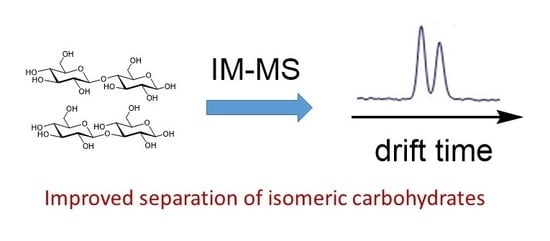
Applications of Ion Mobility-Mass Spectrometry in Carbohydrate Chemistry and Glycobiology
Abstract
1. Introduction
2. Complexity and Standard Analytical Techniques for Carbohydrates
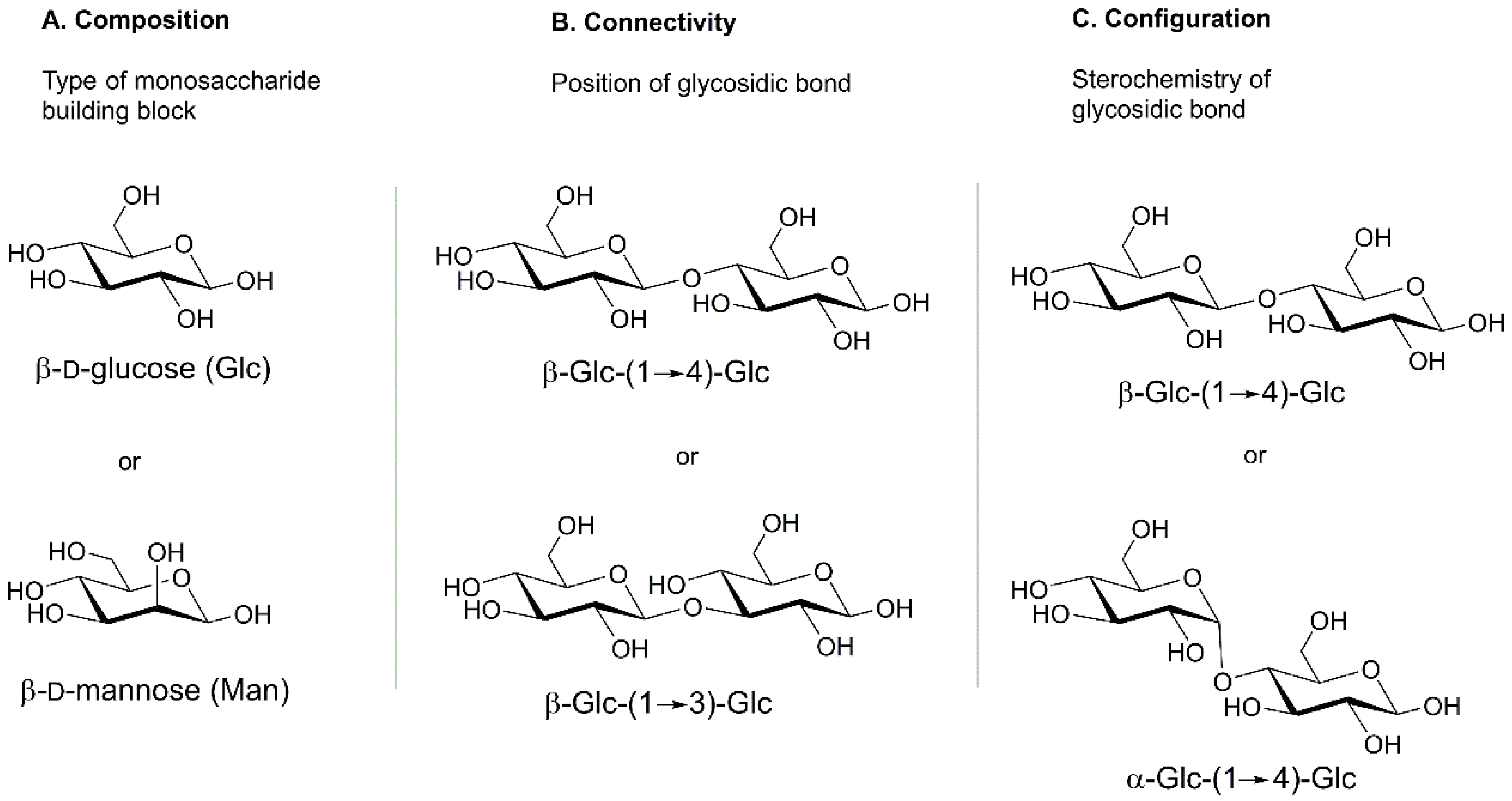
2.1. Complexity of Carbohydrates
2.2. Standard Analytical Techniques for Carbohydrates
3. Overview of Ion Mobility-Mass Spectrometry
3.1. Introduction of IM-MS
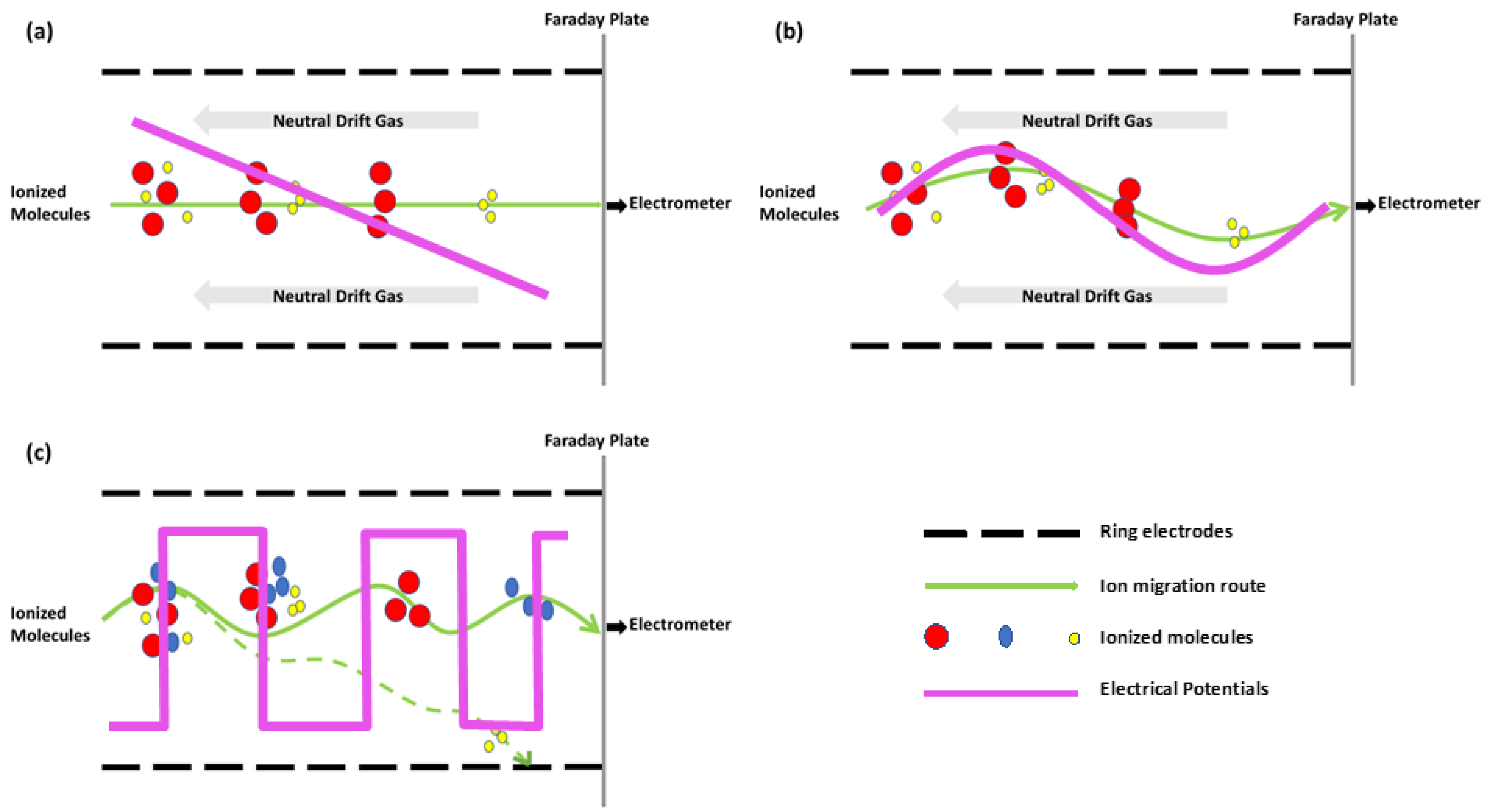
3.2. Principles of IM-MS
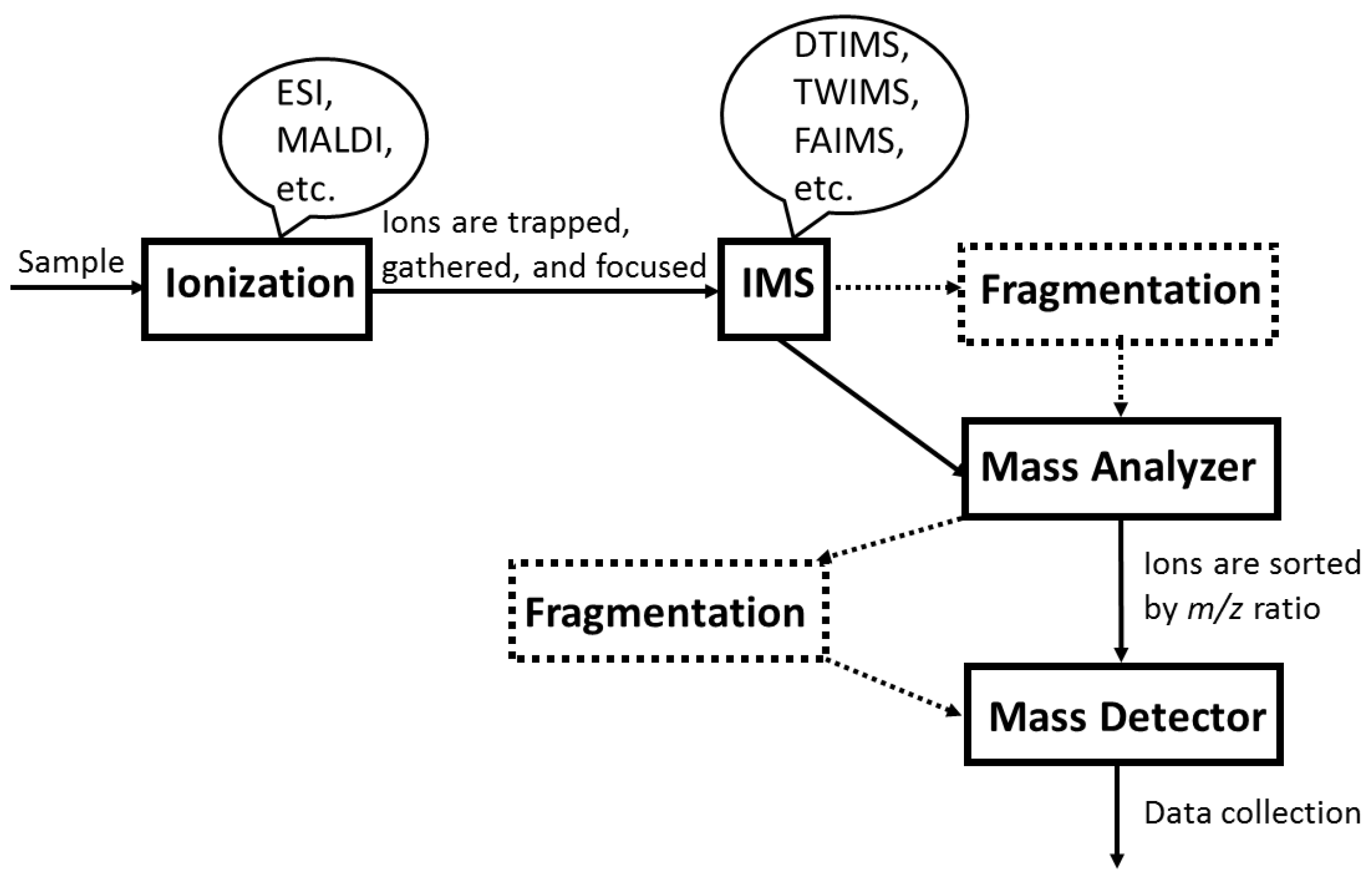
3.3. IM-MS Instrumentation
3.4. Limitations of IM-MS
4. Applications of IM-MS to Carbohydrates
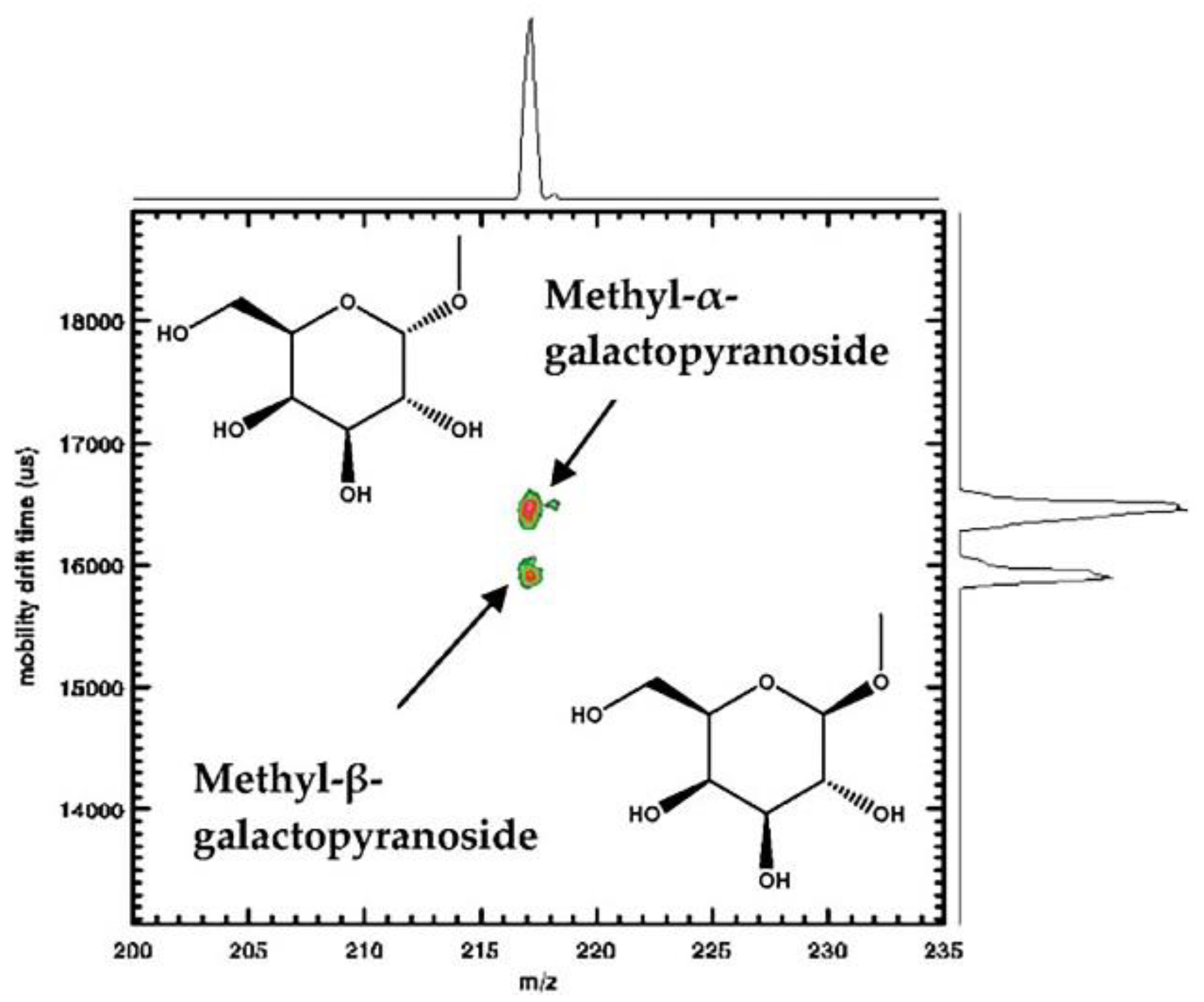
4.1. Mono- and Oligosaccharides
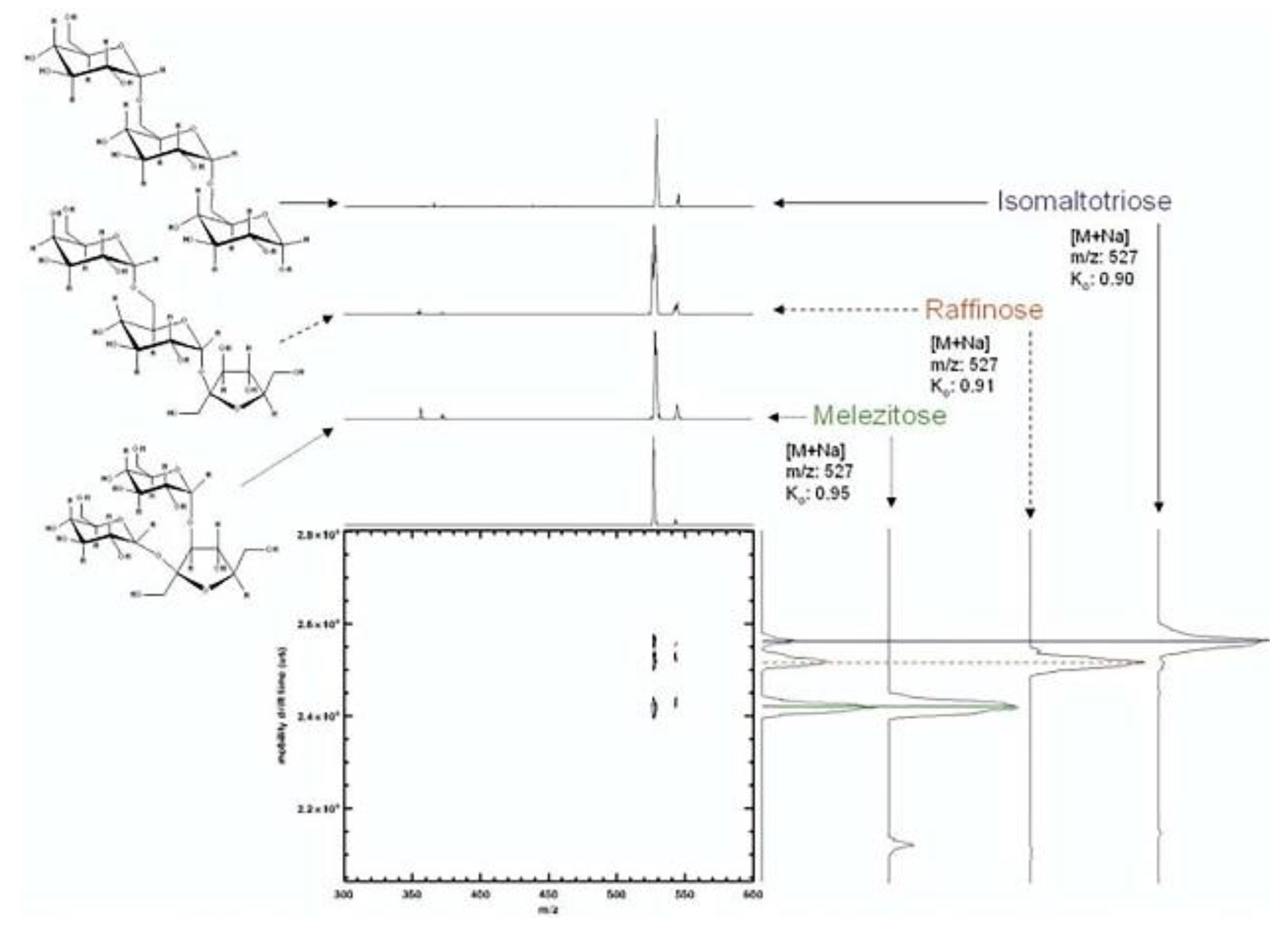
4.2. Complex carbohydrates
4.3. Oligonucleotides
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gray, C.J.; Thomas, B.; Upton, R.; Migas, L.G.; Eyers, C.E.; Barran, P.E.; Flitsch, S.L. Applications of ion mobility mass spectrometry for high throughput, high resolution glycan analysis. Biochim. Biophys. Acta 2016, 1860, 1688–1709. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bendiak, B.; Siems, W.F.; Gang, D.R.; Hill, H.H. Ion mobility mass spectrometry analysis of isomeric disaccharide precursor, product and cluster ions. Rapid Commun. Mass Spectrom. 2013, 27, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Clemmer, D. Characterizing oligosaccharides using injected-ion mobility mass spectrometry. Anal. Chem. 1997, 69, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Clowers, B.H.; Dwivedi, P.; Steiner, W.E.; Hill, H.H.; Bendiak, B. Separation of sodiated isobaric disaccharides and trisaccharides using electrospray ionization-atmospheric pressure ion mobility-time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2005, 16, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Pagel, K. Glycan analysis by ion mobility-mass spectrometry. Angew. Chem. Int. Ed. 2017, 56, 8342–8349. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, A.; Guttman, M. Bridging the structural gap of glycoproteomics with ion mobility spectrometry. Curr. Opin. Chem. Biol. 2018, 42, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.A.; Clowers, B.H. Contemporary glycomic approaches using ion mobility–mass spectrometry. Curr. Opin. Chem. Biol. 2018, 42, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Manz, C.; Pagel, K. Glycan analysis by ion mobility-mass spectrometry and gas-phase spectroscopy. Curr. Opin. Chem. Biol. 2018, 42, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Glover, M.S.; Li, L. Recent advances in ion mobility–mass spectrometry for improved structural characterization of glycans and glycoconjugates. Curr. Opin. Chem. Biol. 2018, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.; Kim, K.S. Carbohydrate chemistry. Chem. Soc. Rev. 2013, 42, 4267–4269. