |
| SOD | superoxide dismutase |
| tBH | tert-butyl-hydroperoxide |
| UV | ultra violet |
References
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Prince, M.; Guerchet, M.; Prina, M. World Alzheimer Report 2013; Alzheimer’s Disease International: London, UK, 2013. [Google Scholar]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Prince, M.; Jackson, J. World Alzheimer Report; Alzheimer’s Disease International: London, UK, 2009. [Google Scholar]
- Rafii, M.S.; Aisen, P.S. Recent developments in Alzheimer’s disease therapeutics. BMC Med. 2009, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.H. (FDA) Namzaric Drug Approval (NDA 206439) 2014. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/in (accessed on 8 December 2018).
- Espinoza-Fonseca, L.M. The benefits of the multi-target approach in drug design and discovery. Bioorg. Med. Chem. 2006, 14, 896–897. [Google Scholar] [CrossRef] [PubMed]
- CenterWatch FDA Approved Drugs by Medical Condition. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/medical-conditions/ (accessed on 8 November 2018).
- Aguzzi, A.; Lakkaraju, A.K.K.; Frontzek, K. Toward therapy of human prion diseases. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Sureda, F.X.; Junyent, F.; Verdaguer, E.; Auladell, C.; Pelegri, C.; Vilaplana, J.; Folch, J.; Canudas, A.M.; Zarate, C.B.; Pallès, M.; et al. Antiapoptotic drugs: A therapautic strategy for the prevention of neurodegenerative diseases. Curr. Pharm. Des. 2011, 17, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Lublin, A.L.; Link, C.D. Alzheimer’s disease drug discovery: in vivo screening using Caenorhabditis elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov. Today Technol. 2013, 10, e115–e119. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, O.M.; Esteban, G.; Chioua, M.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Soriano, E.; Samadi, A.; Unzeta, M.; et al. Multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s disease: Design, synthesis, biochemical evaluation, ADMET, molecular modeling, and QSAR analysis of novel donepezil-pyridyl hybrids. Drug Des. Devel. Ther. 2014, 8, 1893–1910. [Google Scholar] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Kaminsky, Y.G.; Bragin, V.; Kosenko, E.A.; Klochkov, S.G.; Bachurin, S.O.; Benberin, V.V. Flavones from the root of Scutellaria baicalensis Georgi—drug of the future in neurodegeneration and neuroprotection? In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin, Germany, 2014; pp. 2305–2323. [Google Scholar]
- Zhang, Z.; Xie, M.; Ye, K. Asparagine endopeptidase is an innovative therapeutic target for neurodegenerative diseases. Expert Opin. Ther. Targets 2016, 20, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Zunke, F.; Moise, A.C.; Belur, N.R.; Gelyana, E.; Stojkovska, I.; Dzaferbegovic, H.; Toker, N.J.; Jeon, S.; Fredriksen, K.; Mazzulli, J.R. Reversible conformational conversion of α-synuclein into toxic assemblies by glucosylceramide. Neuron 2018, 97, 92–107.e10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Mathur, D.; Vinish, M.; Sharma, R.; Bhatia, K.; Pannu, V.; Anand, A. Hype and hopes of stem cell research in neurodegenerative diseases. In Regenerative Medicine: Laboratory to Clinic; Springer: Singapore, 2017; pp. 209–231. [Google Scholar]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G.; Tong, G.; et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005, 11, 551–555. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimer’s Dis. 2010, 20, S357–S367. [Google Scholar] [CrossRef]
