Abstract
A series of novel pyridine and fused pyridine derivatives have been prepared starting from 6-(3,4-dimethylphenyl)-2-hydrazinyl-4-(thiophen-2-yl)-pyridine-3-carbonitrile 1 which on treatment with appropriate formic acid, acetic acid/acetic anhydride, benzoyl chloride and/or carbon disulfide afforded the corresponding triazolopyridine derivatives 2–5. Also, treatment of hydrazide 1 with diethyloxalate, chloroacetyl chloride, chloroacetic acid and/or 1,2-dichloroethane yielded the corresponding pyridotriazine derivatives 7–10. Further transformation of compound 1 with a different active methylene group, namely acetyl acetone, diethylmalonate, ethyl cyanoacetate, ethyl benzoylacetate and/or ethyl acetoacetate, produced the pyridine–pyrazole hybrid derivatives 11–15. These newly synthesized compounds (1–15) were subjected to in silico molecular docking screenings towards GlcN-6-P synthase as the target protein. The results revealed moderate to good binding energies of the ligands on the target protein. All the newly prepared products exhibited antimicrobial and antioxidant activity.
1. Introduction
Pyridine derivatives have received great attention because of their presence in various drugs and biologically active molecules [1,2,3,4]. In our previous work, we found that heterocyclic compounds implicate the pyridine nucleus and showed wide promising biological activities such as anti-cancer [5,6,7], anti-oxidant [8,9], anti-microbial [5,9,10] and anti-viral [2] activities. In addition, the literature reports that pyridine derivatives are potent anti-inflammatory [11,12,13,14], Ca2+ channel antagonists [15], anti-cancer [16,17,18], anti-plasmodial [19], anti-microbial [20,21], anti-malarial [22] anti-biotic [23], analgesic [24,25], anti-oxidant [26] agents. Also, compounds containing triazolopyridine nucleuses are associated with diverse activities such as anti-fungal [27,28,29,30], insecticidal [30], herbicidal [31], anti-convulsant [32] and anti-bacterial [33,34] activities. Several pyridotriazine analogues were reported to possess various biological activities such as anti-Alzheimer’s [35], anti-fungal [36], anti-microbial [37], anti-thrombotic [38], and hypotensive agents, while also acting as antagonists of serotonin receptors 5-HT2 and 5-HT2a [39,40]. On the other hand, pyrazole hybrid heterocyclic compounds were reported as anti-bacterial [41,42,43,44], anti-inflammatory [42,43,44,45,46], anti-oxidant [46,47], anti-cancer [48,49,50] agents, etc.
In designing a drug candidate, interaction between drug and receptor is mostly understood by employing molecular docking studies. This provides information about the interaction along with predicting the activity and affinity of the targeted drug candidate on the selected receptor [51]. Enzymes involved in the process of biosynthesis of the cell walls of the microbes are considered to be good targets for docking while designing novel compounds such as anti-microbial candidates. In this regard, the enzyme glucosamine-6-phosphate synthase (GlmS, GlcN-6-P synthase, L-glutamine: D-fructose-6P amido-transferase, EC 2.6.1.16) came out to be an attractive target for docking studies in anti-bacterial and anti-fungal drug design [52] as it is involved in the formation of N-acetyl Glucosamine (the core amino sugar) which is an important building block of the fungal and the bacterial cell wall [53,54]. Nicotinonitrile-based analogues have been reported as potent anti-bacterial and anti-fungal agents [55,56,57]. Also, some nicotinonitrile derivatives have been reported as inhibitors of GlcN-6-P synthase [57]. Thus, it was thought to be worthwhile to predict drug–receptor interaction employing in silico molecular docking screenings of the synthesized compounds on the selected target i.e., GlcN-6-P synthase.
In line with our previous work [58,59,60,61] and our observations, we propose the modification of new heterocyclic compounds containing a pyridine nucleus in the hope that they could possess good anti-oxidant ability and anti-microbial activity.
2. Results and Discussion
2.1. Chemistry
In the course of our investigation, we have found that 6-(3,4-dimethylphenyl)-2-hydrazinyl-4-(thiophen-2-yl)-pyridine-3-carbonitrile [62] is an excellent building block for the synthesis of a numerous heterocyclic ring systems. Thus, 2-hydrazino-nicotinonitrile 1 reacted with formic acid or acetic acid/acetic anhydride or benzoyl chloride and/or carbon disulfide and afforded the corresponding triazolopyridine derivatives 2–5 (Scheme 1). The infrared (IR) spectra of new triazolopyridine derivatives 2–5 showed an absorption band characteristic for (CN) at 2213, 2211, 2209 and 2212 cm−1, respectively. In addition to the disappearance of the characteristic band for NH, NH2 groups of starting 2-hydrazino-nicotinonitrile 1. The 1H nuclear magnetic resonance (NMR) spectrum of compound 2 showed signals at δ = 2.26, 2.28 for two CH3 group, 7.23–7.34 & 7.51–7.64 for aromatic–H, 7.92 for pyridine–H and 8.11 for triazole–H. The 1H-NMR spectrum of compound 5 showed signals at δ = 2.26, 2.27 for two CH3 group, 7.23–7.31 & 7.50–7.62 for aromatic–H, 7.89 for pyridine–H and 8.87 for the NH group, and its mass spectrum showed the molecular ion peak at m/z (%): 362 (M+, 45%) while its base peak was m/z 304 (M+–NCS, 100%) (cf. the Materials and Methods Section).
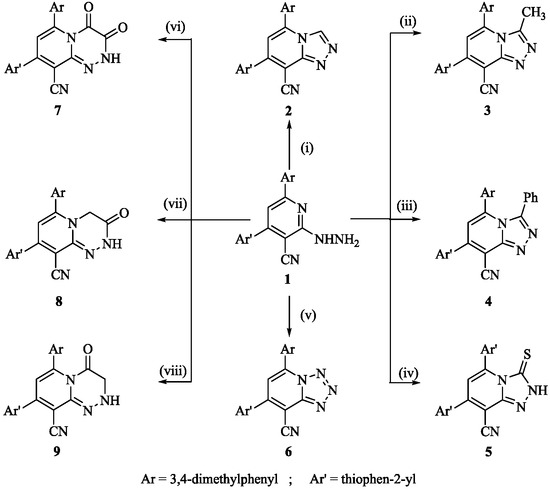
Scheme 1.
Synthesis of compounds 2–9; reagent and conditions: (i) HCOOH, heat; (ii) CH3COOH, (CH3CO)2O, heat; (iii) PhCOCl, heat; (iv) CS2/KOH, heat; (v) NaNO2/HCl, rt; (vi) (COOEt)2, THF, heat; (vii) ClCH2COCl, DMF, heat; (viii) ClCH2COOH, DMF, heat.
2-Hydrazino-nicotinonitrile 1 upon diazotization with a solution of sodium nitrite in hydrochloric acid yielded the corresponding tetrazolopyridine derivative 6 (Scheme 1). The IR and 1H-NMR spectra of the latter compound revealed the absence of characteristic signals for the NH and NH2 groups and its mass spectrum showed molecular ion peak at m/z (%):331 (M+, 37%), 303 (M+–N2, 100%) (cf. the Materials and Methods Section).