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Grabenauer, M.; Holland, R.J.; Carpenter, C.J.; Wormald, M.R.; Giles, K.; Harvey, D.J.; Bateman, R.H.; Scrivens, J.H.; Bowers, M.T. Characterization of simple isomeric oligosaccharides and the rapid separation of glycan mixtures by ion mobility mass spectrometry. Int. J. Mass Spectrom. 2010, 298, 119–127. [Google Scholar] [CrossRef]
- Dwivedi, P.; Bendiak, B.; Clowers, B.H.; Hill, H.H. Rapid resolution of carbohydrate isomers by electrospray ionization ambient pressure ion mobility spectrometry-time-of-flight mass spectrometry (ESI-APIMS-TOFMS). J. Am. Soc. Mass Spectrom. 2007, 18, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Bendiak, B.; Clowers, B.; Hill, H.H. Ion mobility-mass spectrometry analysis of isomeric carbohydrate precursor ions. Anal. Bioanal. Chem. 2009, 394, 1853–1867. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Hahm, H.S.; Seeberger, P.H.; Pagel, K. Identification of carbohydrate anomers using ion mobility-mass spectrometry. Nature 2015, 526, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Giles, K.; Bendiak, B.; Kaplan, K.; Siems, W.F.; Hill, H.H. Resolving structural isomers of monosaccharide methyl glycosides using drift tube and traveling wave ion mobility mass spectrometry. Anal. Chem. 2012, 84, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bendiak, B.; Siems, W.F.; Gang, D.R.; Hill, H.H. Determining the isomeric heterogeneity of neutral oligosaccharide-alditols of bovine submaxillary mucin using negative ion traveling wave ion mobility mass spectrometry. Anal. Chem. 2015, 87, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Schultz, A.J.; Hill, H.H., Jr. Metabolic profiling of human blood by high-resolution ion mobility mass spectrometry (IM-MS). Int. J. Mass Spectrom. 2010, 298, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Stow, S.M.; Lareau, N.M.; Hines, K.M.; McNees, C.R.; Goodwin, C.R.; Bachmann, B.O.; Mclean, J.A. Structural Separations for Natural Product Characterization by Ion Mobility–Mass Spectrometry Fundamental Theory to Emerging Applications; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; pp. 397–431. [Google Scholar]