- Knight, J.A. Free radicals: Their history and current status in aging and disease. Ann. Clin. Lab. Sci. 1998, 28, 331–346. [Google Scholar]
- Hornykiewicz, O. A brief history of levodopa. J. Neurol. 2010, 257, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-L.; Wang, R.; Tang, X.-C. Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur. J. Pharmacol. 2005, 519, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Lee, Y.-J.; Hong, J.T.; Lee, H.-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Chege, P.M.; McColl, G. Caenorhabditis elegans: A model to investigate oxidative stress and metal dyshomeostasis in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 1–15. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Introduction: Biochemistry, physiology and ecological functions of secondary metabolites. In Annual Plant Reviews Volume 40 Biochemistry of Plant Secondary Metabolites; Wink, M., Ed.; Wiley-Blackwell: West Sussex, Oxford UK, 2010; pp. 1–19. [Google Scholar]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Stability of phytochemicals as sources of anti-inflammatory nutraceuticals in beverages—A review. Food Res. Int. 2013, 50, 480–486. [Google Scholar] [CrossRef]
- Khlifi, D.; Sghaier, R.M. Anti-inflammatory and acetylcholinesterase inhibition activities of Globularia Alypum. J. Med. Bioeng. 2013, 2, 232–237. [Google Scholar] [CrossRef]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Gohari, A.R. Importance of Brassica napus as a medicinal food plant. J. Med. Plants Res. 2012, 6, 2700–2703. [Google Scholar] [CrossRef]
- Yue, J.; Shang, P.; Wang, G.; Liu, D.; Xu, L.; Zhou, W. Nutritional and antioxidant properties of rapeseed (Brassica Napus) cultivars with high and low erucic acid content. J. Food Nutr. Res. 2014, 2, 918–924. [Google Scholar] [CrossRef][Green Version]
- Wink, M.; Abbas, S. Epigallocatechin gallate (EGCG) from green tea (Camellia sinensis) and other natural products mediate stress resistance and slows down aging processes in caenorhabditis elegans. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier Science Publishing Co., Inc.: London, UK, 2013; pp. 1105–1115. [Google Scholar]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health--influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.; Hadadeh, O.; Chamaa, F.; Cheaito, K.; Darwish, B.; Makkawi, A.-K.; Abou-Kheir, W. Modeling human neurological and neurodegenerative diseases: From induced pluripotent stem cells to neuronal differentiation and its applications in neurotrauma. Front. Mol. Neurosci. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; D’Avanzo, C.; Kim, D.Y.; Irimia, D. Three-dimensional models of the human brain development and diseases. Adv. Healthc. Mater. 2018, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Marton, R.M.; Paşca, S.P. Neural differentiation in the third dimension: Generating a human midbrain. Cell. Stem Cell. 2016, 19, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. In Neuronal Cell Culture: Methods and Protocols; Amini, S., White, K.M., Eds.; Springer Science + Business Media: New York, NY, USA 2013; pp. 9–21. [Google Scholar]
