Synthesis of New Triarylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Oxide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages
Abstract
1. Introduction
2. Results and Discussion
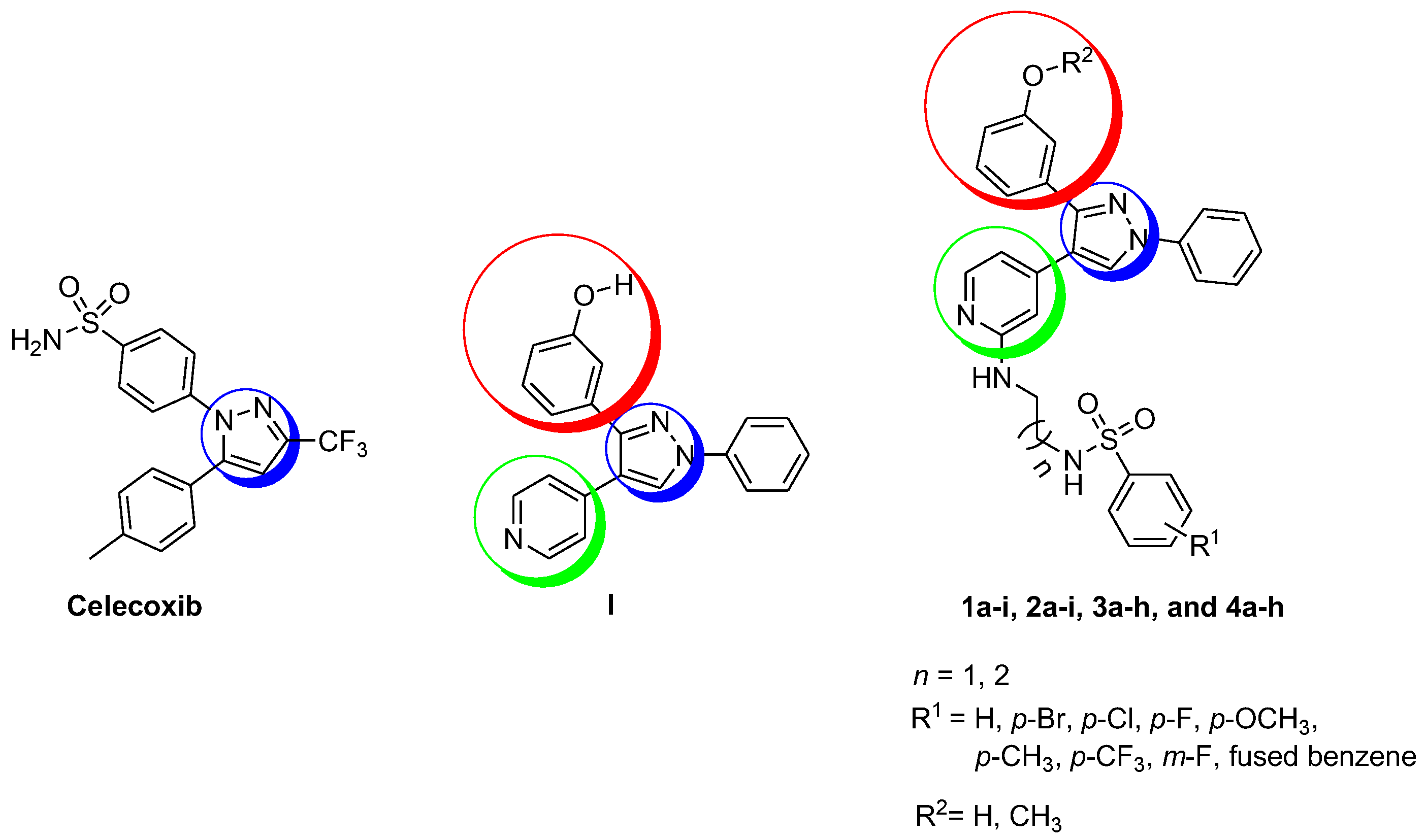
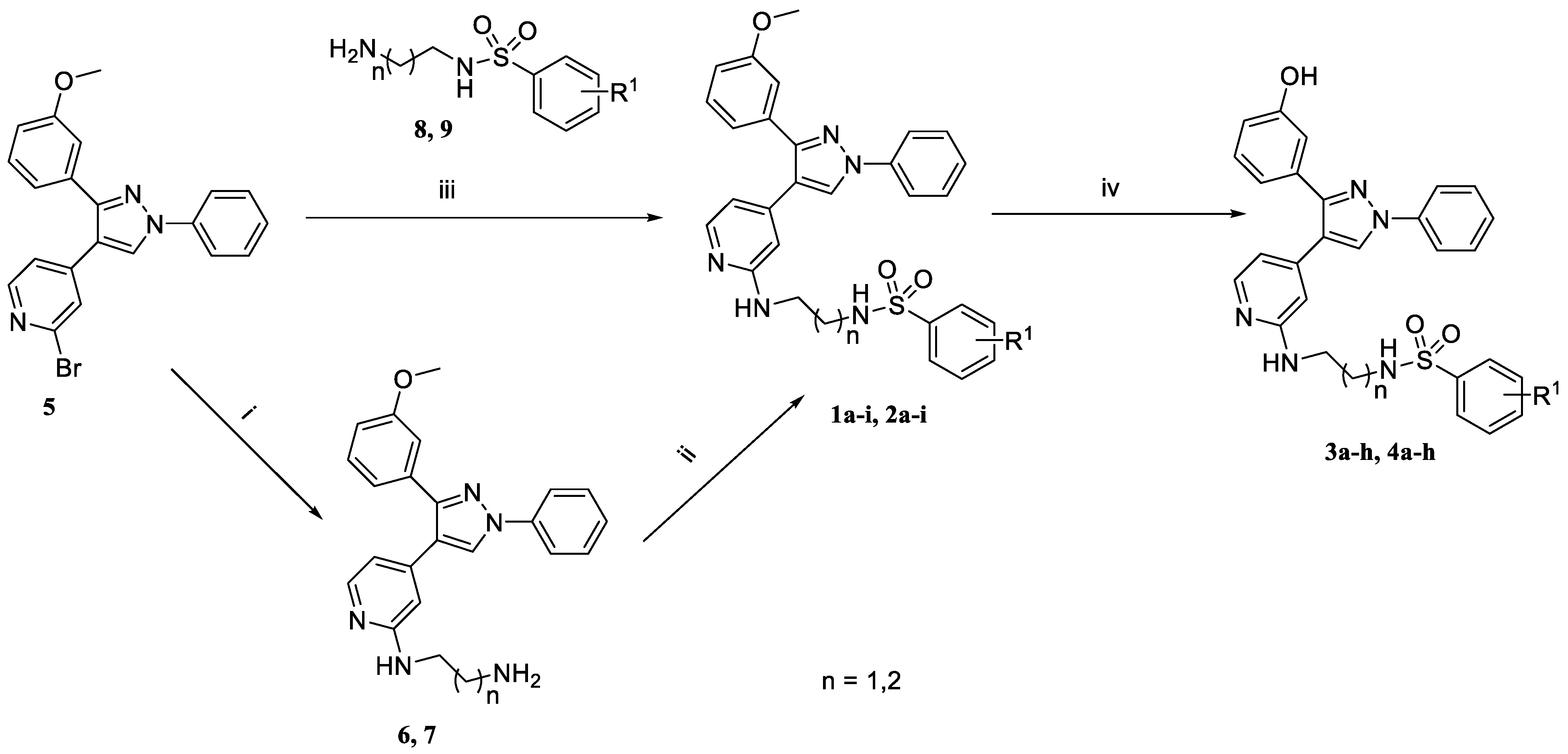
2.1. Chemistry