- Pagel, K.; Harvey, D.J. Ion mobility-mass spectrometry of complex carbohydrates: collision cross sections of sodiated N-linked glycans. Anal. Chem. 2013, 85, 5138–5145. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.S.; Martin, D.W.; McDaniel, E.W. Mass spectrographic identification of the ion observed in hydrogen mobility experiments. Phys. Rev. Lett. 1961, 6, 110–111. [Google Scholar] [CrossRef]
- McAfee, K.B., Jr.; Edelson, D. Identification and mobility of ions in a townsend discharge by time-resolved mass spectrometry. Proc. Phys. Soc. 1963, 81, 382–384. [Google Scholar] [CrossRef]
- Fenn, L.S.; McLean, J.A. Biomolecular structural separations by ion mobility-mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 905–909. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.A. The mass-mobility correlation redux: the conformational landscape of anhydrous biomolecules. J. Am. Soc. Mass Spectrom. 2009, 20, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Vakhrushev, S.Y.; Langridge, J.; Campuzano, I.; Hughes, C.; Peter-Katalinic, J. Identification of monosialylated N-glycoforms in the CDG urinome by ion mobility tandem mass spectrometry: the potential for clinical applications. Clin. Proteomics 2008, 4, 47–57. [Google Scholar] [CrossRef]
- Pritchard, L.K.; Harvey, D.J.; Bonomelli, C.; Crispin, M.; Doores, K.J. Cell- and protein-directed glycosylation of native cleaved HIV-1 envelope. J. Virol. 2015, 89, 8932–8944. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Andaya, A.; Leary, J.A. Preparation, separation, and conformational analysis of differentially sulfated heparin octasaccharide isomers using ion mobility mass spectrometry. Anal. Chem. 2012, 84, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Struwe, W.B.; Benesch, J.L.; Harvey, D.J.; Pagel, K. Collision cross sections of high-mannose N-glycans in commonly observed adduct states – identification of gas-phase conformers unique to [M − H](−) ions. Analyst 2015, 140, 6799–6803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Singh, A.; Li, L.; Linhardt, R.J.; Xu, Y.; Liu, J.; Woods, R.J.; Amster, I.J. Investigating changes in the gas-phase conformation of Antithrombin III upon binding of Arixtra using traveling wave ion mobility spectrometry (TWIMS). Analyst 2015, 140, 6980–6989. [Google Scholar] [CrossRef] [PubMed]
- Reading, E.; Munoz-Muriedas, J.; Roberts, A.D.; Dear, G.J.; Robinson, C.V.; Beaumont, C. Elucidation of drug metabolite structural isomers using molecular modeling coupled with ion mobility mass spectrometry. Anal. Chem. 2016, 88, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Ewing, R.G.; Atkinson, D.A.; Eiceman, G.A.; Ewing, G.J. A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta 2001, 54, 515–529. [Google Scholar] [CrossRef]