On the other hand, treatment of 2-Hydrazino-nicotinonitrile 1 with diethyloxalate, chloroacetyl chloride, chloroacetic acid and/or 1,2-dichloroethane yielded the corresponding pyridotriazine derivatives 7–10, respectively (Scheme 1 and Scheme 2). The IR spectrum of dioxopyridotriazine derivative 7, for example, showed absorption bands at 3150; 2209; 1713 and 1675 cm−1 for (NH); (CN) and two (C=O) groups respectively. Its 1H-NMR spectrum showed signals at δ = 2.26, 2.28 for two CH3 groups, 7.25–7.34 & 7.49–7.61 for Aromatic–H, 7.90 for pyridine–H, and 9.02 for the NH group; and its mass spectrum showed a molecular ion peak at m/z (%):374 (M+, 22%). Upon spectral measurements the isomeric structures of 8 and 9 were deduced (cf. the Materials and Methods Section). The IR spectrum of pyridotriazine derivative 10 displays absorption bands attributed to (NH) and (CN) and its 1H-NMR spectrum showed a signal at δ = 4.51–4.88 for 2CH2 groups in addition to two CH3 groups, Aromatic–H and pyridine–H (cf. the Materials and Methods Section).
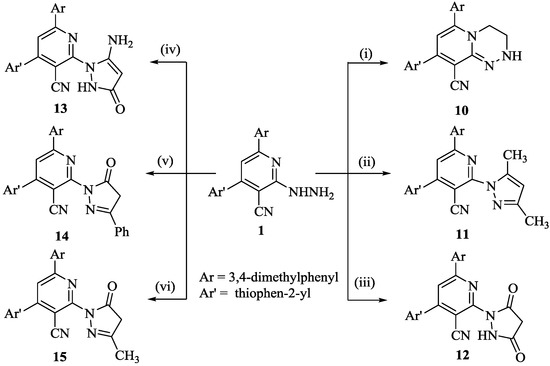
Scheme 2.
Synthesis of compounds 10–15; reagent and conditions: (i) ClCH2CH2Cl, DMF, heat; (ii) CH3COCH2COCH3, EtOH, heat; (iii) CH2(COOEt)2, EtOH, heat; (iv) NCCH2CO2Et, EtOH, heat; (v) PhCOCH2CO2Et, EtOH, heat; (vi) 1-CH3COCH2CO2Et, EtOH, heat; 2- EtONa, heat.
Further transformation of compound 1 with a different active methylene group, namely acetyl acetone, diethylmalonate, ethyl cyanoacetate, ethyl benzoylacetate and/or ethyl acetoacetate, produced the pyridine–pyrazole hybrid derivatives 11–15 (Scheme 2). The 1H-NMR spectrum of compound 11 displays signals at δ = 2.30, 2.67 for two new CH3 groups, 5.96 for pyrazole–H in addition to two CH3 group, Aromatic–H and pyridine–H (cf. the Materials and Methods Section). The IR spectrum of compound 12 revealed absorption bands attributed to (NH); (CN) and two (C=O) groups at 3220; 2215; 1729 and 1680 cm−1, respectively, and its 1H-NMR spectrum showed the signals of CH2 and NH protons for the pyrazole ring at δ = 5.13 and 9.08, respectively, in addition to two CH3 group, Aromatic–H and pyridine–H (cf. the Materials and Methods Section). The IR spectrum of compound 13 showed bands at 3233; 3117; 2210 and 1685 cm−1 for (NH2); (NH); (CN) and (C=O) groups, respectively, and its 1H-NMR spectrum showed the signals of CH; NH and NH2 protons for the pyrazole ring at δ = 6.12; 7.89 and 9.13, respectively, in addition to two CH3 group, Aromatic–H and pyridine–H (cf. the Materials and Methods Section). The IR spectra of both 14 and 15 showed bands for (CN) and (C=O) groups at 2217; 1665 and 2215; 1778, respectively. The 1H-NMR spectrum showed the signals of CH2 for the pyrazole ring at δ = 2.93; 3.10 and 3.71; 3.96, respectively (cf. the Materials and Methods Section).
2.2. In Silico Molecular Docking Screenings
In silico molecular docking screenings were performed after achieving synthesis and characterization of the compounds. GlcN-6-P synthase was considered as the target receptor. In order to determine the best in silico conformation, comparative and automated docking studies were performed with the newly synthesized drug candidates. All the synthesized compounds (1–15) were subjected to docking studies on the target protein GlcN-6-P synthase. Figure 1 shows the docked images of these newly synthesized ligand drug candidates on the target protein. The minimum binding energies and estimated inhibition constants of the synthesized compounds are documented in Table 1. The results of in silico studies revealed a moderate to good pattern of affinity and activity of the synthesized compounds on the target protein which is supported by in vitro antimicrobial screenings as the compounds were active against one and/or the other tested microorganisms. Compounds with higher binding energies came out to be more potent than the standard drug taken. Their binding energies ranged from −6.08 to −7.84 kJ/mol with an estimated inhibition constant ranging from 1.79 to 35 micromol.
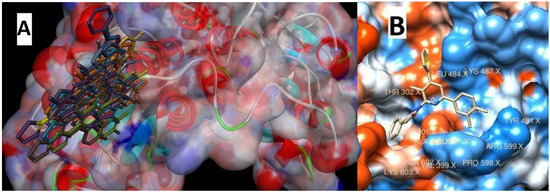
Figure 1.
Docking into active site of glucosamine-6-phosphate (GlcN-6-P) synthase (PDB ID: 2VF5). (A) Docked poses of compounds 1–15 on the GlcN-6-P; (B) the interaction between the compound no. 14 and GlcN-6-P.

Table 1.
In silico molecular docking results of the synthesized compounds (1–15).
2.3. Pharmacological Screening
2.3.1. Anti-Microbial Activity
All synthesized compounds 1–15 were tested for their preliminary in vitro anti-microbial activity against different microorganisms according to reported methods [63] representing fungi (Aspergillus flavus; Regional Center for Mycology and Biotechnology (RCMB) 002002 and Candida albicans; RCMB 005003 (1) ATCC 10231), Gram-positive bacteria (Staphylococcus aureus; RCMB010010 and Bacillus subtilis; RCMB 015 (1) NRRL B-543) and Gram-negative bacteria (Escherichia coli; (RCMB 010052) ATCC 25955 and Salmonella typhimurium; RCMB 006 (1) ATCC 14028). All microorganisms used were obtained from the RCMB, Al-Azhar University, Egypt. The mean zone of inhibition in mm beyond well diameter (6 mm) produced a range of pathogenic microorganisms. The results are depicted in the following Table 2.

Table 2.
Anti-microbial activities of newly synthesized compounds 1–15.
2.3.2. Anti-Oxidant Activity Using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Scavenging
The antioxidant activity of extract was determined at the RCMB at Al-Azhar University by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay in triplicate and average values were considered. All new prepared pyridine derivatives were screened for anti-oxidant activity according to the reported method [64].
Figure 2 displays DPPH radical scavenging ability of Tocopherol and all synthesized compounds 1–15 at different concentrations; compounds 14 and 15 showed excellent anti-oxidant activity at all concentrations due to the introduced 5-oxo-3-Phenyl/Methyl-dihydropyrazole ring. Furthermore, it is apparent that compound 1 which has hydrazide group at the periphery of the molecular chain, has moderate anti-oxidant activity at all concentrations; derivatives 2–7, 9, 11–13 possessed weak anti-oxidant activity at all concentrations while derivatives 8 and 10 showed very weak anti-oxidant activity at all concentrations. All of these results agree with the IC50 values shown in Table 3.