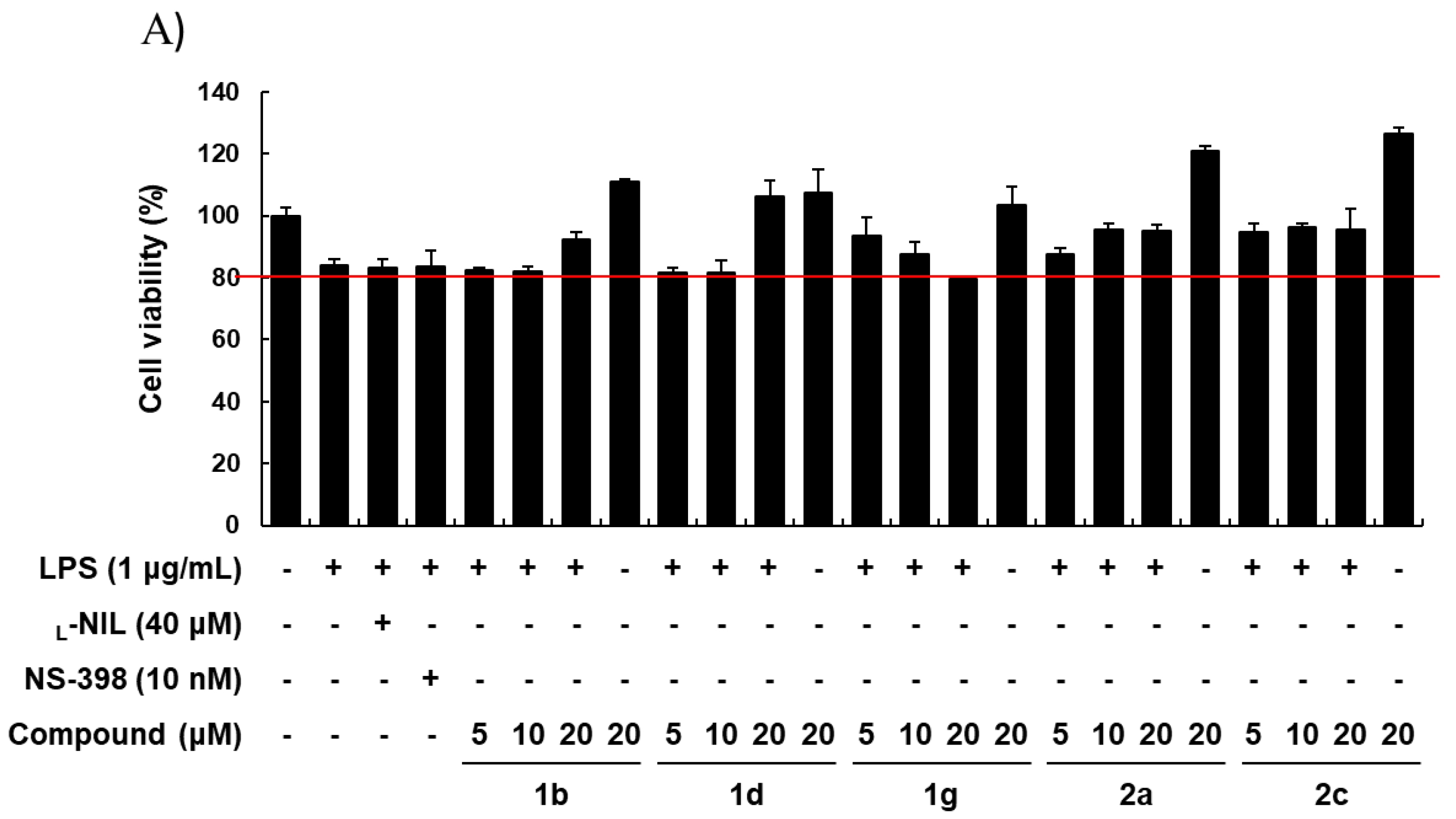
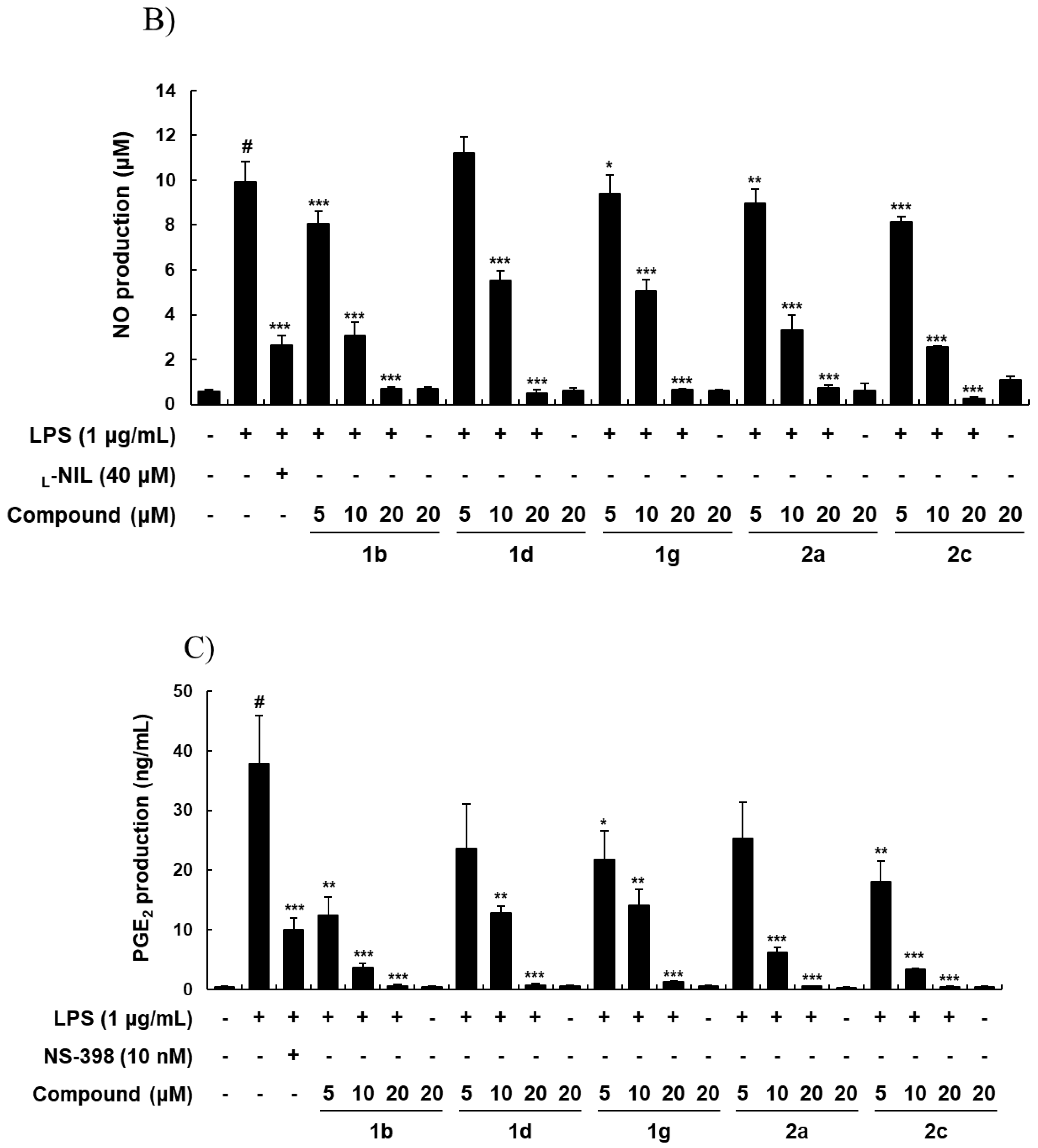
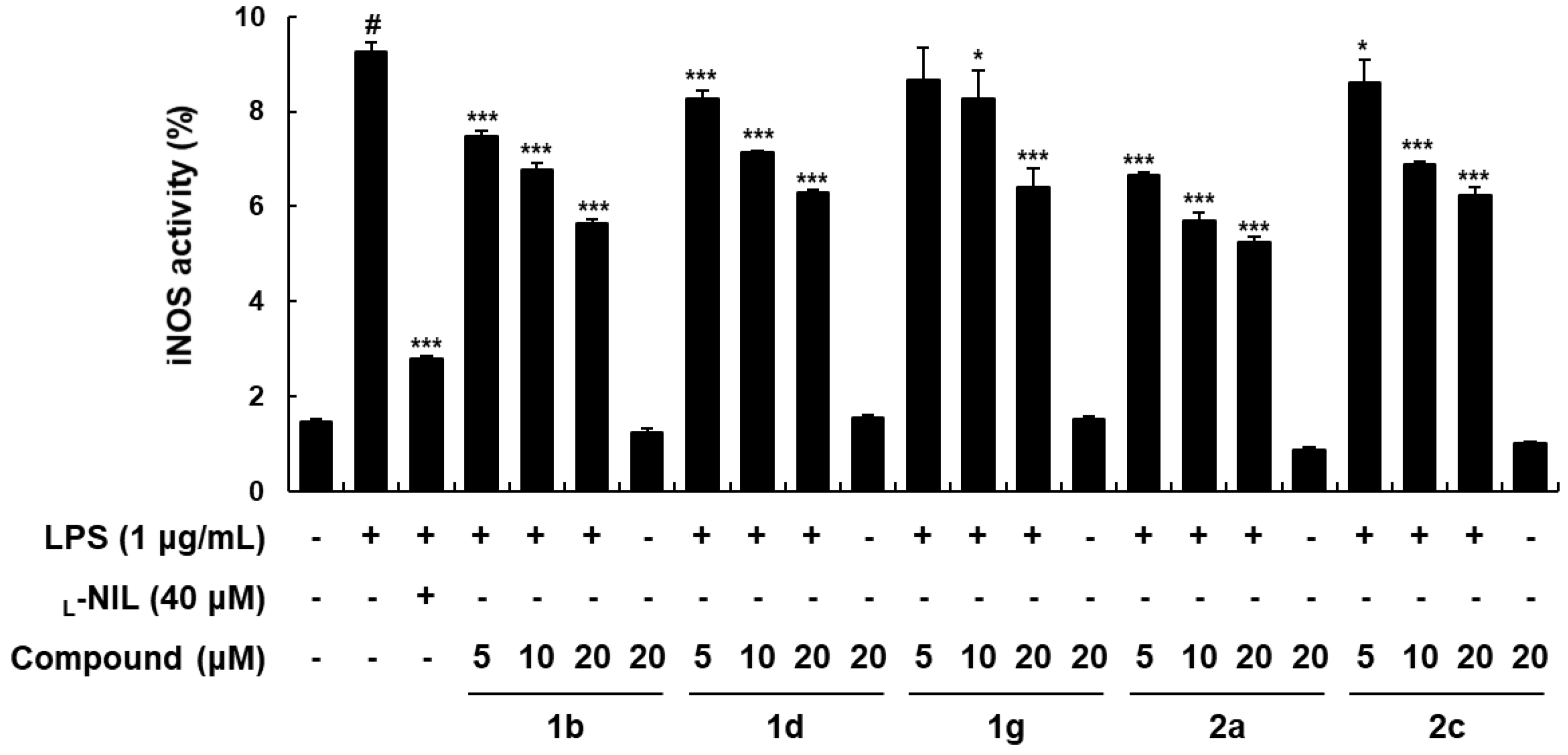
2.2. Biology
3. Materials and Methods
3.1. General
3.2. Synthesis of N-(2-aminoethyl)benzenesulfonamide (8a), N-(2-aminoethyl)substituted benzenesulfonamides (8b–i), N-(3-aminopropyl)benzenesulfonamide (9a), and N-(3-aminopropyl)benzenesulfonamides (9b–i).
3.3. Synthesis of 2-bromo-4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridine (5)
3.4. Synthesis of N1-(4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl) ethane1,2-diamine (6) and N1-(4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl) propane-1,3-diamine (7)
3.5. General Procedure for Synthesis of the Target Compounds N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)arylsulfonamides (1a–i) and N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)arylsulfonamides (2a–i).