- Mäkinen, M.A.; Anttalainen, O.A.; Sillanpää, M.E.T. Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal. Chem. 2010, 82, 9594–9600. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bendiak, B.; Kaplan, K.; Davis, E.; Siems, W.F.; Hill, H.H. Evaluation of ion mobility-mass spectrometry for determining the isomeric heterogeneity of oligosaccharide-alditols derived from bovine submaxillary mucin. Int. J. Mass Spectrom. 2013, 352, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, L.; Fasciotti, M.; Gennäs, G.B.A.; Kotiaho, T.; Daroda, R.J.; Eberlin, M.; Kostiainen, R. Separation of steroid isomers by ion mobility mass spectrometry. J. Chromatogr. A 2013, 1310, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Fenn, L.S.; Kliman, M.; Mahsut, A.; Zhao, S.R.; McLean, J.A. Characterizing ion mobility-mass spectrometry conformation space for the analysis of complex biological samples. Anal. Bioanal. Chem. 2009, 394, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, D.; Kurulugama, R.T.; Plasencia, M.D.; Stokes, S.T.; Kyselova, Z.; Goldman, R.; Mechref, Y.; Novotny, M.V.; Clemmer, D.E. Profiling of human serum glycans associated with liver cancer and cirrhosis by IMS−MS. J. Proteome Res. 2008, 7, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, M.D.; Isailovic, D.; Merenbloom, S.I.; Mechref, Y.; Clemmer, D.E. Resolving and assigning N-linked glycan structural isomers from ovalbumin by IMS-MS. J. Am. Soc. Mass Spectrom. 2008, 19, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Valentine, S.J.; Reilly, J.P.; Clemmer, D.E. Analyzing a mixture of disaccharides by IMS-VUVPD-MS. Int. J. Mass Spectrom. 2012, 309, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Che, F.-Y.; Deng, H.-T.; Ding, S.-J. Mass spectrometry applications in biomedical research. BioMed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, V.N.; Reinhold, B.B.; Costello, C.E. Carbohydrate molecular weight profiling, sequence, linkage, and branching data: ES-MS and CID. (electrospray-mass spectrometry collision-induced dissociation). Anal. Chem. 1995, 67, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Siems, W.F.; Klasmeier, J.; Hill, H.H. Separation of isomeric peptides using electrospray ionization/high-resolution ion mobility spectrometry. Anal. Chem. 2000, 72, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Thalassinos, K.; Slade, S.E.; Jennings, K.R.; Scrivens, J.H.; Giles, K.; Wildgoose, J.; Hoyes, J.; Bateman, R.H.; Bowers, M.T. Ion mobility mass spectrometry of proteins in a modified commercial mass spectrometer. Int. J. Mass Spectrom. 