Differential Accumulation of Aroma Compounds in Normal Green and Albino-Induced Yellow Tea (Camellia sinensis) Leaves
Abstract
1. Introduction
2. Results
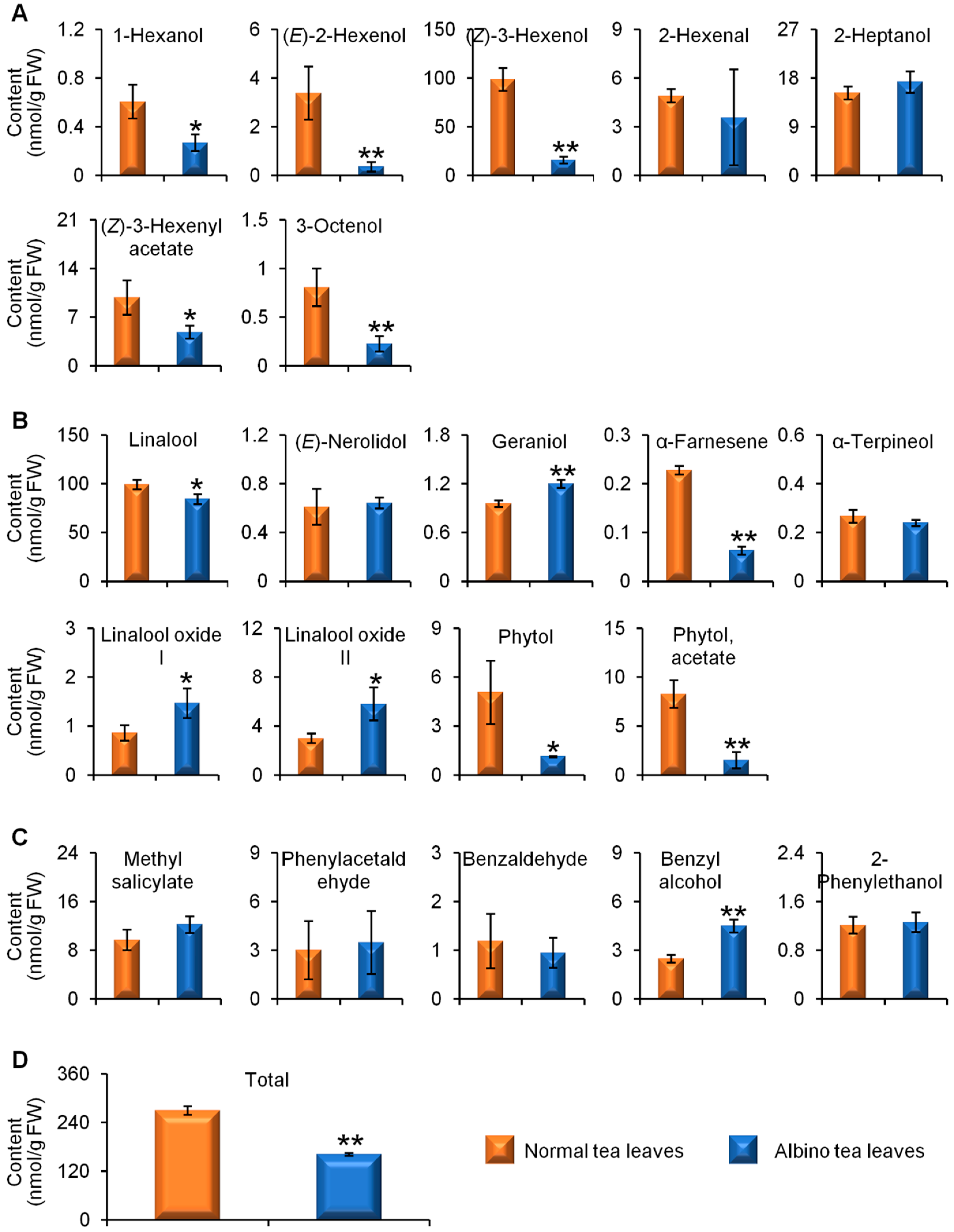
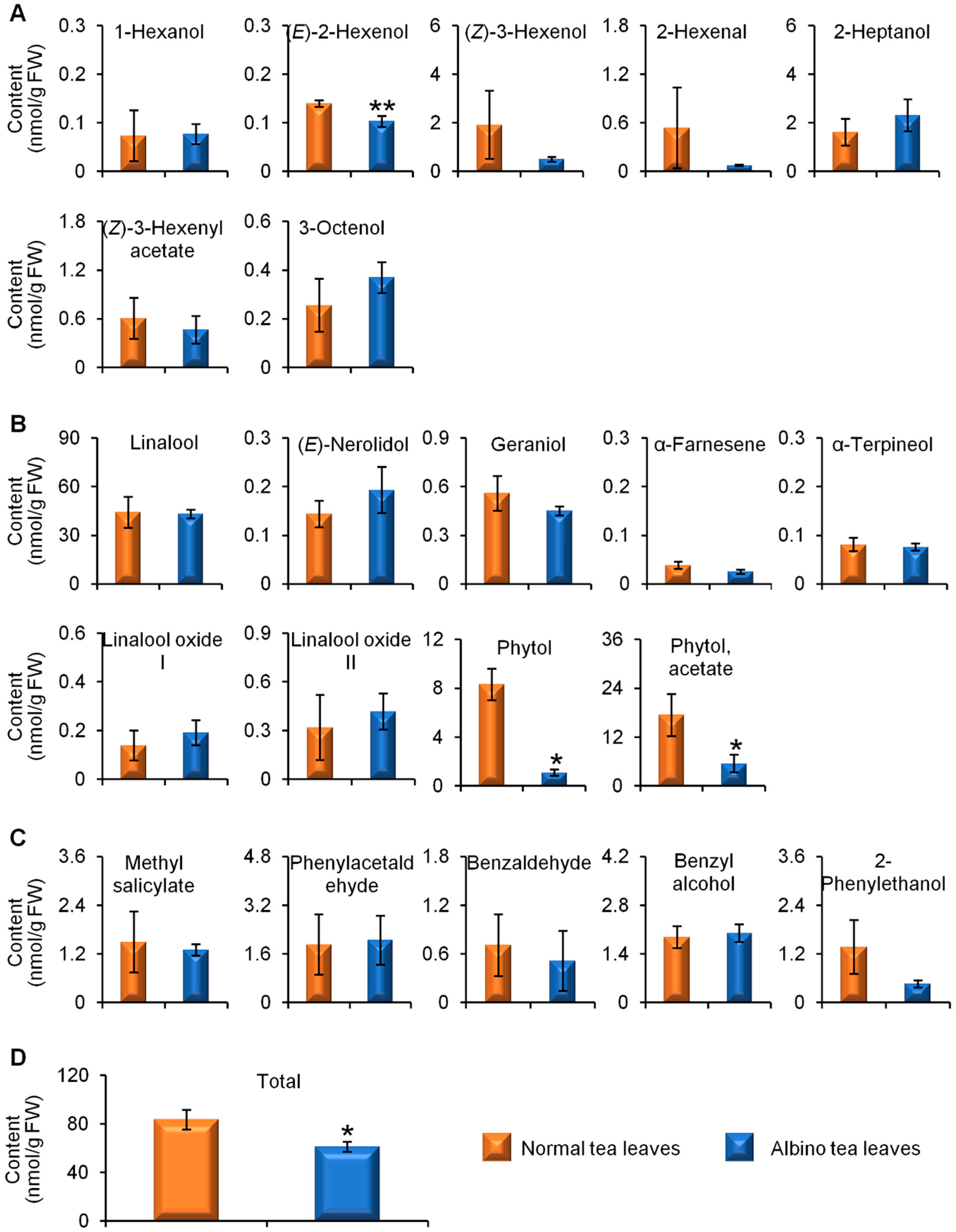
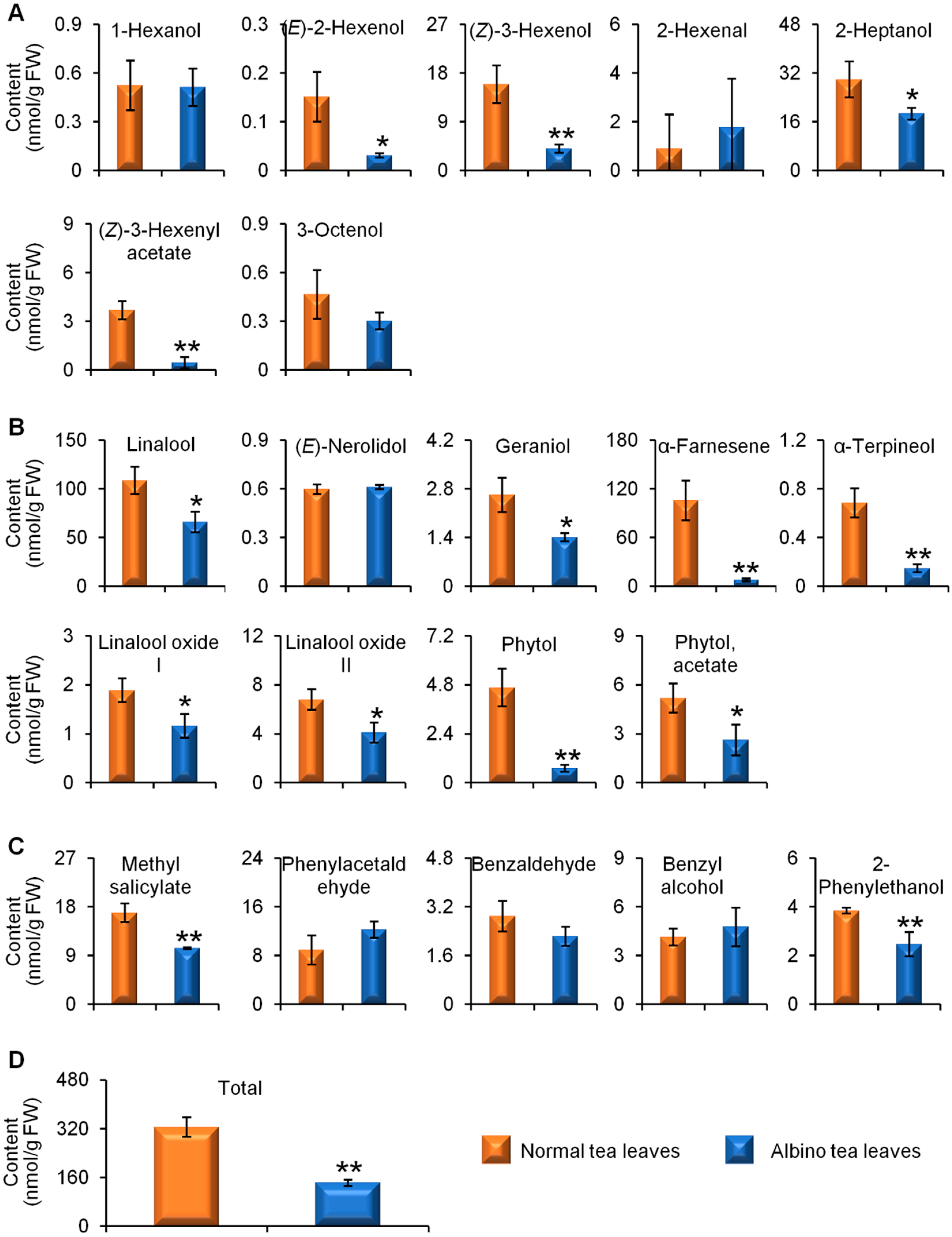
2.1. Comparisons of Endogenous Free Aroma Compound Contents between Albino-Induced Yellow Leaves and Normal Green Leaves
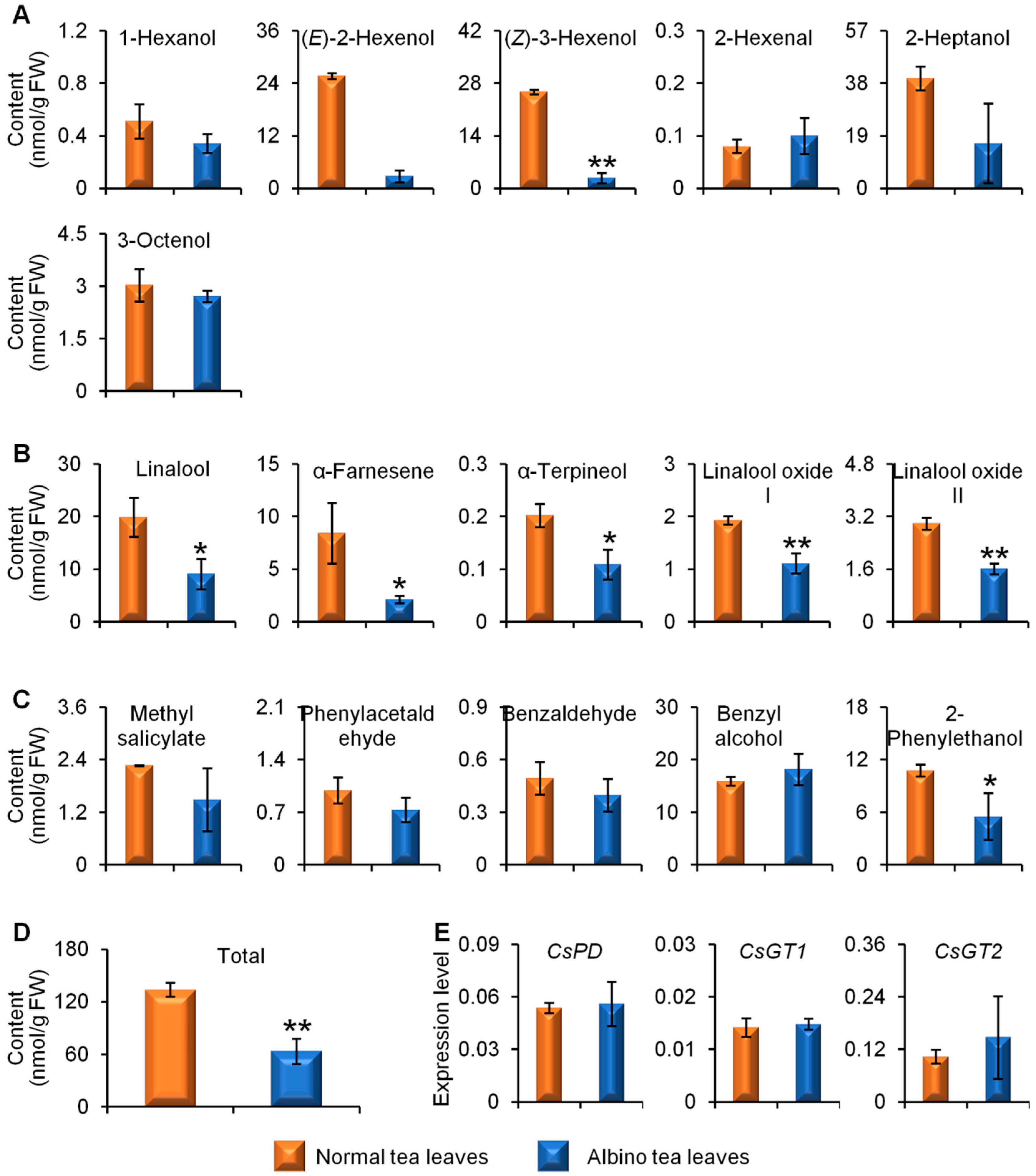
2.2. Comparison of Glycosidically Bound Aroma Compound Contents and Related Gene Expression Levels between Albino-Induced Yellow Leaves and Normal Green Leaves
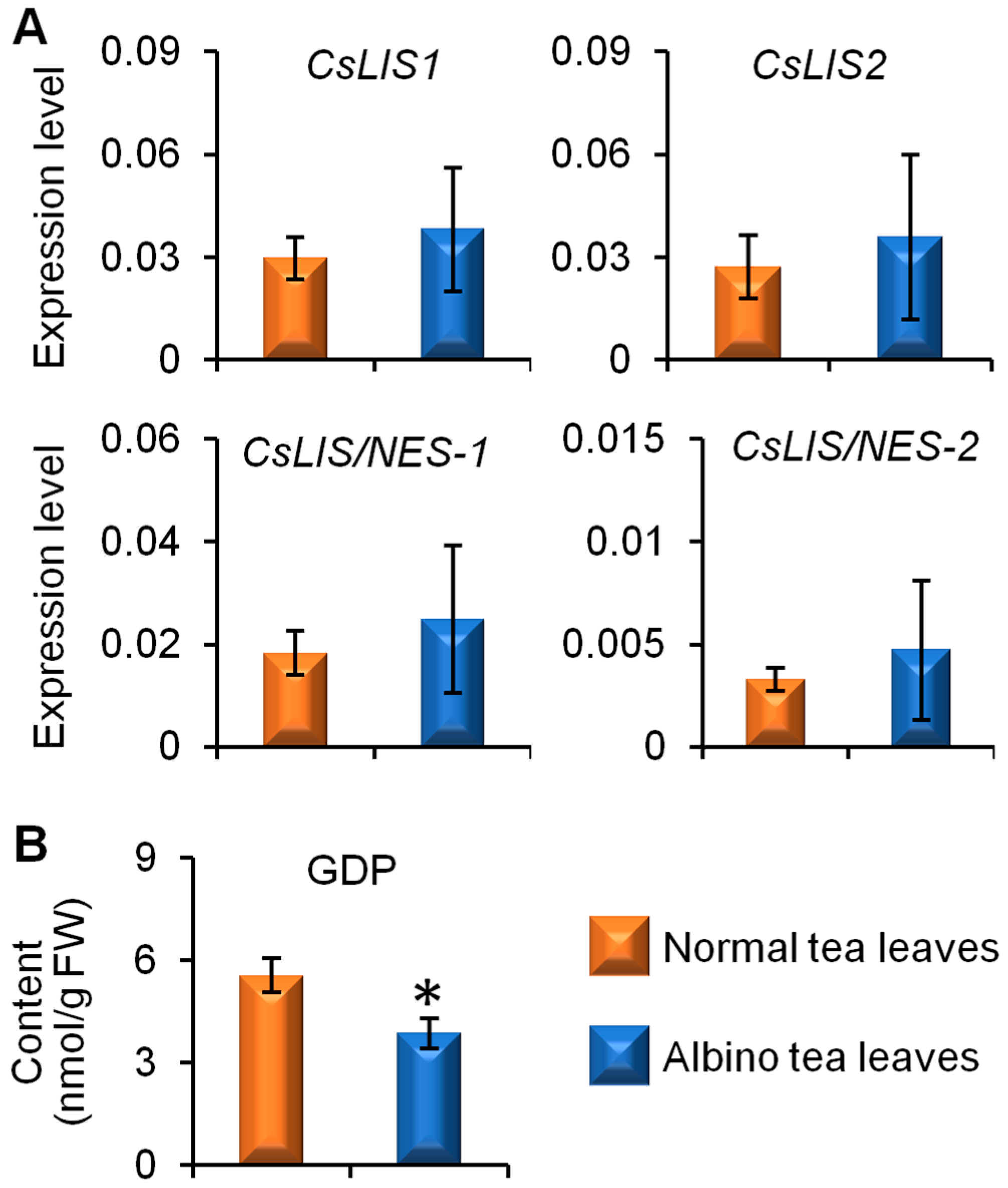
2.3. Comparison of Linalool Synthase Gene Expression Levels and GDP Content between Albino-Induced Yellow Tea Leaves and Normal Green Tea Leaves
3. Discussion
4. Materials and Methods
4.1. Chemicals and Regents
4.2. Plant Materials
4.3. Extraction and Analysis of Endogenous Aroma Compounds in Tea Leaves
4.4. Extraction and Analysis of GBVs in Tea Leaves
4.5. Transcript Expression Analysis of Key Genes Involved in Formation of Tea Aroma Compounds
4.6. Extraction and Analysis of GDP in Tea Leaves
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VFADs | Aroma fatty acid derivatives |
| VTs | Aroma terpenes |
| VPBs | Aroma phenylpropanoids/benzenoids |