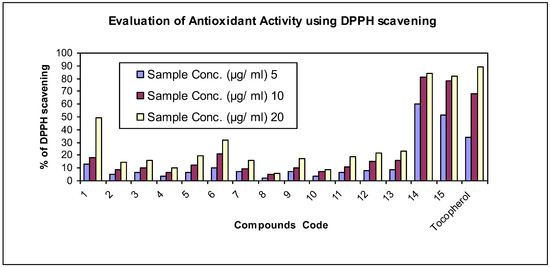
Figure 2.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability of Tocopherol and all synthesized compounds 1–15.

Table 3.
The IC50 value of antioxidant activity for newly synthesized compounds 1–15.
2.3.3. Examination of the Structural Activity Relationship (SAR)
The excellent antioxidant activity of the synthesized compounds 14 and 15 depends on the electronic environment and structural skeleton of the molecule. The promising activity of these synthesized motifs is mainly due to the introduction of the 5-oxo-3-Phenyl/Methyl-dihydropyrazole ring into the skeleton.It was observed that replacement on the 3rd position in the dihydropyrazole ring by the phenyl ring or the methyl group dramatically enhances the anti-oxidant activity. Furthermore, it is apparent that compound 1 and compound 6 bearing the hydrazide and tetrazole group, respectively, at the periphery of the molecular chain, have moderate antioxidant activity.
3. Materials and Methods
3.1. General Information
Melting points were measured using an Electro-Thermal IA 9100 digital melting point apparatus (Büchi, Flawil, Switzerland) and are uncorrected. Infrared spectra were recorded on a Perkin-Elmer 1600 FTIR (Perkin-Elmer, Waltham, MA, USA) discs. NMR spectra were determined on a Jeol-Ex-500 NMR spectrometer (JEOL, Tokyo, Japan) and chemical shifts were expressed as part per million; (δ values, ppm) against TMS as internal reference, National Research Center, Cairo, Egypt. The mass spectra were run at 70 eV with a Finnigan SSQ 7000 spectrometer (Thermo Electron Corporation, Madison, WI, USA) using EI and the values of m/z are indicated in Dalton. Elemental analyses were performed on a Perkin-Elmer 2400 analyzer (Perkin-Elmer) and were found within the accepted range (± 0.30) of the calculated values. Reaction monitoring and verification of the purity of the compounds was done by TLC on silica gel pre-coated aluminum sheets (type 60 F254, Merck, Darmstadt, Germany). All solvents and chemical reagents were purchased from Aldrich (Munich, Germany). Compound 1 was prepared according to a reported method [62].
3.2. Chemistry
3.2.1. 5-(3,4-Dimethylphenyl)-7-(thiophen-2-yl)-[1,2,4]triazolo[4,3-a]pyridine-8-carbonitrile (2)
A mixture of compound 1 (0.01 mole) and formic acid (20 mL) was refluxed for 20 h. The reaction mixture was filtered off on hot and the separated solid was recrystallized from dioxane to give 2. Yield 73%, m.p. 162–164 °C. IR (KBr, ν, cm−1): 2213 (CN). 1H-NMR spectrum (dimethyl sulfoxide (DMSO)-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 7.23–7.34 (m, 3H, Ar–H), 7.51–7.64 (m, 3H, Ar–H), 7.92 (s, 1H, pyridine–H), 8.11(s, 1H, triazole–H). 13C-NMR spectrum (CDCl3, δ ppm): 19.80, 20.56, 114.16, 115.23, 115.45, 117.41, 120.05, 124.18, 124.29, 126.78, 127.89, 128.58, 128.67, 128.77, 129.35, 131.56, 131.68, 144.38, 159.43; MS, m/z (%): 330 (M+, 52%). Analysis for C19H14N4S (330.41): C, 69.07; H, 4.27; N, 16.96; S, 9.70. Found: C, 68.81; H, 4.06; N, 16. 79; S, 9.41.
3.2.2. 5-(3,4-Dimethylphenyl)-3-methyl-7-(thiophen-2-yl)-[1,2,4]triazolo[4,3-a]pyridine-8-carbonitrile (3)
A mixture of compound 1 (0.01 mole) and acetic acid/acetic anhydride (30 mL; 2:1) was refluxed for 8 h. The reaction mixture was cooled and poured onto iced-water. The separated solid was filtered off, dried and recrystallized from ethanol to give 3. Yield 78%, m.p. 199–201 °C. IR (KBr, ν, cm−1): 2211 (CN). 1H-NMR spectrum (DMSO-d6, δ ppm): 2.21 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.28 (s, 3H, CH3), 7.22–7.31 (m, 3H, Ar–H), 7.48–7.60 (m, 3H, Ar–H), 7.88 (s, 1H, pyridine–H). 13C-NMR spectrum (CDCl3, δ ppm): 17.82, 19.27, 20.83, 114.24, 115.62, 115.81, 117.47, 121.20, 124.51, 124.67, 126.95, 127.87, 128.57, 128.76, 129.66, 129.70, 131.57, 131.81, 144.61, 161.61; MS, m/z (%): 344 (M+, 67%), 329 (M+–CH3, 100%). Analysis for C20H16N4S (344.43): C, 69.74; H, 4.68; N, 16.27; S, 9.31. Found: C, 68.47; H, 4.41; N, 16.00; S, 9.05.
3.2.3. 5-(3,4-Dimethylphenyl)-3-phenyl-7-(thiophen-2-yl)-[1,2,4]triazolo[4,3-a]pyridine-8-carbonitrile (4)
A mixture of compound 1 (0.01 mole), benzoyl chloride (5 mL) and trimethylamine (0.5 mL) was refluxed in ethanol (20 mL) for 8 h. The solvent was removed under reduced pressure and the residue was recrystallized from methanol to give 4. Yield 59%, m.p. 251–253 °C. IR (KBr, ν, cm−1): 2209 (CN). 1H-NMR spectrum (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 7.22–7.31 (m, 3H, Ar–H), 7.48–7.60 (m, 3H, Ar–H), 7.81–8.48 (m, 6H, 5Ar–H+ pyridine–H).13C-NMR spectrum (CDCl3, δ ppm): 18.96, 21.15, 113.10, 114.17, 114.38, 116.48, 119.74, 123.23, 126.60, 126.72, 126.97, 127.08, 127.33, 127.52, 127.71, 130.32, 130.49, 133.50, 133.68, 134.11, 134.21, 134.31, 143.39, 151.68, 153.78; MS, m/z (%): 406 (M+, 73%), 329 (M+–Ph, 100%). Analysis for C25H18N4S (406.5): C, 73.87; H, 4.46; N, 13.78; S, 7.89. Found: C, 73.61; H, 4.17; N, 13.49; S, 7.59.
3.2.4. 5-(3,4-Dimethylphenyl)-7-(thiophen-2-yl)-3-thioxo-2,3-dihydro-[1,2,4]triazolo[4,3-a]pyridine-8-carbonitrile (5)
To an aqueous solution of 1 (0.01 mole) in ethanol (20 mL), carbon disulfide (10 mL) was added then the reaction mixture was refluxed in a water-bath for 3 h, cooled, poured onto iced-water and neutralized with 2–3 drops of hydrochloric acid (35%). The precipitate was filtered off, left to dry and recrystallized from methanol to give 5. Yield 69 %; m.p. 136–138 °C. IR spectrum (KBr, ν, cm−1): 3115 (NH); 2212 (CN). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.27 (s, 3H, CH3), 7.23–7.31 (m, 3H, Ar–H), 7.50–7.62 (m, 3H, Ar–H), 7.89 (s, 1H, pyridine–H), 8.87(s, 1H, NH; D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 18.81, 21.10, 133.67, 114.74, 114.96, 117.06, 120.32, 123.81, 127.18, 127.29, 127.55, 127.65, 127.90, 128.10, 128.29, 130.90, 131.07, 143.96, 160.70; Ms, m/z (%): 362 (M+, 45), 304 (M+–NCS, 100). Analysis for C19H14N4S2: C, 62.96; H, 3.89; N, 15.46; S, 17.69. Found: C, 62.68; H, 3.60; N, 15.18; S, 17.41.