- Gordon, J.; Amini, S.; White, M.K. General overview of neuronal cell culture. Methods Mol. Biol. 2013, 1078, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.-M.; Jang, I.; Lee, M.-H.; Kim, D.K.; Jeon, H.; Cha, D.S. Sorbus alnifolia protects dopaminergic neurodegeneration in Caenorhabditis elegans. Pharm. Biol. 2016, 55, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-B.; Park, J.-S.; Lim, S.-B. Antioxidant activity and cell toxicity of pressurised liquid extracts from 20 selected plant species in Jeju, Korea. Food Chem. 2010, 122, 546–552. [Google Scholar] [CrossRef]
- Lee, B.K.; Jung, Y.-S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxid. Med. Cell. Longev. 2016, 2016, 2495624. [Google Scholar] [CrossRef] [PubMed]
- Fredotovíc, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Cikeš-Čulić, V.; Puizina, J. Chemical composition and biological activity of allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Lee, C.H.; Yoo, K.-Y.; Choi, J.H.; Park, O.K.; Lim, S.S.; Kang, I.-J.; Kwon, D.Y.; Park, J.; Yi, J.-S.; et al. Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J. Med. Food 2009, 12, 990–995. [Google Scholar] [CrossRef]
- Bhanot, A.; Shri, R. A comparative profile of methanol extracts of Allium cepa and Allium sativum in diabetic neuropathy in mice. Pharmacogn. Res. 2010, 2, 374–384. [Google Scholar] [CrossRef]
- Jaiswal, N.; Rizvi, S.I. Onion extract (Allium cepa L.), quercetin and catechin up-regulate paraoxonase 1 activity with concomitant protection against low-density lipoprotein oxidation in male Wistar rats subjected to oxidative stress. J. Sci. Food Agric. 2014, 94, 2752–2757. [Google Scholar] [CrossRef] [PubMed]
- Pate, K.M.; Rogers, M.; Reed, J.W.; van der Munnik, N.; Vance, S.Z.; Moss, M.A. Anthoxanthin polyphenols attenuate a β oligomer-induced neuronal responses associated with Alzheimer’s disease. CNS Neurosci. Ther. 2017, 23, 135–144. [Google Scholar] [CrossRef] [PubMed]
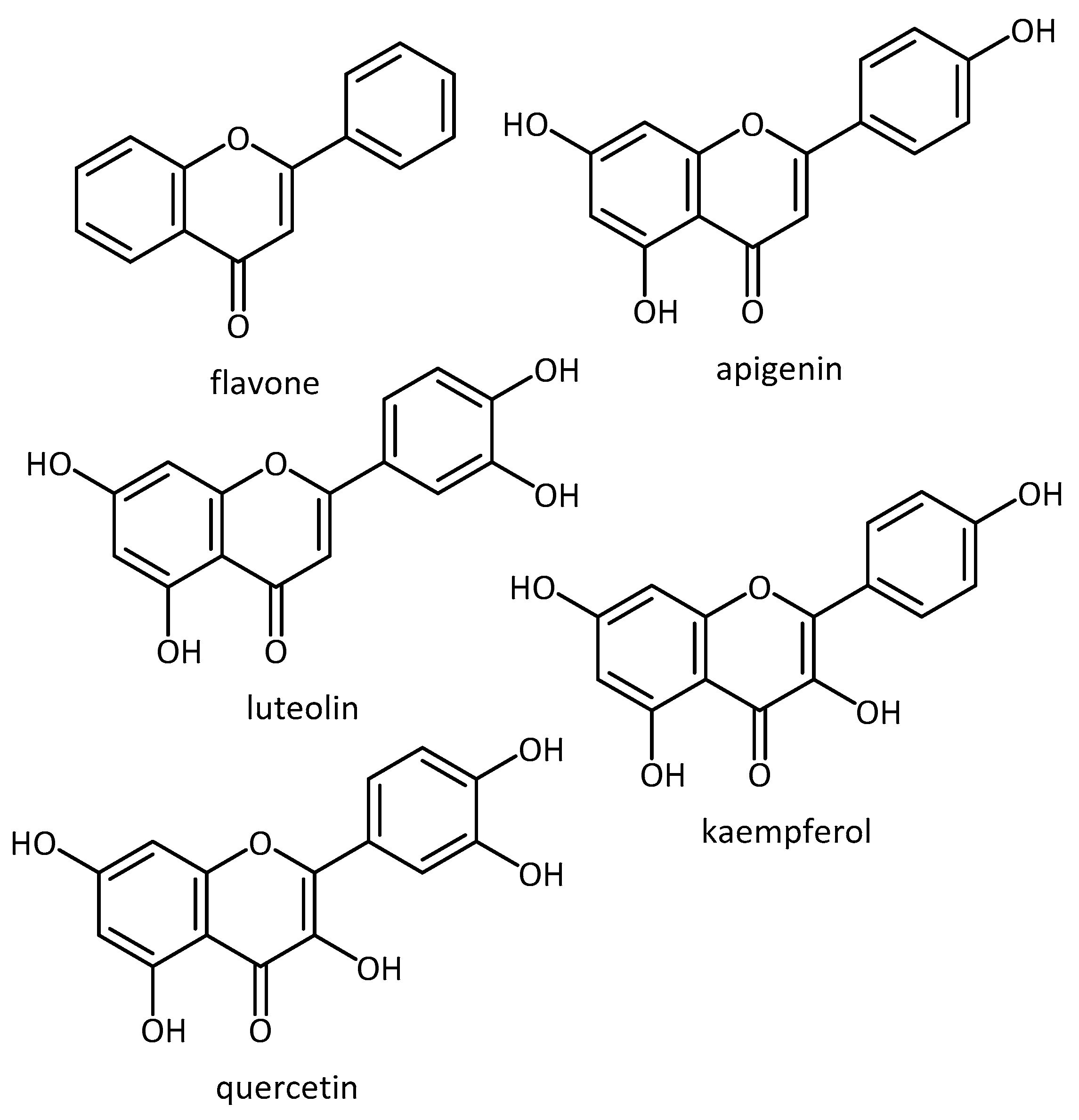
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.-L.; Shu, L.; Su, Z.-Y.; Kong, A.-N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Angeles, D.C.; Ho, P.; Dymock, B.W.; Lim, K.-L.; Zhou, Z.-D.; Tan, E.-K. Antioxidants inhibit neuronal toxicity in Parkinson’s disease-linked LRRK2. Ann. Clin. Transl. Neurol. 2016, 3, 288–294. [Google Scholar] [CrossRef]
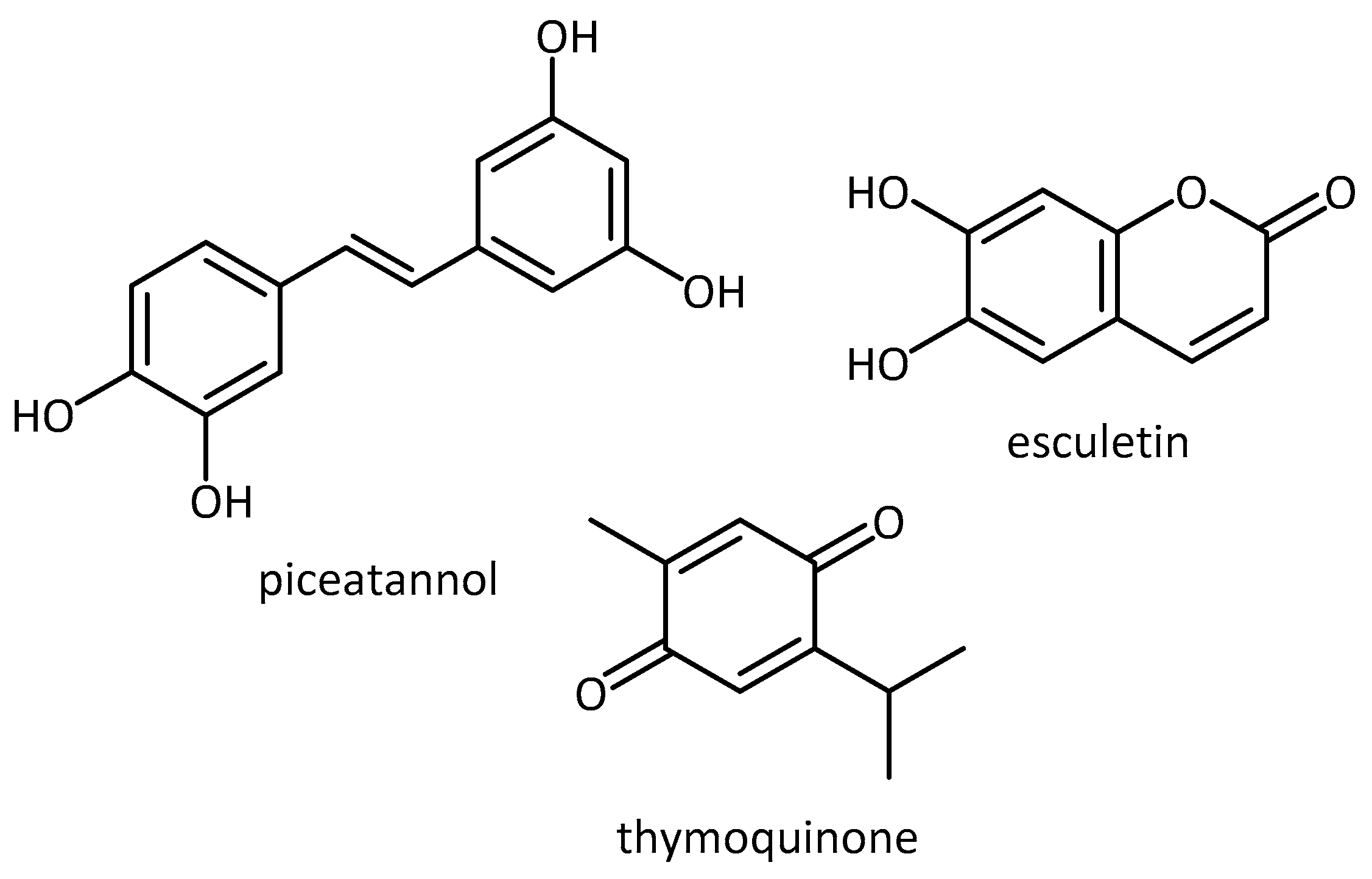
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S.; Shahri, A.M.P.; Samini, F. The neuroprotective effects of thymoquinone: A review. Dose. Response 2018, 16, 1559325818761455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ha, T.-Y.; Ahn, J.; Kim, S. Analysis and distribution of esculetin in plasma and tissues of rats after oral administration. Prev. Nutr. Food Sci. 2014, 19, 321–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
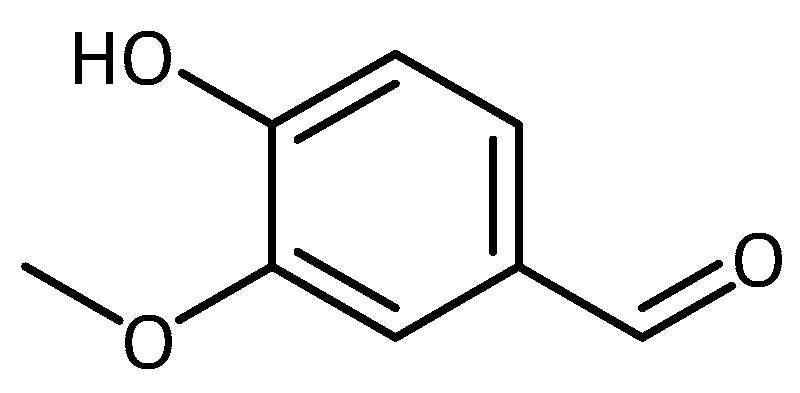