| PVPP | Polyvinylpolypyrrolidone |
| GDP | Geranyl diphosphate |
| RI | Retention indices |
| GBVs | Glycosidically bound aromas |
| EF1 | Encoding elongation factor 1 |
| PD | β-Primeverosidase |
| GT | Glycosyltransferase |
| LIS | Linalool synthase |
| LIS/NES | Linalool synthase/(E)-nerolidol synthase |
References
- Bushman, J.L. Green tea and cancer in humans: A review of the literature. Nutr. Cancer 1998, 31, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Trevisanato, S.I. Tea and health. Nutr. Rev. 2000, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.C. Tea Biochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the aroma compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Zeng, L.T.; Watanabe, N.; Yang, Z.Y. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.R.; Li, M.; Zhang, L.J.; Liang, Y.R. Studies on classification of albino tearesources. J. Tea 2015, 41, 126–129. (In Chinese) [Google Scholar]
- Wang, W.; Guo, Y.L. Development and application of albino tea varieties. J. Food Saf. Qual. 2017, 8, 3104–3110. (In Chinese) [Google Scholar]
- Du, Y.Y.; Chen, H.; Zhong, W.L.; Wu, L.Y.; Ye, J.H.; Lin, C.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Effect of temperature on accumulation of chlorophylls and leaf ultrastructure of low temperature induced albino tea plant. Afr. J. Biotechnol. 2008, 7, 1881–1885. [Google Scholar] [CrossRef]