2004, 236, 55–63. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Sowa, S.; Lin, S.; Bell, S.E. Ion mobility spectrometry for continuous on-site monitoring of nicotine vapors in air during the manufacture of transdermal systems. J. Hazard. Mater. 1995, 43, 13–30. [Google Scholar] [CrossRef]
- Enders, J.R.; McLean, J.A. Chiral and structural analysis of biomolecules using mass spectrometry and ion mobility-mass spectrometry. Chirality 2009, 21, E253–E264. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Ugarov, M.; Egan, T.; Koomen, J.; Gillig, K.; Fuhrer, K.; Gonin, M.; Schultz, J. Lipid/peptide/nucleotide separation with MALDI-ion mobility-TOF MS. Anal. Chem. 2004, 76, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Borsdorf, H.; Nazarov, E.G.; Eiceman, G.A. Atmospheric pressure ionization and gas phase ion mobility studies of isomeric dihalogenated benzenes using different ionization techniques. Int. J. Mass Spectrom. 2004, 232, 117–126. [Google Scholar] [CrossRef]
- Von Helden, G.; Wyttenbach, T.; Bowers, M.T. Conformation of macromolecules in the gas phase: use of matrix-assisted laser desorption methods in ion chromatography. Science 1995, 267, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, T.; von Helden, G.; Bowers, M.T. Gas-phase conformation of biological molecules: Bradykinin. J. Am. Chem. Soc. 1996, 118, 8355–8364. [Google Scholar] [CrossRef]
- Clemmer, D.E.; Hudgins, R.R.; Jarrold, M.F. Naked protein conformations: Cytochrome c in the gas phase. J. Am. Chem. Soc. 1995, 117, 10141–10142. [Google Scholar] [CrossRef]
- Srebalus Barnes, C.; Hilderbrand, A.; Valentine, S.; Clemmer, D. Resolving isomeric peptide mixtures: A combined HPLC/ion mobility-TOFMS analysis of a 4000-component combinatorial library. Anal. Chem. 2002, 74, 2–36. [Google Scholar] [CrossRef]
- Niu, S.; Ruotolo, B.T. Collisional unfolding of multiprotein complexes reveals cooperative stabilization upon ligand binding. Protein Sci. 2015, 24, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Valentine, S.J.; Plasencia, M.D.; Liu, X.; Krishnan, M.; Naylor, S.; Udseth, H.R.; Smith, R.D.; Clemmer, D.E. Toward plasma proteome profiling with ion mobility-mass spectrometry. J. Proteome Res. 2006, 5, 2977–2984. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Wu, P.; Klopsch, S.; Puzon, G.; Xun, L.; Hill, H. Metabolic profiling by ion mobility mass spectrometry (IMMS). Metabolomics 2008, 4, 63–80. [Google Scholar] [CrossRef]
