Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin
Abstract
1. Introduction
2. Source, Extraction and Detection Method
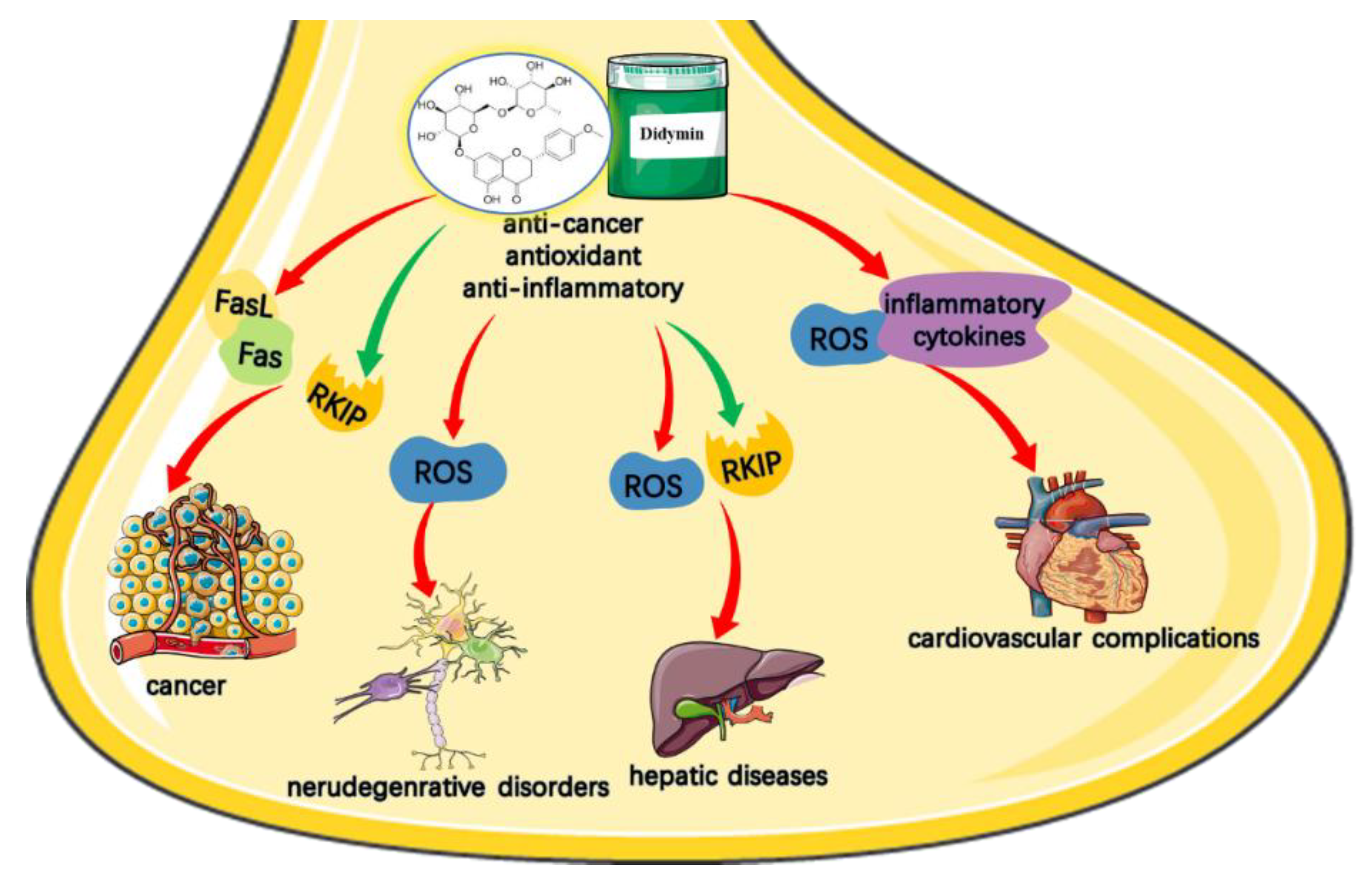
3. Therapeutic Bioactivities: Protective Effects and Health Benefits
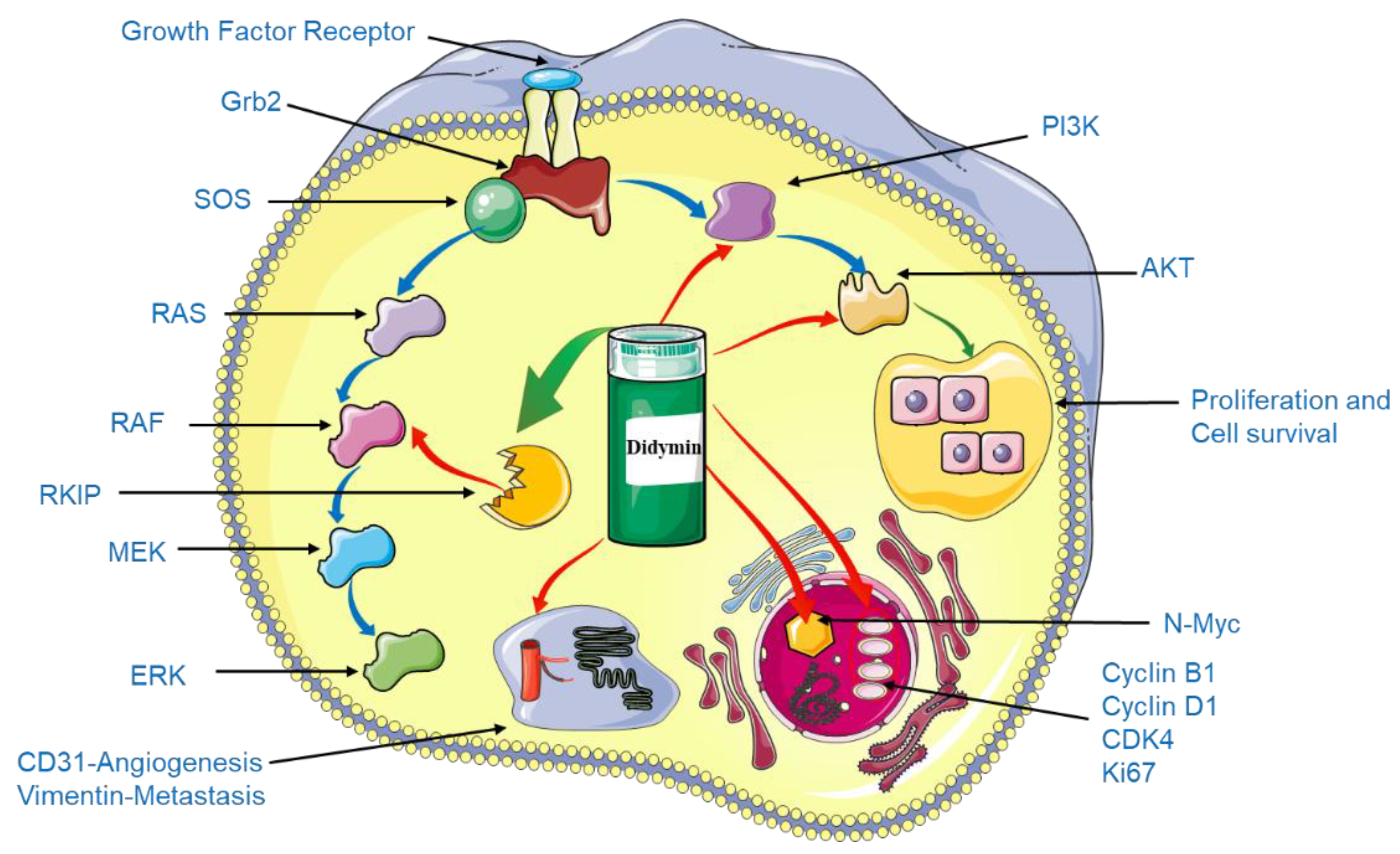
3.1. Didymin and Anti-Tumor Property
3.2. Didymin and Neuroprotective Property
3.3. Didymin for Anxiolytic-Like and Antinociceptive Actions
3.4. Didymin for Hepatic Cytoproct Activity
3.5. Didymin and Cardiovascular Activities
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hung, J.Y.; Chang, W.A.; Tsai, Y.M.; Hsu, Y.L.; Chiang, H.H.; Chou, S.H.; Huang, M.S.; Kuo, P.L. Tricetin, a dietary flavonoid, suppresses benzo(a)pyreneinduced human nonsmall cell lung cancer bone metastasis. Int. J. Oncol. 2015, 46, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Ziegler, D.S. Too many targets, not enough patients: Rethinking neuroblastoma clinical trials. Nat. Rev. Cancer 2018, 18, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D. Citrus Fruits: Production, Consumption and Health Benefits; Nova Science Publishers: New York, NY, USA, 2016. [Google Scholar]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Lasa, A.; Aguirre, L.; Fernandez-Quintela, A.; Milton, I.; Portillo, M.P. Potential application of non-flavonoid phenolics in diabetes: Antiinflammatory effects. Curr. Med. Chem. 2015, 22, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Blandizzi, C.; Antonioli, L. Dietary flavonoids as a potential intervention to improve redox balance in obesity and related co-morbidities: A review. Nutr. Res. Rev. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Xavier, C.; Tejada, S. Neuroprotective effects of flavonoid compounds on neuronal death associated to alzheimer’s disease. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.; Barbosa, K.B.; Volp, A.C.; Puchau, B.; Bressan, J.; Zulet, M.A.; Martinez, J.A. Vitamin c and fibre consumption from fruits and vegetables improves oxidative stress markers in healthy young adults. Br. J. Nutr. 2012, 107, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Fu, J.; Nall, D.; Rodova, M.; Shankar, S.; Srivastava, R.K. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int. J. Cancer 2012, 131, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Li, L.; Chen, M.; Lagunero, F.T.; Go, V.L.W.; Boros, L.G. Diverse mechanisms of growth inhibition by luteolin, resveratrol, and quercetin in mia paca-2 cells: A comparative glucose tracer study with the fatty acid synthase inhibitor c75. Metabolomics 2012, 8, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Latif, R. Flavonoids as novel neuroprotective nutraceuticals. Saudi J. Health Sci. 2015, 4, 1–4. [Google Scholar] [CrossRef]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and its neuroprotective effects on brain ischemia and neurodegenerative diseases. Curr. Drug Targets 2018. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhao, Z.; Chen, Y.; Li, Z.; Tian, Y.; Liu, Z.; Liu, B.; Song, J. Quercetin protects rat cortical neurons against traumatic brain injury. Mol. Med. Rep. 2018, 17, 7859–7865. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Singhal, S.S.; Singhal, S.; Singhal, P.; Singhal, J.; Horne, D.; Awasthi, S. Didymin: An orally active citrus flavonoid for targeting neuroblastoma. Oncotarget 2017, 8, 29428–29441. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Hsu, Y.L.; Ko, Y.C.; Tsai, Y.M.; Yang, C.J.; Huang, M.S.; Kuo, P.L. Didymin, a dietary flavonoid glycoside from citrus fruits, induces fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer 2010, 68, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Bok, S.H.; Jang, M.K.; Lee, M.K.; Nam, K.T.; Park, Y.B.; Rhee, S.J.; Choi, M.S. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001, 69, 2855–2866. [Google Scholar] [CrossRef]
- Lin, X.; Bai, F.; Nie, J.; Lu, S.; Lu, C.; Zhu, X.; Wei, J.; Lu, Z.; Huang, Q. Didymin alleviates hepatic fibrosis through inhibiting erk and pi3k/akt pathways via regulation of raf kinase inhibitor protein. Cell. Physiol. Biochem. 2016, 40, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Massenti, R.; Lo Bianco, R.; Sandhu, A.K.; Gu, L.; Sims, C. Huanglongbing modifies quality components and flavonoid content of ‘valencia’ oranges. J. Sci. Food Agric. 