- Du, Y.Y.; Shin, S.; Wang, K.R.; Lu, J.L.; Liang, Y.R. Effect of temperature on the expression of genes related to the accumulation of chlorophylls and carotenoids in albino tea. J. Hortic. Sci.Biotechnol. 2009, 84, 365–369. [Google Scholar] [CrossRef]
- Wang, K.K.; Li, N.N.; Du, Y.Y.; Liang, Y.R. Effect of sunlight shielding on leaf structure and amino acids concentration of light sensitive albino tea plan. Afr. J. Biotechnol. 2013, 12, 5535–5539. [Google Scholar]
- Song, L.; Ma, Q.; Zou, Z.; Sun, K.; Yao, Y.; Tao, J.; Kaleri, N.A.; Li, X. Molecular link between leaf coloration and gene expression of flavonoid and carotenoid biosynthesis in Camellia sinensis cultivar ‘Huangjinya’. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Han, Z.X.; Feng, L.; Gao, L.P.; Gao, M.J.; Gruber, M.Y.; Zhang, Z.L.; Xia, T.; Wan, X.C.; Wei, S. Metabolic flux redirection and transcriptomic reprogramming in the albino tea cultivar ‘Yu-Jin-Xiang’ with an emphasis on catechin production. Sci. Rep. 2017, 7, 45062. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Fu, X.M.; Liao, Y.Y.; Xu, X.L.; Zeng, L.T.; Tang, J.C.; Li, J.L.; Lai, J.H.; Yang, Z.Y. Differential accumulation of specialized metabolite l-theanine in green and albino-induced yellow tea (Camellia sinensis) leaves. Food Chem. 2019, 276, 93–100. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.A.; Liu, S.Q.; Li, J.; Yang, X.H.; Liu, Y.S.; Liu, Z.H. Proteomic analysis of young leaves at three developmental stages in an albino tea cultivar. Proteome Sci. 2011, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Fu, X.M.; Wang, X.Q.; Liao, Y.Y.; Zeng, L.T.; Dong, F.; Yang, Z.Y. Studies on the biochemical formation pathway of the amino acid l-theanine in tea (Camellia sinensis) and other plants. J. Agric. Food Chem. 2017, 65, 7210–7216. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Kobayashi, E.; Katsuno, T.; Asanuma, T.; Fujimori, T.; Ishikawa, T.; Tomomura, M.; Mochizuki, K.; Watase, T.; Nakamura, Y.; et al. Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 2012, 135, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Fu, X.M.; Mei, X.; Zhou, Y.; Cheng, S.H.; Zeng, L.T.; Dong, F.; Yang, Z.Y. Proteolysis of chloroplast proteins is responsible for accumulation of amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Nakanishi, H.; Ema, J.I.; Ma, S.J.; Noguchi, E.; Inohara-Ochiai, M.; Fukuchi-Mizutani, M.; Nakao, M.; Sakata, K. Cloning of β-primeverosidase from tea leaves, a key enzyme in tea aroma formation. Plant Physiol. 