- Dwivedi, P.; Puzon, G.; Tam, M.; Langlais, D.; Jackson, S.; Kaplan, K.; Siems, W.F.; Schultz, A.J.; Xun, L.; Woods, A.; Hill, H.H. Metabolic profiling of Escherichia coli by ion mobility-mass spectrometry with MALDI ion source. J. Mass Spectrom. 2010, 45, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Raja, U.K.B.; Injeti, S.; Culver, T.; McCabe, J.W.; Angel, L.A. Probing the stability of insulin oligomers using electrospray ionization ion mobility mass spectrometry. Eur. J. Mass Spectrom. 2015, 21, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Kliman, M.; May, J.C.; McLean, J.A. Lipid analysis and lipidomics by structurally selective ion mobility-mass spectrometry. Biochim. Biophys. Acts Mol. Cell Biol. Lipids 2011, 1811, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nishima, W.; Re, S.; Sugita, Y. Confident identification of isomeric N-glycan structures by combined ion mobility mass spectrometry and hydrophilic interaction liquid chromatography. Rapid Commun. Mass Spectrom. 2012, 26, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- May, J.C.; Goodwin, C.R.; Lareau, N.M.; Leaptrot, K.L.; Morris, C.B.; Kurulugama, R.T.; Mordehai, A.; Klein, C.; Barry, W.; Darland, E.; et al. Conformational ordering of biomolecules in the gas phase: Nitrogen collision cross sections measured on a prototype high resolution drift tube ion mobility-mass spectrometer. Anal. Chem. 2014, 86, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, N.; Crawford, C.; Hauck, B.; Quinton, J.; Seims, W.; Hill, H.; Clark, A. An assessment of computational methods for obtaining structural information of moderately flexible biomolecules from ion mobility spectrometry. J. Am. Soc. Mass Spectrom. 2012, 23, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Baker, E.S.; Zheng, X.; Garimella, S.V.B.; Hamid, A.M.; Ibrahim, Y.M.; Deng, L.; Webb, I.K.; Prost, S.A.; Sandoval, J.A.; Norheim, R.V.; Anderson, G.A.; Tolmachev, A.V. Ultra-high resolution ion mobility separations utilizing traveling waves in a 13 m serpentine path length structures for lossless ion manipulations module. Anal. Chem. 2016, 88, 8957–8964. [Google Scholar]