- Kumar, S.S.; Priyadarsini, K.I.; Sainis, K.B. Inhibition of peroxynitrite-mediated reactions by vanillin. J. Agric. Food Chem. 2004, 52, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta—Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Scipioni, M.; Kay, G.; Megson, I.; Kong Thoo Lin, P. Novel vanillin derivatives: Synthesis, anti-oxidant, DNA and cellular protection properties. Eur. J. Med. Chem. 2018, 143, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Chen, J.-C.; Li, C.-C.; Lo, H.-Y.; Ho, T.-Y.; Hsiang, C.-Y. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J. Pharmacol. Exp. Ther. 2009, 330, 370–376. [Google Scholar] [CrossRef]
- Dhanalakshmi, C.; Manivasagam, T.; Nataraj, J.; Justin Thenmozhi, A.; Essa, M.M. Neurosupportive role of vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 626028. [Google Scholar] [CrossRef]
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an in vivo model for human neurodegenerative disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef]
- Jahromi, S.R.; Haddadi, M.; Shivanandappa, T.; Ramesh, S.R. Attenuation of neuromotor deficits by natural antioxidants of Decalepis hamiltonii in transgenic Drosophila model of Parkinson’s disease. Neuroscience 2015, 293, 136–150. [Google Scholar] [CrossRef]
- Srivastava, A.; Harish, S.R.; Shivanandappa, T. Antioxidant activity of the roots of Decalepis hamiltonii (Wight & Arn.). LWT—Food Sci. Technol. 2006, 39, 1059–1065. [Google Scholar] [CrossRef]
- Srivastava, A.; Jagan Mohan Rao, L.; Shivanandappa, T. Isolation of ellagic acid from the aqueous extract of the roots of Decalepis hamiltonii: Antioxidant activity and cytoprotective effect. Food Chem. 2007, 103, 224–233. [Google Scholar] [CrossRef]
- Briffa, M.; Ghio, S.; Neuner, J.; Gauci, A.J.; Cacciottolo, R.; Marchal, C.; Caruana, M.; Cullin, C.; Vassallo, N.; Cauchi, R.J. Extracts from two ubiquitous Mediterranean plants ameliorate cellular and animal models of neurodegenerative proteinopathies. Neurosci. Lett. 2017, 638, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Martínez, U.; Reyes-Esparza, J.; Rodríguez-Fragoso, L. Cactus (Opuntia ficus-indica): A review on its antioxidants properties and potential pharmacological use in chronic diseases. Nat. Prod. Chem. Res. 2014, 2, 6. [Google Scholar] [CrossRef]
- Khaled, N.; Hiba, M.; Asma, C. Antioxidant and antifungal activities of Padina Pavonica and Sargassum Vulgare from the Lebanese Mediterranean coast. Adv. Environ. Biol. 2012, 6, 42–48. [Google Scholar]
- Alexander, A.G.; Marfil, V.; Li, C. Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Front. Genet. 2014, 5, 1–21. [Google Scholar] [CrossRef]
- Chen, X.; Barclay, J.W.; Burgoyne, R.D.; Morgan, A. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chem. Cent. J. 2015, 9, 1–20. [Google Scholar] [CrossRef]
- Wei, C.-C.; Yu, C.-W.; Yen, P.-L.; Lin, H.-Y.; Chang, S.-T.; Hsu, F.-L.; Liao, V.H.-C. Antioxidant activity, delayed aging, and reduced amyloid-β toxicity of methanol extracts of tea seed pomace from Camellia tenuifolia. J. Agric. Food Chem. 2014, 62, 10701–10707. [Google Scholar] [CrossRef]