3.2.5. 5-(3,4-Dimethylphenyl)-7-(thiophen-2-yl)tetrazolo[1,5-a]pyridine-8-carbonitrile (6)
To an ice-cold solution of compound 1 (0.01 mole) in hydrochloric acid (35%, 10 mL), a solution of sodium nitrite [prepared by dissolving sodium nitrite (0.01 mole) in water (3 mL)] was added drowsily in an ice-bath. The reaction mixture was allowed to stand overnight at room temperature and then was poured onto water. The formed solid was filtered off; washed with water; dried and recrystallized from ethanol to give 6. Yield 47%, m.p.175–177 °C. IR (KBr, ν, cm−1): 2213 (CN). 1H-NMR spectrum (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 7.23–7.31 (m, 3H, Ar–H), 7.50–7.62 (m, 3H, Ar–H), 7.89 (s, 1H, pyridine–H). 13C-NMR spectrum (CDCl3, δ ppm): 18.61, 20.81, 114.81, 115.80, 116.01, 118.00, 121.56, 124.99, 125.18, 127.37, 128.16, 128.77, 129.16, 129.96, 132.29, 136.96, 138.72, 145.07; MS, m/z (%): 331 (M+, 37%), 303 (M+–N2, 100%). Analysis for C18H13N5S (331.39): C, 65.24; H, 3.95; N, 21.13; S, 9.68. Found C, 64.95; H, 3.67; N, 20.88; S, 9.41.
3.2.6. 6-(3,4-Dimethylphenyl)-3,4-dioxo-8-(thiophen-2-yl)-3,4-dihydro-2H-pyrido[2,1-c][1,2,4] triazine-9-carbonitrile (7)
A mixture of compound 1 (0.01 mole) and diethyl oxalate (0.01 mole) in THF (50 mL) was refluxed for 24 h and then cooled. The solid obtained was filtered off and recrystallized from acetic acid to give 7. Yield 56%, m.p. 233–235 °C. IR spectrum (KBr, ν, cm−1): 3150 (NH); 2209 (CN); 1713 (C=O); 1675 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 7.25–7.34 (m, 3H, Ar–H), 7.49–7.61(m, 3H, Ar–H), 7.90 (s, 1H, pyridine–H), 9.02 (s, 1H, NH, D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 19.33, 21.53, 115.53, 116.52, 116.73, 118.72, 122.28, 125.71, 125.90, 128.08, 128.50, 128.87, 129.49, 129.88, 130.67, 133.01, 137.67, 139.44, 159.68, 162.69; MS, m/z (%): 374 (M+, 22). Analysis for C20H14N4O2S: C, 64.16; H, 3.77; N, 14.96; S, 8.56. Found C, 63.88; H, 3.48; N, 14.68; S, 8.29.
3.2.7. 6-(3,4-Dimethylphenyl)-3-oxo-8-(thiophen-2-yl)-3,4-dihydro-2H-pyrido[2,1-c][1,2,4]triazine-9-carbonitrile (8)
To a solution of compound 1 (0.01 mole) in DMF (30 mL), chloroacetyl chloride (0.01 mole) was added dropwise under stirring at room temperature. The reaction mixture was then heated for 12 h and after cooling, poured onto iced-water with vigorous stirring. The precipitate was collected by filtration, washed with water, dried and recrystallized from DMF to give 8. Yield 47%; m.p. 208–210 °C. IR spectrum (KBr, ν, cm−1): 3120 (NH); 2210 (CN); 1675 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.27 (s, 3H, CH3), 4.79 (d, J = 9.10; 1H, CH2), 4.87 (d, J = 9.17, 1H, CH2), 7.24–7.32 (m, 3H, Ar–H), 7.48–7.60 (m, 3H, Ar–H), 7.90 (s, 1H, pyridine–H), 8.51 (s, 1H, NH, D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 18.65, 21.10, 62.15, 113.53, 114.52, 114.73, 116.72, 120.28, 123.71, 123.90, 126.08, 126.50, 126.87, 127.49, 127.88, 128.67, 131.01, 135.67, 137.44, 160.69; Ms, m/z (%): 360 (M+, 37). Analysis for C20H16N4OS: C, 66.65; H, 4.47; N, 15.54; S, 8.90. Found: C, 66.37; H, 4.20; N, 15.25; S, 8.66.
3.2.8. 6-(3,4-Dimethylphenyl)-4-oxo-8-(thiophen-2-yl)-3,4-dihydro-2H-pyrido[2,1-c][1,2,4]triazine-9-carbonitrile (9)
To a solution of compound 1 (0.01 mole) in DMF (30 mL), chloroacetic acid (0.01 mole) was added drop wise under stirring at room temperature. The reaction mixture was then heated for 3 h and after cooling, poured onto iced-water with vigorous stirring. The precipitate was collected by filtration, washed with water, dried and recrystallized from DMF to give 9. Yield 60%; m.p. 189–191 °C. IR spectrum (KBr, ν, cm−1): 3175 (NH); 2211(CN); 1667 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 4.17 (d, J = 8.92 Hz, 1H, CH2), 4.22 (d; J = 9.00 Hz; 1H, CH2), 7.25–7.34 (m, 3H, Ar–H), 7.49–7.61(m, 3H, Ar–H), 7.90 (s, 1H, pyridine–H), 8.61 (s, 1H, NH, D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 18.65, 21.10, 48.24, 113.53, 114.52, 114.73, 116.72, 120.28, 123.71, 123.90, 126.08, 126.50, 126.87, 127.49, 127.88, 128.67, 131.01, 135.67, 137.44, 167.48; Ms, m/z (%): 360 (M+, 45). Analysis for C20H16N4OS: C, 66.65; H, 4.47; N, 15.54; S, 8.90. Found: C, 66.40; H, 4.18; N, 15.27; S, 8.64.
3.2.9. 6-(3,4-Dimethylphenyl)-8-(thiophen-2-yl)-3,4-dihydro-2H-pyrido[2,1-c][1,2,4]triazine-9-carbonitrile (10)
A mixture of compound 1 (0.01 mole) and 1,2-dichloroethane (0.01 mole) in DMF (30 mL) was refluxed for 4 h, then poured onto crushed ice. The solid which separated was filtered off and dried then recrystallized from THF to give 10. Yield 58%, m.p. 260–262 °C. IR (KBr, ν, cm−1): 3209 (NH); 2210 (CN). 1H-NMR spectrum (DMSO-d6, δ ppm): 2.25 (s, 3H, CH3), 2.28 (s, 3H, CH3), 4.51–4.82 (m, 4H, 2CH2), 7.20–7.30 (m, 3H, Ar–H), 7.53–7.61 (m, 3H, Ar–H), 7.86 (s, 1H, pyridine–H), 8.67(s, 1H, NH, D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 18.65, 21.10, 41.24, 43.44, 113.53, 114.52, 114.73, 114.82, 116.72, 120.28, 123.71, 123.90, 126.08, 126.50, 127.49, 127.88, 128.67, 131.01, 135.67,137.44; MS, m/z (%): 346 (M+, 57%). Analysis for C20H18N4S (346.45): C, 69.34; H, 5.24; N, 16.17; S, 9.26. Found: C, 69.09; H, 4.95; N, 15.87; S, 8.98.