3.5.1. Method A
3.5.2. Method B
3.6. General Procedure for Synthesis of N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)benzenesulfonamide (3a), N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)benzenesulfonamide (3b–h), N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl) amino)propyl)benzenesulfonamide (4a) and N-(3-((4-(3-(3-hydroxyphenyl) -1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) (substituted)benzenesulfonamide (4b–h)
3.7. Biological Evaluation
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Chettibi, S.; Ferguson, M.W.J. Inflammation: Basic Principles and Clinical Correlates, 3rd ed.; Gallin, J.I., Snyderman, R., et al., Eds.; Lipincott, Williams and Wilkinson: Philadelphia, PA, USA, 1999; pp. 865–881. [Google Scholar]
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol 2000, 18, 217–242. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Muller, A.; Zlotnik, A. Chemokines: Agents for the immunotherapy of cancer? Nat. Rev. Immunol. 2002, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Richardson, J.C.; Gentleman, S.M.; Brooks, D.J. Inflammatory risk factors and pathologies associated with Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Prasad, S.; Yadav, V.R.; Lavasanifar, A.; Aggarwal, B.B. Cancer and diet: How are they related? Free Rad. Res. 2011, 45, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Bae, E.A.; Hyun, Y.J.; Kim, D.H. Dextran sulfate sodium and 2,4,6-trinitrobenzene sulfonic acid induce lipid peroxidation by the proliferation of intestinal gram-negative bacteria in mice. J. Inflamm. 2010, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Qui, H.; Johansson, A.S.; Sjostrom, M.; Wan, M.; Schroder, O.; Palmblad, J.; Haeggstrom, J.Z. Differential induction of BLT receptor expression on human endothelial cells by lipopolysacharide, cytokines, and leukotriene B4. Proc. Natl. Acad. Sci. USA 2006, 103, 6913–6918. [Google Scholar]
- Marletta, M.A. Nitric oxide synthase structure and mechanism. J. Biol. Chem. 1993, 268, 12231–12234. [Google Scholar] [PubMed]
- Grisham, M.B.; Jourd, H.D.; Wink, D.A.I. Physiological chemistry of nitric oxide and its metabolites: Implications in inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, 315–321. [Google Scholar] [CrossRef]
- Morikawa, A.; Koide, N.; Kato, Y.; Sugiyama, T.; Chakravortty, D.; Yoshida, T.; Yokochi, T. Augmentation ofnitric oxide production by gamma interferon in a mouse vascular endothelial cell line and its modulationby tumor necrosis factor alpha and lipopolysaccharide. Infect. Immun. 2000, 68, 6209–6214. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guerrero, C.; Hernandez-Vargas, P.; Lopez-Franco, O.; Ortiz-Munoz, G.; Egido, J. Mesangial cells and glomerular inflammation: From the pathogenesis to novel therapeutic approaches. Curr. Drug Targets Inflamm. Allergy 2005, 4, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Vasilakaki, S.; Pastukhov, O.; Mavromoustakos, T.; Huwiler, A.; Kokotos, G. Small peptides able to suppress prostaglandin e2 generation in renal mesangial cells. Molecules 2018, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Dawson, V.L.; Dawson, T.M. Neurobiology of nitricoxide. Crit. Rev. Neurobiol. 1996, 10, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Brune, K.J. Cyclooxygenase-2–10 years later. Pharmacol. Exp. Ther. 2002, 300, 367–375. [Google Scholar] [CrossRef]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A promising structure in medicinal chemistry. ChemMedChem. 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Yasuhiro, Y.; Hirokazu, K.; Yoji, M.; Yoshinori, O.; Masashi, F.; Noriko, Y.I.; Jun, I.; Taiji, Y.; Makoto, T.; Mitsuaki, O. Novel potent and selective Ca2+ release-activated Ca2+ (CRAC) channel inhibitors. Part 3: Synthesis and CRAC channel inhibitory activity of 4’-[(trifluoromethyl)pyrazol-1-yl] carboxanilides. Bioorg. Med. Chem. 2008, 16, 9457–9466. [Google Scholar]
- Matwijczuk, A.; Karcz, D.; Pustuła, K.; Makowski, M.; Górecki, A.; Kluczyk, D.; Karpińska, M.M.; Niewiadomy, A.; Gagoś, M. Spectroscopic and theoretical studies of fluorescence effects in bio-active: 4-(5-(methyl-1,3,4-thiadiazol-2-yl))benzene-1,3-diol and 4-(5-(methylamino-1,3,4-thiadiazol-2-yl)) benzene-1,3-diol compounds: Effect of molecular aggregation and amino group position. J. Luminescence 2018, 201, 44–56. [Google Scholar]