- Pohl, F.; Goua, M.; Bermano, G.; Russell, W.R.; Scobbie, L.; Maciel, P.; Kong Thoo Lin, P. Revalorisation of rapeseed pomace extracts: An in vitro study into its anti-oxidant and DNA protective properties. Food Chem. 2018, 239, 323–332. [Google Scholar] [CrossRef]
- Pohl, F.; Goua, M.; Bermano, G.; Russell, W.R.; Maciel, P.; Kong Thoo Lin, P. Study into the polyphenol content and antioxidant activity of rapeseed pomace extracts. Proc. Nutr. Soc. 2016, 75, E59. [Google Scholar] [CrossRef]
- Eaton, S.L.; Wishart, T.M. Bridging the gap: Large animal models in neurodegenerative research. Mamm. Genome 2017, 28, 324–337. [Google Scholar] [CrossRef]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.-T. Animal models of Parkinson’s disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.A.C.; Orr, H.T.; Clark, H.B. Genetically engineered mouse models of the trinucleotide-repeat spinocerebellar ataxias. Brain Res. Bull. 2012, 88, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Keifer, J.; Summers, C.H. Putting the “biology” back into “neurobiology”: The strength of diversity in animal model systems for neuroscience research. Front. Syst. Neurosci. 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gengler, S.; Hamilton, A.; Hölscher, C. Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer’s disease is impaired in old but not young mice. PLoS ONE 2010, 5, e9764. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Youn, J.E.; Kim, H.-S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr.) varieties. J. Food Sci. Technol. 2014, 51, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Cunha-Santos, J.; Duarte-Neves, J.; Carmona, V.; Guarente, L.; Pereira de Almeida, L.; Cavadas, C. Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway. Nat. Commun. 2016, 7, 11445. [Google Scholar] [CrossRef]
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A focus on several neurodegenerative diseases. Oxid. Med. Cell. Longev. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
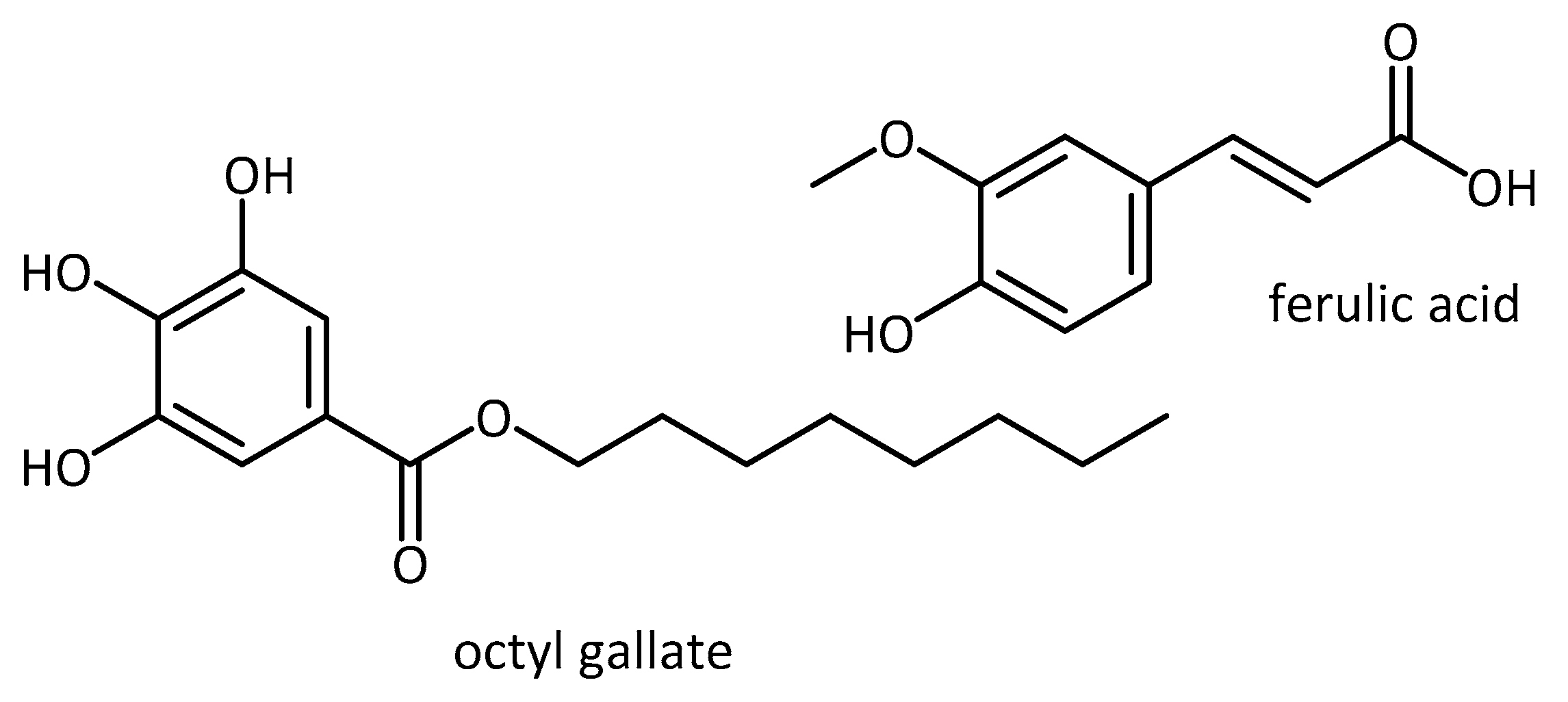
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef] [PubMed]
- Phonsatta, N.; Deetae, P.; Luangpituksa, P.; Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Le Comte, J.; Villeneuve, P.; Decker, E.A.; Visessanguan, W.; Panya, A. Comparison of antioxidant evaluation assays for investigating antioxidative activity of gallic acid and its alkyl esters in different food matrices. J. Agric. Food Chem. 2017, 65, 7509–7518. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
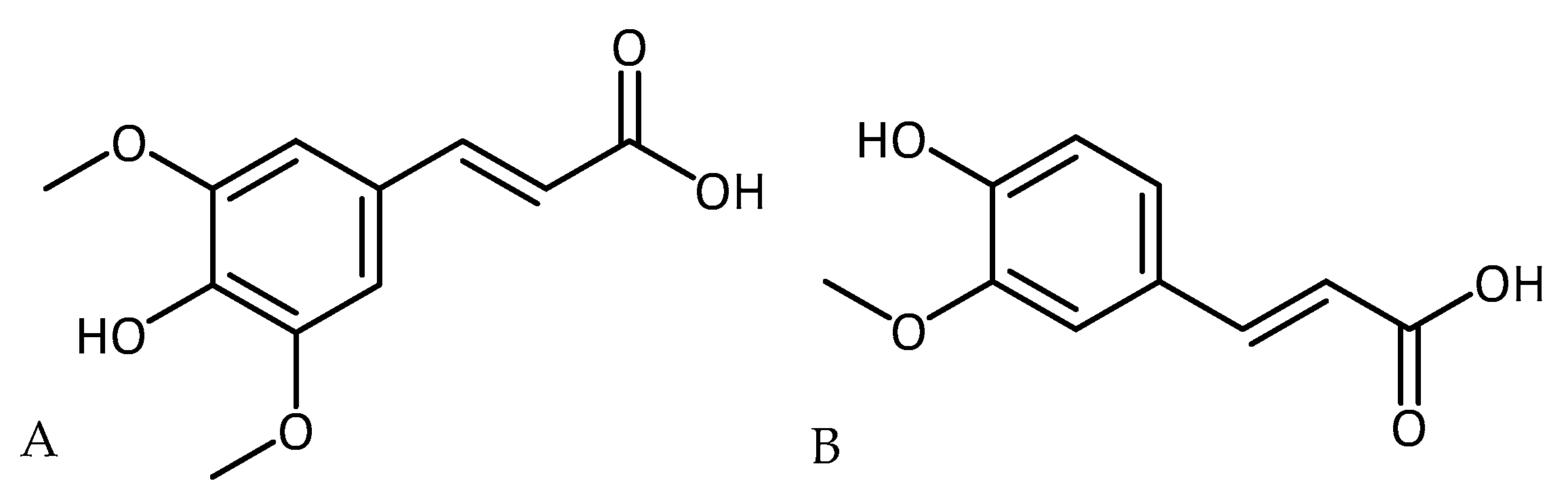
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Devel. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2015, 1–10. [Google Scholar] [CrossRef]
- Zare, K.; Eidi, A.; Roghani, M.; Rohani, A.H. The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-parkinsonian rat. Metab. Brain Dis. 2015, 30, 205–213. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Q.-W.; Zhu, C.-X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid β deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 183–191. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Zhan, Z.; Li, X.; Zhou, F.; Xing, A.; Jiang, C.; Chen, Y.; An, L. Beneficial effects of sulforaphane treatment in Alzheimer’s disease may be mediated through reduced HDAC1/3 and increased P75NTR expression. Front. Aging Neurosci. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Hettinger, C.L.; Zhang, D.; Rezvani, K.; Wang, X.; Wang, H. Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington’s disease. J. Neurochem. 2014, 129, 539–547. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, B.; Wang, X.; Wu, L.; Yang, Y.; Cheng, X.; Hu, Z.; Cai, X.; Yang, J.; Sun, X.; et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Piermarini, E.; Pastore, A.; Vasco, G.; Schirinzi, T.; Carrozzo, R.; Bertini, E.; Piemonte, F. Nrf2-inducers counteract neurodegeneration in frataxin-silenced motor neurons: Disclosing new therapeutic targets for Friedreich’s Ataxia. Int. J. Mol. Sci. 2017, 18, 2173. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the Clinician’s expectation be matched by the reality? Oxid. Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Facciolà, A.; Bertuccio, M.P.; Picerno, I.; Di Pietro, A. In vitro assessment of the indirect antioxidant activity of Sulforaphane in redox imbalance vanadium-induced. Nat. Prod. Res. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Selvaraju, S.; Essa, M.M.; Vaishnav, R.; Al-Adawi, S.; Al-Asmi, A.; Al-Senawi, H.; Abd Alrahman Alobaidy, A.; Lakhtakia, R.; Guillemin, G.J. Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid. Med. Cell. Longev. 2013, 2013, 685909. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Chang, J.-C.; Lin, W.-Y.; Li, C.-C.; Hsieh, M.; Chen, H.-W.; Wang, T.-S.; Liu, C.-S.; Liu, K.-L. Treatment with caffeic acid and resveratrol alleviates oxidative stress induced neurotoxicity in cell and drosophila models of spinocerebellar ataxia type3. Sci. Rep. 2017, 7, 11641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Banskota, A.; Critchley, A.; Hafting, J.; Prithiviraj, B. Neuroprotective effects of the cultivated chondrus crispus in a C. elegans model of Parkinson’s disease. Mar. Drugs 2015, 13, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-W.; Tsai, R.-T.; Liu, S.-P.; Chen, C.-S.; Tsai, M.-C.; Chien, S.-H.; Hung, H.-S.; Lin, S.-Z.; Shyu, W.-C.; Fu, R.-H. Neuroprotective effects of betulin in pharmacological and transgenic Caenorhabditis elegans models of Parkinson’s disease. Cell. Transplant. 2017, 26, 1903–1918. [Google Scholar] [CrossRef]
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β(1–42) protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266. [Google Scholar] [CrossRef]
- Karakida, F.; Ikeya, Y.; Tsunakawa, M.; Yamaguchi, T.; Ikarashi, Y.; Takeda, S.; Aburada, M. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol. Pharm. Bull. 2007, 30, 514–519. [Google Scholar] [CrossRef]
- Wang, J.; Santa-Maria, I.; Ho, L.; Ksiezak-Reding, H.; Ono, K.; Teplow, D.B.; Pasinetti, G.M. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Langley, M.; Kanthasamy, A.G.; Reddy, M.B. Epigallocatechin gallate has a neurorescue effect in a mouse model of parkinson disease. J. Nutr. 2017, 147, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health (NIH) New NCCIH Funding Opportunities for Natural Product Clinical Trials Webinar Summary|NCCIH. Available online: https://nccih.nih.gov/news/events/telecon/natural-product-CT-webinar (accessed on 9 June 2018).