3.2.10. 2-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(3,4-dimethylphenyl)-4-(thiophen-2-yl)nicotinonitrile (11)
A mixture of compound 1 (0.01 mole) and acetyl acetone (0.02 mole) in ethanol (20 mL) was refluxed for 10 h. The separated solid was filtered off, dried and recrystallized from dioxane to give 11. Yield 79%, m.p. 224–226 °C. IR (KBr, ν, cm−1): 2210 (CN). 1H-NMR spectrum (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.67 (s, 3H, CH3), 5.96 (s, 1H, pyrazole–H), 7.22–7.31 (m, 3H, Ar–H), 7.52–7.61 (m, 3H, Ar–H), 7.88 (s, 1H, pyridine–H). 13C-NMR spectrum (CDCl3, δ ppm): 15.81, 16.89, 18.78, 20.04, 111.81, 113.48, 115.13, 118.93, 121.66, 122.25, 123.97, 124.83, 126.90, 128.99, 130.90, 132.37, 132.77,134.68, 136.16, 137.61, 138.68, 141.39, 142.87; MS, m/z (%): 384 (M+, 41%). Analysis for C23H20N4S (384.5): C, 71.85; H, 5.24; N, 14.57; S, 8.34. Found: C, 71.57; H, 4.97; N, 14.28; S, 8.06.
3.2.11. 6-(3,4-Dimethylphenyl)-2-(3,5-dioxopyrazolidin-1-yl)-4-(thiophen-2-yl)nicotinonitrile (12)
A mixture of compound 1 (0.01 mole) and diethylmalonate (0.01 mole) was fused for 1 h then absolute ethanol (20 mL) was added drop wise and reflux continued for additional 5 h. The solid product formed was filtered off and recrystallized from dioxane to give 12. Yield 70%; m.p. 289–291 °C. IR spectrum (KBr, ν, cm−1): 3220 (NH); 2215 (CN); 1729 (C=O); 1680 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 5.13 (s, 2H, CH2), 7.20–7.28 (m, 3H, Ar–H), 7.47–7.58 (m, 3H, Ar–H), 7.89 (s, 1H, pyridine–H); 9.08 (s, 1H, NH; D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 19.00, 20.04, 50.08, 111.77, 115.40, 118.78, 122.15, 123.97, 124.72, 125.52, 127.33, 129.43, 132.53, 134.89, 137.46, 140.01, 141.83, 142.91, 145.00, 157.76, 167.95; Ms, m/z (%): 388 (M+, 29). Analysis for C21H16N4O2S: C, 64.93; H, 4.15; N, 14.42; S, 8.25. Found: C, 64.66; H, 3.87; N, 14.15; S, 7.96.
3.2.12. 2-(5-Amino-3-oxo-2,3-dihydropyrazol-1-yl)-6-(3,4-dimethylphenyl)-4-(thiophen-2-yl)nicotinonitrile (13)
A mixture of compound 1 (0.01 mole) and ethyl cyanoacetate (0.02 mole) was fused for 1 h then absolute ethanol (20 mL) was added drop wise and reflux continued for additional 2 h. The solid product formed was filtered off and recrystallized from dioxane to give 13. Yield 51%; m.p. 219–221 °C. IR spectrum (KBr, ν, cm−1): 3233 (NH2); 3117 (NH); 2210 (CN); 1685 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 6.12 (s, 1H, pyrazole–H), 6.80 (s, 2H, NH2; D2O exchangeable), 7.21–7.29 (m, 3H, Ar–H), 7.49–7.57 (m, 3H, Ar–H), 7.89 (s, 1H, pyridine–H); 9.13 (s, 1H, NH; D2O exchangeable). 13C-NMR spectrum (CDCl3, δ ppm): 19.00, 20.04, 94.50, 111.83, 114.72, 118.66, 121.80, 123.93, 124.95, 125.20, 127.33, 129.39, 131.00, 132,80, 134.61, 135.98, 141.45, 143.08, 144.64, 145.97, 169.81; Ms, m/z (%): 387 (M+, 19). Analysis for C21H17N5OS: C, 65.10; H, 4.42; N, 18.08; S, 8.28. Found: C, 64.82; H, 4.17; N, 17.79; S, 8.01.
3.2.13. 6-(3,4-Dimethylphenyl)-2-(5-oxo-3-phenyl-4,5-dihydropyrazol-1-yl)-4-(thiophen-2-yl)nicotinonitrile (14)
A mixture of compound 1 (0.01 mole) and ethyl benzoylacetate (0.02 mole) was fused for 1 h then absolute ethanol (20 mL) was added drop wise and reflux continued for additional 4 h. The solid product formed was filtered off and recrystallized from dioxane to give compound 14. Yield 63%; m.p. 279–281 °C. IR spectrum (KBr, ν, cm−1): 2217 (CN); 1665 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.26 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.93 (d, J = 8.98 Hz, 1H, CH2), 3.10 (d, J = 8.96 Hz, 1H, CH2), 7.22–7.31 (m, 3H, Ar–H), 7.48–7.60 (m, 3H, Ar–H), 7.81-8.48 (m, 6H, 5Ar–H+ pyridine–H). 13C-NMR spectrum (CDCl3, δ ppm): 19.43, 20.90, 36.89, 113.91, 117.13, 120.93, 122.88, 123.46, 124.39, 125.10, 126.00, 126.92, 127.07, 129.32, 131.30, 132.54, 133.26, 133.58, 134.75, 136.72, 137.44, 138.87, 139.57, 140.63, 143.07, 145.12,175.60; Ms, m/z (%): 448 (M+, 34), 371 (M+–Ph, 100). Analysis for C27H20N4OS: C, 72.30; H, 4.49; N, 12.49; S, 7.15. Found: C, 72.02; H, 4.21; N, 12.20; S, 6.89.
3.2.14. 6-(3,4-Dimethylphenyl)-2-(3-methyl-5-oxo-4,5-dihydropyrazol-1-yl)-4-(thiophen-2-yl)nicotinonitrile (15)
To a solution of compound 1 (0.01 mole) and ethyl acetoacetate (0.01 mole) was fused for 1 h then absolute ethanol (20 mL) was added drop wise and reflux continued for additional 2 h. The solid product which formed was refluxed with sodium ethoxide (20 mL) for 0.5 h. The solid precipitate was filtered off and recrystallized from acetic acid to give compound 15. Yield 51%, m.p. 263–265 °C. IR spectrum (KBr, ν, cm−1): 2215(CN), 1678 (C=O). 1H-NMR (DMSO-d6, δ ppm): 2.20 (s, 3H, CH3); 2.24 (s, 3H, CH3), 2.27 (s, 3H, CH3), 3.71 (d, J = 8.87 Hz, 1H, CH2), 3.96 (d, J = 8.86 Hz, 1H, CH2), 7.21–7.29 (m, 3H, Ar–H), 7.49–7.57 (m, 3H, Ar-H), 7.89 (s, 1H, pyridine–H). 13C-NMR spectrum (CDCl3, δ ppm): 18.99, 20.96, 21.65, 45.48, 113.99, 116.72, 120.95, 126.17, 126.84, 127.07, 128.44, 129.89, 131.37, 133.07, 134.38, 136.36, 138.13, 139.60, 141.82, 143.38, 144.82, 175.45; MS, m/z (%): 386 (M+, 46), 371(M+–CH3, 100). Analysis for C22H18N4OS: C, 68.37; H, 4.69; N, 14.50; S, 8.30. Found: C, 68.10; H, 4.40; N, 14.23; S, 8.03.