2002, 130, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, F.; Kunimasa, A.; Zhang, Y.Q.; Cheng, S.H.; Lu, J.M.; Zhang, L.; Murata, A.; Mayer, F.; Fleischmann, P.; et al. Occurrence of glycosidically conjugated 1-phenylethanol and its hydrolase β-primeverosidase in tea (Camellia sinensis) flowers. J. Agr. Food Chem. 2014, 62, 8042–8050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, L.T.; Gui, J.D.; Liao, Y.Y.; Li, J.L.; Tang, J.C.; Meng, Q.; Dong, F.; Yang, Z.Y. Functional characterizations of β-glucosidases involved in aroma compound formation in tea (Camellia sinensis). Food Res. Int. 2017, 96, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, S.; Ono, E.; Horikawa, M.; Murata, J.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Mizutani, M.; Watanabe, N.; et al. Aroma glycosylation in tea plants: sequential glycosylationsfor the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.D.; Fu, X.M.; Zhou, Y.; Katsuno, T.; Mei, X.; Deng, R.F.; Xu, X.L.; Zhang, L.Y.; Dong, F.; Watanabe, N.; et al. Does enzymatic hydrolysis of glycosidically bound aroma compounds really contribute to the formation of aroma compounds during the oolong tea manufacturing process? J. Agr. Food Chem. 2015, 63, 6905–6914. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Chen, Y.Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z.Y. Regulation of formation of aroma compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015, 5, 16858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, L.T.; Liu, X.Y.; Gui, J.D.; Mei, X.; Fu, X.M.; Dong, F.; Tang, J.C.; Zhang, L.Y.; Yang, Z.Y. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem. 2017, 231, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Liu, X.Y.; Zhou, Y.; Wang, X.Q.; Zeng, L.T.; Fu, X.M.; Li, J.L.; Tang, J.C.; Dong, F.; Yang, Z.Y. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda). Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Liu, J.J.; He, Z.R.; Wang, F.M.; Yang, H.; Yan, Y.F.; Gao, M.J.; Gruber, M.Y.; Wan, X.C.; Wei, S. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant Cell Environ. 2018, 41, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Zhou, Y.; Fu, X.M.; Liao, Y.Y.; Yuan, Y.F.; Jia, Y.X.; Dong, F.; Yang, Z.Y. Biosynthesis of jasmine lactone in tea (Camellia sinensis) leaves and its formation in response to multiple stresses. J. Agric. Food Chem. 2018, 66, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zeng, L.T.; Liao, Y.Y.; Zhou, Y.; Xu, X.L.; Dong, F.; Yang, Z.Y. An alternative pathway for the formation of aromatic aroma compounds derived from l-phenylalanine via phenylpyruvic acid in tea (Camellia sinensis (L.) O. Kuntze) leaves. Food Chem. 2019, 270, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Zhou, Y.; Fu, X.M.; Mei, X.; Cheng, S.H.; Gui, J.D.; Dong, F.; Tang, J.C.; Ma, S.Z.; Yang, Z.Y. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017, 237, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.D.; Tan, J.F.; Peng, Q.H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds n-Alkanes (C8–C40), benzaldehyde, benzyl alcohol, (Z)-3-hexenyl acetate, methyl salicylate, 2-phenylethanol, ethyl n-decanoate, geraniol, 1-hexanol, (Z)-3-hexenol, phenylacetaldehyde, α-farnesene, (E)-nerolidol, linalool, and linalool oxide are available from the authors. |
| Gene | Accession Number | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|---|
| CsEF1 | KA280301.1 | TTGGACAAGCTCAAGGCTGAACG | ATGGCCAGGAGCATCAATGACAGT |
| CsPD | AB088027.1 | CCAAAGGTTCGGAATTGTCTATG | GCGCTTTTAGTCATACACCGA |
| CsGT1 | AB847092 | TGAAGAAGGAAGCAGAAGAAGC | GGCTCATGATTCAACCGG |
| CsGT2 | AB847093 | GAGGACATAAGGATTAAAGCGAG | TTTTCAACCCACTTAAATATTTCAATA |
| CsLIS1 | KF006849 | ACATTGCAAGGATGGTTCC | ATGAGCATTACAGGTGCTAGCT |
| CsLIS2 | KY033151 | GTCAATGTTCCGTGATACTGTTTC | ACACCAAGATAGACACCCTACTTTC |