- Postuma, R.B.; Anang, J.; Pelletier, A.; Joseph, L.; Moscovich, M.; Grimes, D.; Furtado, S.; Munhoz, R.P.; Appel-Cresswell, S.; Moro, A.; et al. Caffeine as symptomatic treatment for Parkinson disease (Café-PD): A randomized trial. Neurology 2017, 89, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-T.; Qian, Z.-M.; He, X.; Gong, Q.; Wu, K.-C.; Jiang, L.-R.; Lu, L.-N.; Zhu, Z.; Zhang, H.-Y.; Yung, W.-H.; et al. Reducing iron in the brain: A novel pharmacologic mechanism of huperzine A in the treatment of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Ke, Y. Huperzine A: Is it an effective disease-modifying drug for Alzheimer’s disease? Front. Aging Neurosci. 2014, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Walsh, S.; Little, J.T.; Behan, K.; Reynolds, B.; Ward, C.; Jin, S.; Thomas, R.; Aisen, P.S. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 2011, 76, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Miroddi, M.; Navarra, M.; Quattropani, M.C.; Calapai, F.; Gangemi, S.; Calapai, G. Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer’s disease. CNS Neurosci. Ther. 2014, 20, 485–495. [Google Scholar] [CrossRef]
- DeFeudis, F.V.; Drieu, K. Ginkgo biloba extract (EGb 761) and CNS functions: Basic studies and clinical applications. Curr. Drug Targets 2000, 1, 25–58. [Google Scholar] [CrossRef]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef]
- Yao, Z.; Drieu, K.; Papadopoulos, V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from β-amyloid-induced cell death by inhibiting the formation of β-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001, 889, 181–190. [Google Scholar] [CrossRef]
- Lugasi, A.; Horvahovic, P.; Dworschák, E. Additional information to the in vitro antioxidant activity of Ginkgo biloba L. Phyther. Res. 1999, 13, 160–162. [Google Scholar] [CrossRef]
- Maitra, I.; Marcocci, L.; Droy-Lefaix, M.T.; Packer, L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem. Pharmacol. 1995, 49, 1649–1655. [Google Scholar] [CrossRef]
- Ellnain-Wojtaszek, M.; Kruczyński, Z.; Kasprzak, J. Investigation of the free radical scavenging activity of Ginkgo biloba L. leaves. Fitoterapia 2003, 74, 1–6. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.P.; Klein, W.L.; Link, C.D.; Luo, Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.V.; Luo, Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J. Alzheimers Dis. 2003, 5, 287–300. [Google Scholar] [CrossRef]
- Liu, X.; Hao, W.; Qin, Y.; Decker, Y.; Wang, X.; Burkart, M.; Schötz, K.; Menger, M.D.; Fassbender, K.; Liu, Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain. Behav. Immun. 2015, 46, 121–131. [Google Scholar] [CrossRef]
- Rainer, M.; Mucke, H.; Schlaefke, S. Ginkgo biloba extract EGb 761® in the treatment of dementia: A pharmacoeconomic analysis of the Austrian setting. Wien. Klin. Wochenschr. 2013, 125, 8–15. [Google Scholar] [CrossRef]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef]
- Hoerr, R.; Nacu, A. Neuropsychiatric symptoms in dementia and the effects of Ginkgo biloba extract EGb 761® treatment: Additional results from a 24-week randomized, placebo-controlled trial. Open Access J. Clin. Trials 2016, 8, 1–6. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo evaluation of memory (GEM) study investigators Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 300, 2253–2262. [Google Scholar] [CrossRef]
- Tan, M.-S.; Yu, J.-T.; Tan, C.-C.; Wang, H.-F.; Meng, X.-F.; Wang, C.; Jiang, T.; Zhu, X.-C.; Tan, L. Efficacy and adverse effects of Ginkgo biloba for cognitive impairment and dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2015, 43, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, D.; Liu, Z. Synergistic, additive and antagonistic effects of Potentilla fruticosa combined with EGb761 on antioxidant capacities and the possible mechanism. Ind. Crops Prod. 2015, 67, 227–238. [Google Scholar] [CrossRef]
- Skroza, D.; Generalić Mekinić, I.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J. Food Compos. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Admasu, T.D.; Chaithanya Batchu, K.; Barardo, D.; Ng, L.F.; Lam, V.Y.M.; Xiao, L.; Cazenave-Gassiot, A.; Wenk, M.R.; Tolwinski, N.S.; Gruber, J. Drug synergy slows aging and improves healthspan through IGF and SREBP lipid signaling. Dev. Cell 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- de los Ríos, C.; Egea, J.; Marco-Contelles, J.; León, R.; Samadi, A.; Iriepa, I.; Moraleda, I.; Gálvez, E.; García, A.G.; López, M.G.; et al. Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1,8-naphthyidine derivatives. J. Med. Chem. 2010, 53, 5129–5143. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
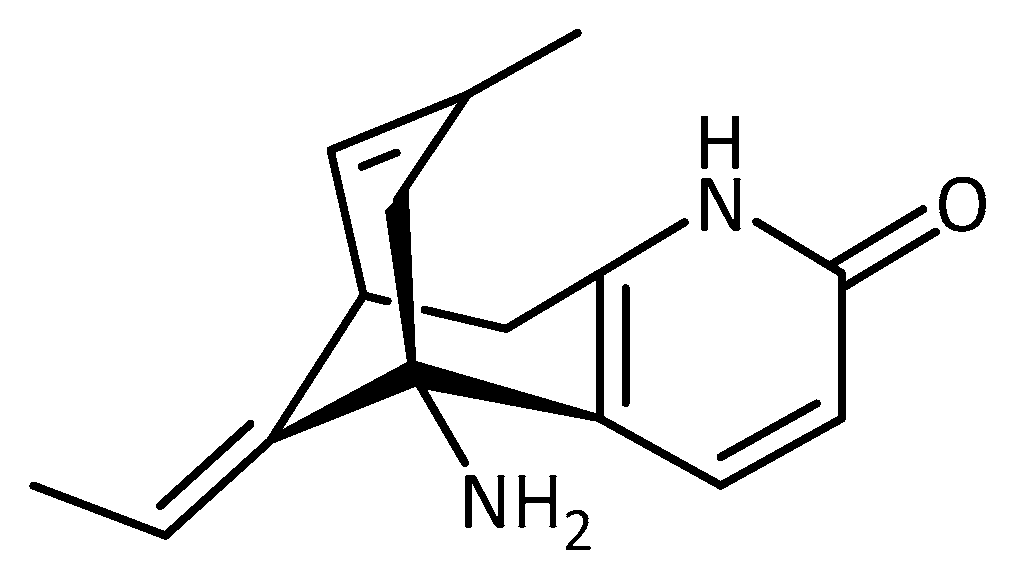
| Nitrogen-Containing | Without Nitrogen |
|---|---|
| Alkaloids | Terpenes (Mono-, Sesqui-, Di-, Tri-, Tetraterpenes) |
| Non-protein amino acids | Steroids, saponins |
| Amines | Flavonoids, tannins |
| Cyanogenic glycosides | Phenylpropanoids, lignin, coumarins, lignans |
| Glucosinolates | Polyacetylenes, fatty acids, waxes |
| Alkamides | Polyketides |
| Lectins, peptides, polypeptides | Carbohydrates, organic acids |
| In Vitro/ In Vivo | Origin of antioxidant/s | Model system | Condition | Molecular Outcome |
|---|---|---|---|---|
| In vitro | Korean mountain ash (Sorbus alnifolia) | PC12 cells | PD | - restored MPP+-induced loss of viability [58] |
| Onion (Allium cepa)/ quercetin | primary cortical neurons derived from mouse embryos | Oxidative stress | - protection of cells mediated through ERK1/2 phosphorylation and p38MAPK dephosphorylation and inhibition of PKC-ε [60] | |