3.3. In Silico Molecular Docking Screenings
A Lamarckian genetic algorithm contained in AutoDock (version 4.0) was employed for in silico molecular docking studies [65] as it is more reliable, efficient, successful and possesses more degree of freedom for ligands as compared to the Monte Carlo method present in old versions of AutoDock. ChemSketch was used for drawing the ligands and that file format was converted into PDB format using Open Babel [66]. Glucosamine-6-phosphate of GlcN-6-P synthase (PDB ID 2VF5) was obtained from Protein Data Bank (http://www.pdb.org/pdb/home/home.do) with the best accurate active region as solved by experimental crystallographic data [67]. This protein was then optimized by removing all the heteroatoms, rotating all the torsions and adding the C-terminal oxygen. Its energy was minimized using by PRODRG server [68]. Ligand was added with polar hydrogen’s along with assignment of Kollman partial charges. We made and adjusted the grid in X, Y, Z-axis so as to cover the entire active site of protein i.e., the 12 amino acids residues (Cys300, Gly301, Thr 302, Ser 303, Ser 347, Gln 348, Ser 349, Thr 352, Val 399, Ala 400, Ala 602 and Lys 603) by setting the grid box size at 70, 64, and 56 Å for x, y and z, respectively, and the grid center to 30.59, 15.822 and 3.497 for x, y and z, respectively, with grid spacing of 0.375 Å. Standard protocol was undertaken for docking studies. The docking results were interpreted according to the .pdb file. The co-ordinates of the minimum energy run were determined using the rmsd table created in the .dlg file. UCSF Chimera 1.11.2 was used to visualize this ligand protein interaction within region of 6.5 Ǻ.
3.4. In Vitro Anti-Microbial Screenings
The Susceptibility Tests were performed according to National Committee for Clinical Laboratory Standards (NCCLS) recommendations (1993). Screening tests regarding the inhibition zone were carried out by the well diffusion method according to Hindler’s method [63]. The inoculum suspension was prepared from colonies grown overnight on an agar plate and inoculated into Mueller–Hinton broth (fungi using malt broth). A sterile swab was immersed in the suspension and used to inoculate Mueller-Hinton agar plates (fungi using malt agar plates). The compounds were dissolved in DMSO with different concentrations (10, 5, 2.5 mg/mL). The inhibition zone was measured around each well after 24 h at 37 °C. Controls using DMSO were adequately done.
3.5. DPPH Radical Scavenging Activity
Freshly prepared (0.004% w/v) methanol solution of DPPH radical was prepared and stored at 10 °C in the dark. A methanol solution of the test compound was prepared. A 40 uL aliquot of the methanol solution was added to 3 ml of DPPH solution. Absorbance measurements were recorded immediately with a ultraviolet (UV)-visible spectrophotometer (Milton Roy, Spectronic 1201). The decrease in absorbance at 515 nm was determined continuously, with data being recorded at 1 min intervals until the absorbance stabilized (16 min). The absorbance of the DPPH radical without anti-oxidant (control) and the reference compound ascorbic acid were also measured. All the determinations were performed in three replicates and averaged. The percentage inhibition (PI) of the DPPH radical was calculated according to the formula:
where AC = Absorbance of the control at t = 0 min and AT = absorbance of the sample + DPPH at t = 16 min.
PI = {(AC − AT)/AC} × 100
4. Conclusions
This study focused on the synthesis of new nicotinonitrile derivatives which were synthesized and characterized using spectral and elemental analyses. All synthesized compounds 1–15 were screened for anti-oxidant activity. Compounds 14 and 15 showed excellent anti-oxidant activity at all concentrations due to the introduced 5-oxo-3-Phenyl/Methyl-dihydropyrazole ring. Furthermore, it is apparent that compound 1 which has a hydrazide group at the periphery of the molecular chain has moderate anti-oxidant activity. In silico studies were undertaken to predict the activity and affinity of the synthesized compounds towards the target protein i.e., Glucosamine-6-phosphate synthase and the results were reported to be ranging from −6.08 to −7.84 kJ/mol along with estimation of inhibition constant, Ki, from 1.79–35 micromol. Also, the prepared compounds were tested for their preliminary in vitro anti-microbial activity against different microorganisms. Some of the compounds came up as active against screened bacterial and fungal strains while other were non-reactive. The in silico and in vitro results are consistent with each other as some of the synthesized compounds have demonstrated moderate to good anti-bacterial and anti-fungal activities as compared to the standard reference drugs.
Author Contributions
E.M.F., W.I.E.-S. and M.E.-S. conceived the research project, participated in all steps of the research, interpreted the results, discussed the experimental data and prepared the manuscript; A.N. conducted the molecular modeling study; and E.A. discussed the experimental data and prepared the manuscript. All authors read, discussed and approved the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Senderowicz, A.M. Targeting cell cycle and apoptosis for the treatment of human malignancies. Curr. Opin. Cell Biol. 2004, 16, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.E.; Shamroukh, A.H.; El-Hashash, M.A.; El-Farargy, A.F.; Yousif, N.M.; Salama, M.A.; Mostafa, A.; El-Shahat, M. Synthesis and Anti-Avian Influenza Virus (H5N1) Evaluation of Some Novel Nicotinonitriles and Their N–Acylic Nucleosides. J. Heterocycl. Chem. 2012, 49, 1130–1135. [Google Scholar] [CrossRef]
- Tanifum, E.A.; Kots, A.Y.; Choi, B.K.; Murad, F.; Gilbertson, S.R. Novel pyridopyrimidine derivatives as inhibitors of stable toxin a (STa) induced cGMP synthesis. Bioorg. Med. Chem. Lett. 2009, 19, 3067–3071. [Google Scholar] [CrossRef] [PubMed]
- Rosowsky, A.; Mota, C.E.; Queener, S.F. Synthesis and antifolate activity of 2, 4-diamino-5,6,7,8-tetrahydropyrido [4,3-d] pyrimidine analogues of trimetrexate and piritrexim. J. Heterocycl. Chem. 1995, 32, 335–340. [Google Scholar] [CrossRef]
- Kotb, E.R.; Abbas, H.A.S.; Flefel, E.M.; Sayed, H.H.; Abdelwahed, N.A.M. Utility of Hantzsch ester in synthesis of some 3,5-bis-dihydropyridine derivatives and studying their biological evaluation. J. Heterocycl. Chem. 2015, 52, 1531–1539. [Google Scholar] [CrossRef]
- Flefel, E.M.; Abbas, H.A.S.; Abdel Magid, R.E.; Zaghary, W.A. Synthesis and Cytotoxic Effect of Some Novel 1,2-Dihydropyridine-3-Carbonitrile and Nicotinonitrile Derivatives. Molecules 2016, 21, 30. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Khaireldin, N.Y.; El-Shahat, M.; El-Hefny, E.A.; El-Saidi, M.M.T.; Ali, M.M.; Mahmoud, A.E. Antiproliferative Activity For Newly Heterofunctionalized Pyridine Analogues. Ponte 2016, 72, 106–118. [Google Scholar]