- Silva, C.F.; Pinto, D.C.; Silva, A.M. Chromones: A promising ring system for new anti-inflammatory drugs. ChemMedChem. 2016, 11, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Huan-Wei, T.; Jiun-Yi, S.; Ting-Yi, K.; Ting-Syun, T.; Yi-An, C.; Alexander, P.; Pi-Tai, C. Excited-state intramolecular proton-transfer reaction demonstrating anti-Kasha behavior. Chem. Sci. 2016, 7, 655–665. [Google Scholar]
- Matwijczuk, A.; Janik, E.; Luchowski, R.; Niewiadomy, A.; Gruszecki, W.I.; Gagoś, M. Spectroscopic studies of the molecular organization of 4-([1,2,4] triazolo [4,3-a] pyridin-3-yl)-6-methylbenzene-1,3-diol in selected solvents. J. Luminescence 2018, 194, 208–218. [Google Scholar] [CrossRef]
- Steel, H.C.; Tintinger, G.R.; Anderson, R. Comparison of the anti-inflammatory activities of imidazole antimycotics in relation to molecular structure. Chem. Biol. Drug Des. 2008, 72, 225–228. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Sim, T.B.; Hong, J.H.; Cho, J.-H.; Yoo, K.H.; Oh, C.-H. Synthesis of 1H-pyrazole-1-carboxamide derivatives and their antiproliferative activity against melanoma cell line. Arch. Pharm. Chem. Life Sci. 2011, 344, 197–204. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Oh, C.-H. Design and synthesis of 3-(3-chloro-4-substituted phenyl)-4-(pyridin-4-yl)-1H-pyrazole-1-carboxamide derivatives and their antiproliferative activity against melanoma cell line. Bull. Korean Chem. Soc. 2011, 32, 821–828. [Google Scholar] [CrossRef]
- El-Gamal, M.I.; Choi, H.S.; Cho, H.G.; Hong, J.H.; Yoo, K.H.; Oh, C.H. Design, synthesis, and antiproliferative activity of 3,4-diarylpyrazole-1-carboxamide derivatives against melanoma cell line. Arch. Pharm. Chem. Life Sci. 2011, 344, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.K.; El-Gamal, M.I.; Choi, H.S.; Baek, D.; Oh, C.H. New diarylureas and diarylamides containing 1,3,4-triarylpyrazole scaffold: Synthesis, antiproliferative evaluation against melanoma cell lines, ERK kinase inhibition, and molecular docking studies. Eur. J. Med. Chem. 2011, 46, 5754–5762. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Park, Y.S.; Chi, D.Y.; Yoo, K.H.; Oh, C.H. New triarylpyrazoles as broad-spectrum anticancer agents: Design, synthesis, and biological evaluation. Eur. J. Med. Chem. 2013, 65, 315–322. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Choi, H.S.; Yoo, K.H.; Baek, D.; Oh, C.H. Antiproliferative diarylpyrazole derivatives as dual inhibitors of the ERK pathway and COX-2. Chem. Biol. Drug Des. 2013, 82, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Kamble, V.M. Synthesis, anti-inflammatory and antimicrobial evaluation of novel 1-acetyl-3,5-diaryl-4,5-dihydro (1H) pyrazole derivatives bearing urea, thiourea and sulfonamide moieties. Bioorg. Med. Chem. Lett. 2012, 22, 6611–6615. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.A.; Abdel-Aziz, N.I.; Abdel-Aziz, A.A.M.; El-Azab, A.S.; ElTahir, K.E.H. Synthesis, biological evaluation and selective COX-2 inhibitors and anti-inflammatory agents Part 2. Bioorg. Med. Chem. 2012, 20, 3306–3316. [Google Scholar] [PubMed]
- Ragab, F.A.; Abdel Gawad, N.M.; Georgey, H.H.; Said, M.F. Synthesis of novel 1,3,4-trisubstituted pyrazoles as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2013, 63, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Malvar, D.; Ferreira, R.; De Castro, R.; De Castro, L.; Freitas, A.C.; Costa, E.; Florentino, I.; Mafra, J.; De Souza, G.; Vandelinde, F. Antinociceptive, anti-inflammatory and antipyretic effects of 1.5-diphenyl-1H-Pyrazole-3- carbohydrazide, a new heterocyclic pyrazole derivative. Life Sci. 2014, 95, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.; Stevens, A.; Gierse, J.; McDonald, J.; Stegeman, R.; Pak, J.; Gildehaus, D.; Miyashiro, J.; Penning, T.; Seibert, K.; Isakson, P.; Stallings, W. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Norgard, B.; Pedersen, L.; Johnsen, S.P.; Tarone, R.E.; McLaughlin, J.K.; Friis, S.; Sorensen, H.T. COX-2-selective inhibitors and the risk of upper gastrointestinal bleeding in high-risk patients with previous gastrointestinal diseases: a population-based case-control study. Aliment. Pharm. Therap. 