- Wojcik, R.; Webb, I.; Deng, L.; Garimella, S.; Prost, S.; Ibrahim, Y.; Baker, E.; Smith, R. Lipid and glycolipid isomer analyses using ultra-high resolution ion mobility spectrometry separations. Int. J. Mol. Sci. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Zekavat, B.; Solouki, T. Chemometric data analysis for deconvolution of overlapped ion mobility profiles. J. Am. Soc. Mass Spectrom. 2012, 23, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Gama, M.R.; Da Costa Silva, R.G.; Collins, C.H.; Bottoli, C.B.G. Hydrophilic interaction chromatography. Trends Anal. Chem. 2012, 37, 48–60. [Google Scholar] [CrossRef]
- Li, H.; Bendiak, B.; Siems, W.F.; Gang, D.R.; Hill, H.H. Carbohydrate structure characterization by tandem ion mobility mass spectrometry (IMMS)2. Anal. Chem. 2013, 85, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Fenn, L.S.; McLean, J.A. Enhanced carbohydrate structural selectivity in ion mobility-mass spectrometry analyses by boronic acid derivatization. Chem. Commun. 2008, 5505–5507. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Hofmann, J.; Pagel, K. Energy-resolved ion mobility-mass spectrometry-a concept to improve the separation of isomeric carbohydrates. J. Am. Soc. Mass Spectrom. 2014, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Barran, P.E.; Deakin, J.A.; Lyon, M.; Uhrin, D. Conformation of glycosaminoglycans by ion mobility mass spectrometry and molecular modelling. Phys. Chem. Chem. Phys. 2005, 7, 3464–3471. [Google Scholar] [CrossRef] [PubMed]
- Fenn, L.S.; McLean, J.A. Structural resolution of carbohydrate positional and structural isomers based on gas-phase ion mobility-mass spectrometry. Phys. Chem. Chem. Phys. 2011, 13, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Struwe, W.B.; Scarff, C.A.; Scrivens, J.H.; Harvey, D.J.; Pagel, K. Estimating collision cross sections of negatively charged N-glycans using traveling wave ion mobility-mass spectrometry. Anal. Chem. 2014, 86, 10789–10795. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Crispin, M.; Bonomelli, C.; Harvey, D.J.; Scrivens, J.H. Ion mobility mass spectrometry for ion recovery and clean-up of MS and MS/MS spectra obtained from low abundance viral samples. J. Am. Soc. Mass Spectrom. 2015, 26, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Struwe, W.B.; Crispin, M.; Scarff, C.A.; Edgeworth, M.; Scrivens, J.H.; Scarff, C.A.; Edgeworth, M.; Pagel, K.; Thalassinos, K. Travelling-wave ion mobility mass spectrometry and negative ion fragmentation of hybrid and complex N-glycans. J. Mass Spectrom. 