- Sayed, H.H.; Morsy, E.M.; Flefel, E.M. Synthesis and reactions of some novel Nicotinonitrile, thiazolotriazole, and Imidazolotriazole derivatives for Antioxidant evaluation. Synth. Commun. 2010, 40, 1360–1370. [Google Scholar] [CrossRef]
- Sayed, H.H.; Flefel, E.M.; Abd El-Fatah, A.M.; El-Sofany, W.I. Focus on the Synthesis and Reactions of Some New Pyridine Carbonitrile Derivatives as Antimicrobial and Antioxidant Agents. Egypt. J. Chem. 2010, 53, 17–35. [Google Scholar]
- Abdelhameed, R.M.; El-Sayed, H.A.; El-Shahat, M.; El-Sayed, A.A.; Darwesh, O.M. Novel triazolothiadiazole and triazolothiadiazine derivatives containing pyridine moiety: Design, synthesis, bactericidal and fungicidal activities. Curr. Bioact. Compd. 2018, 14, 169–179. [Google Scholar] [CrossRef]
- Abo-Ghalia, M.H.; Amr, A.E.G.E.; Abdalah, M.M. Synthesis of some new (Nα-dipicolinoyl)-bis-L-leucyl-DL-norvalyl linear tetra and cyclic octa bridged peptides as new antiinflammatory agents. Zeitschrift für Naturforschung B 2003, 58, 903–910. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Dinodia, M.; Kumar, A. Synthesis, anti-inflammatory and analgesic activity evaluation of some amidine and hydrazone derivatives. Bioorg. Med. Chem. 2006, 14, 4657–4663. [Google Scholar] [CrossRef] [PubMed]
- Komoda, H.; Inoue, T.; Node, K. Anti-inflammatory properties of azelnidipine, a dihydropyridine-based calcium channel blocker. Clin. Exp. Hypertens. 2010, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Flores, Y.K.; Campos-Aldrete, M.E.; Salgado-Zamora, H.; Correa-Basurto, J.; Meléndez-Camargo, M.E. Docking simulations, synthesis, and anti-inflammatory activity evaluation of 2-(N-alkyl) amino-3-nitroimidazo [1,2-a]pyridines. Med. Chem. Res. 2012, 21, 775–782. [Google Scholar] [CrossRef]
- Coburn, R.A.; Wierzba, M.; Suto, M.J.; Solo, A.J.; Triggle, A.M.; Triggle, D.J. 1, 4-Dihydropyridine antagonist activities at the calcium channel: a quantitative structure-activity relationship approach. J. Med. Chem. 1988, 31, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.T.; Piazza, G.A.; Han, E.K.H.; Delohery, T.M.; Li, H.; Finn, T.S.; Gross, P.H. Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines. Biochem. Pharmacol. 1999, 58, 1097–1107. [Google Scholar] [CrossRef]
- Kotb, E.R.; El-Hashash, M.A.; Salama, M.A.; Kalf, H.S.; Abdel Wahed, N.A. Synthesis and reactions of some novel nicotinonitrile derivatives for anticancer and antimicrobial evaluation. Acta Chim. Slov. 2009, 56, 908–919. [Google Scholar]
- Al-Abdullah, E.S. Synthesis and anticancer activity of some novel tetralin-6-yl-pyrazoline, 2-thioxopyrimidine, 2-oxopyridine, 2-thioxo-pyridine and 2-iminopyridine derivatives. Molecules 2011, 16, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Das, K.S.; Dey, S.K.; Maity, S.; Guha, P.; Choubey, M.; Bandyopadhyay, U. Antiplasmodial activity of [(aryl) arylsulfanylmethyl] pyridine. Antimicrob. Agents Chemother. 2008, 52, 705–715. [Google Scholar]
- Patel, N.B.; Agravat, S.N.; Shaikh, F.M. Synthesis and antimicrobial activity of new pyridine derivatives-I. Med. Chem. Res. 2011, 20, 1033–1041. [Google Scholar] [CrossRef]
- Kotb, E.R.; Anwar, M.M.; Abbas, H.A.S.; Abd, E.M.; SI, A. A concise synthesis and antimicrobial activity of a novel series of naphthylpyridine-3-carbonitrile compounds. Acta Pol. Pharm. 2013, 70, 667–679. [Google Scholar] [PubMed]
- Acharya, B.N.; Thavaselvam, D.; Kaushik, M.P. Synthesis and antimalarial evaluation of novel pyridine quinoline hybrids. Med. Chem. Res. 2008, 17, 487–494. [Google Scholar] [CrossRef]
- Mukai, A.; Nagai, A.; Inaba, S.; Takagi, M.; Shin-ya, K. JBIR-54, A new 4-pyridinone derivative isolated from Penicillium daleae Zaleski fE50. J. Antibiot. 2009, 62, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Nigade, G.; Chavan, P.; Deodhar, M. Synthesis and analgesic activity of new pyridine-based heterocyclic derivatives. Med. Chem. Res. 2012, 21, 27–37. [Google Scholar] [CrossRef]
- Amr, A.E.G.E.; Sayed, H.H.; Abdulla, M.M. Synthesis and reactions of some new substituted pyridine and pyrimidine derivatives as analgesic, anticonvulsant and antiparkinsonian agents. Arch. Pharm. Chem. Life Sci. 2005, 338, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Worachartcheewan, A.; Prachayasittikul, S.; Pingaew, R.; Nantasenamat, C.; Tantimongcolwat, T.; Ruchirawat, S.; Prachayasittikul, V. Antioxidant, cytotoxicity, and QSAR study of 1-adamantylthio derivatives of 3-picoline and phenylpyridines. Med. Chem. Res. 2012, 21, 3514–3522. [Google Scholar] [CrossRef]
- Mu, J.X.; Shi, Y.X.; Wu, H.K.; Sun, Z.H.; Yang, M.Y.; Liu, X.H.; Li, B.J. Microwave assisted synthesis, antifungal activity, DFT and SAR study of 1,2,4-triazolo[4,3-a]pyridine derivatives containing hydrazone moieties. Chem. Cent. J. 2016, 10, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhai, Z.; Sun, Z.; Liu, X.; Tan, C.; Weng, J. Synthesis, Crystal Structure and Antifungal Activity of 8-Chloro-3-((4-chlorobenzyl)-thio)[1,2,4]triazolo[4,3-a] pyridine. Chin. J. Struct. Chem. 2016, 35, 651–655. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Shi, Y.X.; Yang, M.Y.; Zhao, W.; Sun, Z.H.; Weng, J.Q.; Zhang, Y.G. Microwave assisted synthesis and antifungal activity of some novel thioethers containing 1,2,4-triazolo[4, 3-a] pyridine moiety. Lett. Drug Des. Discov. 2016, 13, 521–525. [Google Scholar] [CrossRef]
- Xu, F.Z.; Shao, J.H.; Zhu, Y.Y.; Liu, L.W.; Zhao, Y.H.; Shan, W.L.; Xue, W. Synthesis, antifungal and insecticidal activity of novel[1,2,4]triazolo[4, 3-a] pyridine derivatives containing a sulfide substructure. Chem. Pap. 2017, 71, 729–739. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo [4,3-a] pyridine derivatives. Pest Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.P.; Zhang, R.P.; Sun, Y.; Chang, Y.; Sui, X. Synthesis and studies on the anticonvulsant activity of 5-alkoxy-[1,2,4]triazolo[4,3-a]pyridine derivatives. Arzneimittelforschung 2012, 62, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Hussain, K.; Aneja, D.K.; Sharma, C.; Aneja, K.R. A facile iodine (III)-mediated synthesis of 3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a]pyridines via oxidation of 2-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)-1-(pyridin-2-yl)hydrazines and their antimicrobial evaluations. Org. Med. Chem. Lett. 2011, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A.K.; Mirza, Y.; Aneja, K.R.; Prakash, O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo [4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo [4,3-a] quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Maqbool, M.; Manral, A.; Jameel, E.; Kumar, J.; Saini, V.; Shandilya, A.; Jayaram, B. Development of cyanopyridine–triazine hybrids as lead multitarget anti-Alzheimer agents. Bioorg. Med. Chem. 2016, 24, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Abdel-Rahman, R.M.; Abdel-Halim, A.M.; Ibrahim, S.S.; Allimony, H.A. Synthesis, chemical reactivity and fungicidal activity of pyrido [1,2-b][1,2,4] triazine derivatives. J. Braz. Chem. Soc. 2009, 20, 1275–1286. [Google Scholar] [CrossRef]