| Vanillin | SH-SY5Y | Neurodegeneration in general | - attenuated rotenone induced mitochondrial dysfunction, ROS generation, oxidative stress, and apoptosis [76] | |
| Flavonoids | AD | - flavonoids altered oligomer size distribution and conformation and were able to attenuate the oligomer induced intracellular ROS and caspases activation (only for luteolin and quercetin) [65] | ||
| Pomegranate Juice Extracts | Primary human neurons | PD | - ameliorate MPTP-induced neurotoxicity [114] | |
| Caffeic Acid and Resveratrol | SK-N-SH-MJD78 | MJD/SCA3 | - decreased reactive oxygen species (ROS), mutant ataxin-3 and apoptosis and increased autophagy in pro-oxidant tert-butyl hydroperoxide (tBH)-treated cells [115] | |
| Piceatannol, thymoquinone, esculetin | SK-N-SH -G2019S | PD | - increased viability through multi-target approach of antioxidant and kinase inhibitory properties (LRRK2 model of PD) [68] | |
| In vivo | Korean mountain ash (Sorbus alnifolia) | C. elegans | PD | - protection against chemically and genetically induced DAergic neurodegeneration, increased food-sensing functions and prolonged average lifespan [58] |
| Tea Seed Pomace (Camellia tenuifolia) | AD, aging | - decreased intracellular reactive oxygen species, prolonged lifespan and reduced amyloid-β (Aβ) toxicity in transgenic C. elegans expressing human Aβ [86] | ||
| Extract from red seaweed (Chondrus crispus) | PD | - decreased the accumulation of α-synulein and protection from 6-OHDA induced dopaminergic neurodegeneration, improved movement, potentially associated with up-regulation of the stress response genes, sod-3 and skn-1 [116] | ||
| Betulin (e.g., from outer bark of birch trees) | PD | - decreased a-syn accumulation in the transgenic C. elegans model and reduction of 6-OHDA-induced dopaminergic neuron degeneration, improved food-sensing behavioral and reversed life-span decreases in a pharmacological C. elegans model [117] | ||
| Caffeic Acid and Resveratrol | Drosophila melanogaster | MJD/SCA3 | - improved survival and locomotor activity and decreased mutant ataxin-3 and ROS levels in tBH-treated SCA3 Drosophila [115] | |
| Aqueous root extract from swallowroot (Decalepis Hamiltonii), ellagic acid | PD | - significantly improved climbing ability and circadian rhythm of locomotor activity, reduced levels of ROS and LPO and enhanced catalase (CAT) and superoxide dismutase (SOD) activity [78] | ||
| Peacocks tail (brown algae; Padina pavonica) and barbary fig (Opuntia ficus-indica) | AD | - improvement of the survival and mobility of AD models, Padina pavonica extracts improved a pan-neuronal expression of a double dose of Aβ42 (late-onset AD model), Opuntia ficus-indica showed positive effects in a model of early onset AD (flies expressing the Arctic Aβ42) | ||
| Sinapic acid, Sodium sinapate | Mice | AD, dementia | - rescued neuronal cell death and attenuated the increase of iNOS expression, glial cell activations and nitrotyrosine expressions induced by Aβ1–42 protein, attenuated memory impairment as well as cerebral protective and cognition-improving effects [118,119] | |
| Grape seed polyphenol extract | AD | - attenuated the development of tau neuropathology in a TMHT mouse model of AD through mechanisms associated with attenuation of extracellular signal-receptor kinase 1/2 signaling in the brain and interference with the assembly of tau peptides into neurotoxic aggregates [120] | ||
| Epigallocatechin Gallate (EGCG, polyphenol in green tea) | PD | - regulation of the iron-export protein ferroportin in substantia nigra by EGCG, reduction of oxidative stress and neurorescue effect against MPTP-induced functional and neurochemical deficits in mice [121] | ||
| Korean black soybeans/ anthocyanins | AD | - regulation of the PI3K/Akt/GSK3 pathway, activation of the downstream endogenous anti-oxidant Nrf2 transcription factor and its target genes HO-1 and GCLM led to amyloid β oligomer (AβO)-induced elevation of ROS was reduced and neurodegeneration prevented [94] | ||
| resveratrol | MJD/SCA3 | - activation of the histone deacetylase enzyme SIRT1 pathway, showing improvement in motor behaviour when treating animals at a post symptomatic stage of disease development [97] | ||
| Ferulic acid | Rats | PD | - rescued dopamine neurons in substantia nigra pars compacta area and nerve terminals in the striatum from the rotenone insult; restored antioxidant enzymes, prevented depletion of glutathione, and inhibited lipid peroxidation and attenuation of microglial and astrocytic activation [104] | |
| Sinapic acid | PD | - significantly improved turning behavior, prevented loss of dopaminergic neurons in substantia nigra pars compacta, lowered iron reactivity, and attenuated level of malondialdehyde and nitrite [106] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. https://doi.org/10.3390/molecules23123283
Pohl F, Kong Thoo Lin P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules. 2018; 23(12):3283. https://doi.org/10.3390/molecules23123283
Chicago/Turabian StylePohl, Franziska, and Paul Kong Thoo Lin. 2018. "The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials" Molecules 23, no. 12: 3283. https://doi.org/10.3390/molecules23123283
APA StylePohl, F., & Kong Thoo Lin, P. (2018). The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules, 23(12), 3283. https://doi.org/10.3390/molecules23123283