2016, 51, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Both, P.; Green, A.P.; Gray, C.J.; Sardzik, R.; Voglmeir, J.; Fontana, C.; Austeri, M.; Rejzek, M.; Richardson, D.; Field, R.A.; et al. Discrimination of epimeric glycans and glycopeptides using IM-MS and its potential for carbohydrate sequencing. Nat. Chem. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Crispin, M.; Harvey, D.J.; Bitto, D.; Bonomelli, C.; Edgeworth, M.; Scrivens, J.H.; Huiskonen, J.T.; Bowden, T.A. Structural plasticity of the Semliki Forest virus glycome upon interspecies transmission. J. Proteome Res. 2014, 13, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.M.; Saalbach, G.; Bornemann, S. Discrimination of large maltooligosaccharides from isobaric dextran and pullulan using ion mobility mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Abrahams, J.L. Fragmentation and ion mobility properties of negative ions from N-linked carbohydrates: Part 7. Reduced glycans. Rapid Commun. Mass Spectrom. 2016, 30, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Lemmnitzer, K.; Riemer, T.; Groessl, M.; Süβ, R.; Knochenmuss, R.; Schiller, J. Comparison of ion mobility-mass spectrometry and pulsed-field gradient nuclear magnetic resonance spectroscopy for the differentiation of chondroitin sulfate isomers. Anal. Methods 2016, 8, 8483–8491. [Google Scholar] [CrossRef]
- Gidden, J.; Bushnell, J.E.; Bowers, M.T. Gas-phase conformations and folding energetics of oligonucleotides: dTG− and dGT−. J. Am. Chem. Soc. 2001, 123, 5610–5611. [Google Scholar] [CrossRef] [PubMed]
- Koomen, J.M.; Ruotolo, B.T.; Gillig, K.J.; McLean, J.A.; Russell, D.H.; Kang, M.; Dunbar, K.R.; Fuhrer, K.; Gonin, M.; Schultz, J.A. Oligonucleotide analysis with MALDI-ion-mobility-TOFMS. Anal. Bioanal. Chem. 2002, 373, 612–617. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Y.; Schulz, B.L.; Ferro, V. Applications of Ion Mobility-Mass Spectrometry in Carbohydrate Chemistry and Glycobiology. Molecules 2018, 23, 2557. https://doi.org/10.3390/molecules23102557
Mu Y, Schulz BL, Ferro V. Applications of Ion Mobility-Mass Spectrometry in Carbohydrate Chemistry and Glycobiology. Molecules. 2018; 23(10):2557. https://doi.org/10.3390/molecules23102557
Chicago/Turabian StyleMu, Yuqing, Benjamin L. Schulz, and Vito Ferro. 2018. "Applications of Ion Mobility-Mass Spectrometry in Carbohydrate Chemistry and Glycobiology" Molecules 23, no. 10: 2557. https://doi.org/10.3390/molecules23102557
APA StyleMu, Y., Schulz, B. L., & Ferro, V. (2018). Applications of Ion Mobility-Mass Spectrometry in Carbohydrate Chemistry and Glycobiology. Molecules, 23(10), 2557. https://doi.org/10.3390/molecules23102557