| CsLIS/NES-1 | KF006849 | TCCAACCCCTCAATACAGAAAGACTATC | TTGGCTTTGTAGAAGTGCTTCAATCTC |
| CsLIS/NES-2 | - | GAATGACAATCCAGGCATTG | TGGTGAGAATGGATTTGGAG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Zeng, L.; Yu, Z.; Li, J.; Tang, J.; Su, X.; Yang, Z. Differential Accumulation of Aroma Compounds in Normal Green and Albino-Induced Yellow Tea (Camellia sinensis) Leaves. Molecules 2018, 23, 2677. https://doi.org/10.3390/molecules23102677
Dong F, Zeng L, Yu Z, Li J, Tang J, Su X, Yang Z. Differential Accumulation of Aroma Compounds in Normal Green and Albino-Induced Yellow Tea (Camellia sinensis) Leaves. Molecules. 2018; 23(10):2677. https://doi.org/10.3390/molecules23102677
Chicago/Turabian StyleDong, Fang, Lanting Zeng, Zhenming Yu, Jianlong Li, Jinchi Tang, Xinguo Su, and Ziyin Yang. 2018. "Differential Accumulation of Aroma Compounds in Normal Green and Albino-Induced Yellow Tea (Camellia sinensis) Leaves" Molecules 23, no. 10: 2677. https://doi.org/10.3390/molecules23102677
APA StyleDong, F., Zeng, L., Yu, Z., Li, J., Tang, J., Su, X., & Yang, Z. (2018). Differential Accumulation of Aroma Compounds in Normal Green and Albino-Induced Yellow Tea (Camellia sinensis) Leaves. Molecules, 23(10), 2677. https://doi.org/10.3390/molecules23102677