2004, 19, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-L.; El-Gamal, M.I.; Choi, H.Y.; Choi, H.Y.; Lee, K.T.; Oh, C.H. Synthesis of tricyclic fused coumarin sulfonates and their inhibitory effects on LPS-induced nitric oxide and PGE2 productions in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2014, 24, 571–575. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Lee, W.S.; Shin, J.S.; Oh, C.H.; Lee, K.T.; Choi, J.; Myoung, N.; Baek, D. Synthesis of new tricyclic and tetracyclic fused coumarin sulfonate derivatives, and their inhibitory effects on LPS-induced nitric oxide and PGE2 productions in RAW 264.7 macrophages: Part 2. Arch. Pharm. 2016, 349, 853–863. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Abdel-Maksoud, M.S.; Gamal El-Din, M.M.; Shin, J.S.; Lee, K.T.; Yoo, K.H.; Oh, C.H. Synthesis, in vitro antiproliferative and antiinflammatory activities, and kinase inhibitory effects of new 1,3,4-triarylpyrazole derivatives. Anti-Cancer Agents Med. Chem. 2017, 17, 75–84. [Google Scholar] [CrossRef]
- Park, B.-J.; El-Gamal, M.I.; Lee, W.S.; Shin, J.S.; Yoo, K.H.; Lee, K.T.; Oh, C.H. Synthesis and inhibitory effects of triarylpyrazoles on LPS-induced NO and PGE2 productions in RAW 264.7 macrophages. Med. Chem. Res. 2017, 26, 2161–2171. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.S.; Kim, M.R.; El-Gamal, M.I.; Gamal El-Din, M.M.; Oh, C.H. Design, synthesis, in vitro antiproliferative evaluation, and kinase inhibitory effects of a new series of imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 2015, 95, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.-H. Design, synthesis, broad-spectrum antiproliferative activity and kinase inhibitory effect of triarylpyrazole derivatives possessing arylamides or arylureas moieties. Eur. J. Med. Chem. 2016, 119, 122–131. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the final compounds are available from the authors. |

| Compound | n | R1 | R2 | Yield | Compound | n | R1 | R2 | Yield |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 1 | CH3 | H | 65% | 3a | 1 | H | H | 36% |
| 1b | 1 | CH3 | 4-Br | 61% | 3b | 1 | H | 4-Br | 30% |
| 1c | 1 | CH3 | 4-Cl | 60% | 3c | 1 | H | 4-Cl | 41% |
| 1d | 1 | CH3 | 4-F | 67% | 3d | 1 | H | 4-F | 40% |
| 1e | 1 | CH3 | p-OCH3 | 62% | 3e | 1 | H | 4-CH3 | 38% |
| 1f | 1 | CH3 | 4-CH3 | 69% | 3f | 1 | H | 4-CF3 | 40% |
| 1g | 1 | CH3 | 4-CF3 | 74% | 3g | 1 | H | 3-F | 32% |
| 1h | 1 | CH3 | 3-F | 66% | 3h | 1 | H | Fused benzene | 33% |
| 1i | 1 | CH3 | Fused benzene | 71% | 4a | 2 | H | H | 37% |
| 2a | 2 | CH3 | H | 60% | 4b | 2 | H | 4-Br | 42% |
| 2b | 2 | CH3 | 4-Br | 62% | 4c | 2 | H | 4-Cl | 52% |
| 2c | 2 | CH3 | 4-Cl | 62% | 4d | 2 | H | 4-F | 43% |
| 2d | 2 | CH3 | 4-F | 75% | 4e | 2 | H | 4-CH3 | 33% |
| 2e | 2 | CH3 | 4-OCH3 | 71% | 4f | 2 | H | 4-CF3 | 39% |
| 2f | 2 | CH3 | 4-CH3 | 72% | 4g | 2 | H | 3-F | 41% |
| 2g | 2 | CH3 | 4-CF3 | 71% | 4h | 2 | H | Fused benzene | 40% |
| 2h | 2 | CH3 | 3-F | 76% | |||||
| 2i | 2 | CH3 | Fused benzene | 66% |
| Compound | Nitric Oxide % Inhibition | ||
|---|---|---|---|
| 1 µM | 5 µM | 10 µM | |
| 1a | 17.35 ± 0.09 | 35.36 ± 1.14 | 52.93 ± 3.12 |
| 1b | 12.05 ± 0.11 | 24.23 ± 0.98 | 68.66 ± 2.47 |
| 1c | 13.27 ± 0.08 | 24.76 ± 0.17 | 49.89 ± 1.25 |
| 1d | 19.79 ± 0.14 | 29.36 ± 0.77 | 61.28 ± 1.33 |
| 1e | 13.52 ± 0.11 | 16.10 ± 0.09 | 41.47 ± 0.99 |
| 1f | 16.29 ± 0.10 | 26.54 ± 0.65 | 53.09 ± 2.10 |
| 1g | 10.98 ± 0.07 | 23.62 ± 0.54 | 60.80 ± 1.75 |
| 1h | 11.12 ± 0.02 | 26.24 ± 0.99 | 41.25 ± 2.15 |
| 1i | 13.40 ± 0.18 | 19.90 ± 0.24 | 51.51 ± 0.14 |
| 2a | 0.17 ± 0.03 | 30.20 ± 0.27 | 62.76 ± 3.25 |
| 2b | 0.01 ± 0.03 | 28.06 ± 0.66 | 52.44 ± 1.62 |
| 2c | 0.02 ± 0.01 | 24.69 ± 0.81 | 59.09 ± 0.93 |
| 2d | 7.81 ± 0.03 | 23.03 ± 0.89 | 55.00 ± 4.01 |
| 2e | 2.02 ± 0.01 | 23.90 ± 1.12 | 50.20 ± 1.88 |
| 2f | 4.36 ± 0.04 | 19.76 ± 0.96 | 46.32 ± 3.21 |
| 2g | 2.91 ± 0.02 | 26.26 ± 1.01 | 56.03 ± 0.89 |
| 2h | 4.87 ± 0.05 | 24.07 ± 0.91 | 41.54 ± 1.12 |
| 2i | 16.15 ± 0.12 | 25.75 ± 0.49 | 51.93 ± 1.93 |