- Ali, T.E.S.; Ibrahim, M.A. Synthesis and antimicrobial activity of chromone-linked 2-pyridone fused with 1,2,4-triazoles, 1,2,4-triazines and 1,2,4-triazepines ring systems. J. Braz. Chem. Soc. 2010, 21, 1007–1016. [Google Scholar] [CrossRef]
- Pawlak, D.; Pawlak, K.; Chabielska, E.; Małyszko, J.; Takada, A.; Myśliwiec, M.; Buczko, W. A potent 5-hydroxytryptamine receptor (5-HT2A) antagonist, DV-7028, delays arterial thrombosis development in rats. Thromb. Res. 1998, 90, 259–270. [Google Scholar] [CrossRef]
- Pawlak, D.; Adamkiewicz, M.; Malyszko, J.; Takada, A.; Mysliwiec, M.; Buczko, W. Vascular and cardiac effects of DV-7028, a selective, 5-HT2-receptor antagonist in rats. J. Cardiovasc. Pharmacol. 1998, 32, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Usui, H.; Kobayashi, S.; Yoshiwara, H.; Shibano, T.; Tanaka, T.; Kanao, M. Syntheses and 5-HT2 antagonist activity of bicyclic 1,2,4-triazol-3(2H)-one and 1,3,5-triazine-2,4 (3H)-dione derivatives. J. Med. Chem. 1992, 35, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Nagai, K. Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J. Med. Chem. 2004, 47, 3693–3696. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Ashour, H.; Guemei, A.A. Novel Pyrazole Derivatives as Potential Promising Anti-inflammatory Antimicrobial Agents. Arch. Pharm. Chem. Life Sci. 2005, 338, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Gökhan-Kelekçi, N.; Yabanoğlu, S.; Küpeli, E.; Salgın, U.; Özgen, Ö.; Uçar, G.; Bilgin, A.A. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg. Med. Chem. 2007, 15, 5775–5786. [Google Scholar] [CrossRef] [PubMed]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Villar, R.; Vicente, E.; Solano, B.; Monge, A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1, 4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4, 5-dihydro-(1H)-pyrazole analogues. Bioorg. Med. Chem Lett. 2007, 17, 6439–6443. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, P.; Ravi, T.; Subbuchettiar, G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009, 59, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Draghici, C.; Missir, A.V. Synthesis of new pyrazole derivatives and their anticancer evaluation. Eur. J. Med. Chem. 2010, 45, 4914–4919. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.C.; Li, H.Q.; Sun, J.; Zhou, Y.; Zhu, H.L. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2010, 18, 4606–4614. [Google Scholar] [CrossRef] [PubMed]
- Balbi, A.; Anzaldi, M.; Macciò, C.; Aiello, C.; Mazzei, M.; Gangemi, R.; Viale, M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem. 2011, 46, 5293–5309. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Telkar, S.; Arulmoli, T.; Fun, H.K. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arab. J. Chem. 2013, 6, 197–204. [Google Scholar] [CrossRef]
- Chmara, H.; Andruszkiewicz, R.; Borowski, E. Inactivation of glucosamine-6-phosphatesynthetase from Salmonella†typhimurium†LT2 SL 1027 by N-beta-fumarylcarboxyamido-l-2,3-diamino-propionic acid. Biochem. Biophys. Res. Commun. 1984, 120, 865–872. [Google Scholar] [CrossRef]
- Marshall, N.J.; Andruszkiewicz, R.; Gupta, S.; Milewski, S.; Payne, J.W. Structure activity relationships for a series of peptidomimetic antimicrobial prodrugs containing glutamine analogues. J. Antimicrob. Chemother. 2003, 51, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Borowski, E. Novel approaches in the rational design of antifungal agents of low toxicity. Farmaco 2000, 55, 206–208. [Google Scholar] [CrossRef]
- Magdy, M.Y.; Mohammed, A.A.; Mohamed, A.I. Synthesis, DNA affinity, and antimicrobial activity of 4-substituted phenyl-2,2′-bichalcophenes and aza-analogues. Med. Chem. Res. 2012, 21, 4074–4082. [Google Scholar]
- Hassan, A.E.; Ahmed, H.M.; Abd El-Fattah, Z.H.; Rajab, A.; El Sayed, H.E. Synthesis, antitumor and antimicrobial activities of 4-(4-chlorophenyl)-3-cyano-2-(β-O-glycosyloxy)-6-(thien-2-yl)- nicotinonitrile. Eur. J. Med. Chem. 2011, 46, 2948–2954. [Google Scholar]
- Umesh, D.P.; Ajay, P.N.; Pramod, S.N.; Pramod, P.M. Synthesis, antimicrobial and antifungal activity evaluation of 2,6-diaryl-4-SEC-aminonicotinonitriles and 4-sec-amino-6-aryl-2-(pyridin-2-yl)pyridine-3-carbonitriles i.e., Bipyridines and their docking study. J. Pharm. Res. 2012, 5, 1383–1386. [Google Scholar]
- Rashad, A.E.; Shamroukh, A.H.; Yousif, N.M.; Salama, M.A.; Ali, M.M.; Mahmoud, A.E.; El-Shahat, M. New Pyrimidinone and Fused Pyrimidinone Derivatives as Potential Anticancer Chemotherapeutics. Arch. Pharm. Chem. Life Sci. 2012, 345, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Shamroukh, A.H.; El-Shahat, M.; Drabowicz, J.; Ali, M.M.; Rashad, A.E. Anticancer evaluation of some newly synthesized N-nicotinonitrile derivative. Eur. J. Med. Chem. 2013, 69, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Flefel, E.M.; Tantawy, W.A.; El-Sofany, W.I.; El-Shahat, M.; El-Sayed, A.A.; Abd-Elshafy, D.N. Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation. Molecules 2017, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.; Khalil, A.M.; El-Shahat, M.; Khaireldin, N.Y.; Rabie, S.T. Antimicrobial activity of PVC-pyrazolone-silver nanocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2016, 53, 346–353. [Google Scholar] [CrossRef]
- El-Shahat, M. Studies on the Synthesis and Chemical Reactions of Some Mixed and Non-Mixed Heterocyclic Compounds of Expected Biological Activity. Master’s Thesis, Zagazig University, Zagazig, Egypt, 2007. [Google Scholar]
- Hindler, J.A.; Howard, B.J.; Keiser, J.F. Antimicrobial agents and antimicrobial susceptibility testing. In Clinical and Pathogenic Microbiology, 2nd ed.; Mosby: St Louis, MO, USA, 1994. [Google Scholar]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, K.R.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Noel, M.O.; Michael, B.; Craig, A.J.; Chris, M.; Tim, V.; Geoffrey, R.H. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar]
- Mouilleron, S.; Badet-Denisot, M.A.; Golinelli-Pimpaneau, B. Ordering of C-terminal loop and glutaminase domains of glucosamine-6-phosphate synthase promotes sugar ring opening and formation of the ammonia channel. J. Mol. Biol. 2008, 377, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Schüttelkopf, A.W.; Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Cryst. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).