| 3a | 4.78 ± 0.03 | 21.49 ± 0.37 | 48.97 ± 1.10 |
| 3b | 15.82 ± 0.16 | 24.36 ± 0.52 | 48.69 ± 1.74 |
| 3c | 6.34 ± 0.06 | 11.68 ± 0.71 | 33.81 ± 0.89 |
| 3d | 10.58 ± 0.09 | 18.31 ± 0.26 | 48.18 ± 0.74 |
| 3e | 7.15 ± 0.04 | 12.40 ± 0.72 | 31.43 ± 0.33 |
| 3f | 6.08 ± 0.07 | 15.21 ± 0.91 | 36.51 ± 0.59 |
| 3g | 16.19 ± 0.17 | 19.06 ± 0.89 | 37.68 ± 0.85 |
| 3h | 10.33 ± 0.08 | 14.72 ± 0.42 | 16.07 ± 0.48 |
| 4a | 11.11 ± 0.10 | 24.13 ± 0.56 | 59.25 ± 1.31 |
| 4b | 8.19 ± 0.06 | 22.18 ± 0.72 | 63.82 ± 2.14 |
| 4c | 0.86 ± 0.09 | 22.02 ± 0.42 | 46.95 ± 1.34 |
| 4d | 9.02 ± 0.03 | 20.84 ± 0.36 | 48.49 ± 1.79 |
| 4e | 0.69 ± 0.01 | 18.57 ± 0.98 | 59.69 ± 0.70 |
| 4f | 4.87 ± 0.14 | 18.87 ± 1.02 | 53.82 ± 1.87 |
| 4g | ND | 29.49 ± 0.86 | 51.15 ± 1.45 |
| 4h | 0.97± 0.01 | 21.04 ± 0.22 | 50.21 ± 2.01 |
| l-NIL (40 μM) | 77.89 ± 4.25 | ||
| Compound | NO( IC50) a | Cytotoxicity (IC50) a |
|---|---|---|
| 1a | 9.17 ± 0.52 | 245.78 ± 1.91 |
| 1b | 7.90 ± 0.41 | 254.15 ± 2.54 |
| 1c | 10.10 ± 0.63 | 219.39 ± 0.14 |
| 1d | 8.23 ± 0.32 | 169.15 ± 1.64 |
| 1e | 13.40 ± 0.72 | 285.41 ± 4.18 |
| 1f | 9.42 ± 0.12 | 291.01 ± 1.12 |
| 1g | 8.55 ± 0.14 | 261.57 ± 1.57 |
| 1h | 12.62 ± 0.29 | 244.21 ± 2.23 |
| 1i | 9.76 ± 0.21 | >400 |
| 2a | 8.04 ± 0.09 | 346.2 ± 4.21 |
| 2b | 9.50 ± 0.34 | 384.69 ± 1.29 |
| 2c | 8.68 ± 0.22 | 289.92 ± 1.87 |
| 2d | 12.42 ± 0.40 | 260.32 ± 2.24 |
| 2e | 9.96 ± 0.18 | >400 |
| 2f | 9.16 ± 0.27 | 322.54 ± 3.35 |
| 2g | 10.45 ± 0.44 | 252.64 ± 1.87 |
| 2h | 9.19 ± 0.25 | 245.78 ± 2.71 |
| 2i | 9.63 ± 0.48 | >400 |
| 3a | 10.29 ± 0.23 | 29.75 ± 1.91 |
| 3b | 10.55 ± 0.51 | 24.31 ± 0.41 |
| 3c | 15.21 ± 0.17 | 22.69 ± 0.30 |
| 3d | 10.72 ± 0.52 | 26.58 ± 0.47 |
| 3e | 16.14 ± 0.72 | 32.52 ± 0.75 |
| 3f | 22.21 ± 0.31 | 24.1 5 ± 1.61 |
| 3g | 21.42 ± 0.17 | 28.74 ± 0.74 |
| 3h | >30 | 100.21 ± 1.21 |
| 4a | 8.86 ± 0.36 | 16.58 ± 0.91 |
| 4b | 8.34 ± 0.11 | 19.54 ± 0.49 |
| 4c | 13.25 ± 0.52 | 18.79 ± 0.68 |
| 4d | 11.32 ± 0.16 | 12.58 ± 0.22 |
| 4e | 8.82 ± 0.32 | 22.96 ± 0.63 |
| 4f | 9.45 ± 0.15 | 17.73 ± 0.61 |
| 4g | 8.24 ± 0.41 | 9.35 ± 0.32 |
| 4h | 9.96 ± 0.48 | 10.92 ± 0.22 |
| l-NIL | 29.32 ± 0.15 | ND |
| Compound | PGE2 Inhibition (%) a | |||
|---|---|---|---|---|
| 1 μM | 5 μM | 10 μM | IC50 (μM) | |
| 1b | 16.24 ± 0.79 | 52.56 ± 2.21 | 76.92 ± 1.34 | 4.75 ± 0.25 |
| 1d | 10.26 ± 0.81 | 49.57 ± 1.25 | 85.75 ± 2.64 | 5.06 ± 0.21 |
| 1g | 19.94 ± 0.89 | 53.85 ± 1.91 | 79.34 ± 2.19 | 4.55 ± 0.39 |
| 2a | 31.09 ± 1.14 | 50.70 ± 0.99 | 76.89 ± 4.99 | 4.87 ± 0.44 |
| 2c | 17.51 ± 1.51 | 52.80 ± 2.01 | 81.37 ± 2.94 | 4.68 ± 0.37 |
| NS-398 | 90.53 ± 0.53 | 95.78 ± 0.67 | 98.25 ± 1.50 | 6.25 × 10−3 ± 0.41 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Maksoud, M.S.; El-Gamal, M.I.; Gamal El-Din, M.M.; Choi, Y.; Choi, J.; Shin, J.-S.; Kang, S.-Y.; Yoo, K.H.; Lee, K.-T.; Baek, D.; et al. Synthesis of New Triarylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Oxide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Molecules 2018, 23, 2556. https://doi.org/10.3390/molecules23102556
Abdel-Maksoud MS, El-Gamal MI, Gamal El-Din MM, Choi Y, Choi J, Shin J-S, Kang S-Y, Yoo KH, Lee K-T, Baek D, et al. Synthesis of New Triarylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Oxide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Molecules. 2018; 23(10):2556. https://doi.org/10.3390/molecules23102556
Chicago/Turabian StyleAbdel-Maksoud, Mohammed S., Mohammed I. El-Gamal, Mahmoud M. Gamal El-Din, Yunji Choi, Jungseung Choi, Ji-Sun Shin, Shin-Young Kang, Kyung Ho Yoo, Kyung-Tae Lee, Daejin Baek, and et al. 2018. "Synthesis of New Triarylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Oxide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages" Molecules 23, no. 10: 2556. https://doi.org/10.3390/molecules23102556
APA StyleAbdel-Maksoud, M. S., El-Gamal, M. I., Gamal El-Din, M. M., Choi, Y., Choi, J., Shin, J.-S., Kang, S.-Y., Yoo, K. H., Lee, K.-T., Baek, D., & Oh, C.-H. (2018). Synthesis of New Triarylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Oxide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Molecules, 23(10), 2556. https://doi.org/10.3390/molecules23102556