2014, 96, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.R.; Yu, X.; Jayaprakasha, G.K.; Patil, B.S. Influence of storage temperature and low-temperature conditioning on the levels of health-promoting compounds in rio red grapefruit. Food Sci. Nutr. 2016, 5, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-D.; Zhang, J.-Y.; Li, P.-F.; Wu, H.-F.; Zhu, N.-L.; Jiang, H.; Lv, C.-Y.; Wu, L.-L.; Ma, Z.-X.; Xu, X.-D.; et al. Two new abietane diterpenoid glycosides from clinopodium chinense. Nat. Prod. Res. 2016, 30, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.L.; Galtieri, V.; Cutroneo, P.; Tommasini, S.; Ficarra, P.; Ficarra, R. Study of the extraction procedure by experimental design and validation of a lc method for determination of flavonoids in citrus bergamia juice. J. Pharm. Biomed. 2004, 35, 349–363. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Gu, L.; Lv, C.; He, B.; Liu, Z.; Hou, P.; Bi, K.; Chen, X. Simultaneous determination of five free and total flavonoids in rat plasma by ultra hplc-ms/ms and its application to a comparative pharmacokinetic study in normal and hyperlipidemic rats. J. Chromatogr. B 2014, 953, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mouly, P.; Gaydou, E.M.; Auffray, A. Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography. J. Chromatogr. A 1998, 800, 171–179. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Gu, S.; Liu, Z.; Zhang, Y.; Zhang, X. Simultaneous determination of flavonoids in different parts of citrus reticulata ‘chachi’ fruit by high performance liquid chromatography—Photodiode array detection. Molecules 2010, 15, 5378–5388. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. A fragmentation study of dihydroquercetin using triple quadrupole mass spectrometry and its application for identification of dihydroflavonols in citrus juices. Rapid Commun. Mass Spectrom. 2010, 23, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Presti, M.L.; Ohman, M.; Fazio, A.; Dugo, G.; Mondello, L. Determination of flavonoids in citrus juices by micro-hplc-esi/ms. J. Sep. Sci. 2015, 28, 1149–1156. [Google Scholar] [CrossRef]
- Di, D.L.; Taverna, D.; Mazzotti, F.; Benabdelkamel, H.; Attya, M.; Napoli, A.; Sindona, G. Comprehensive assay of flavanones in citrus juices and beverages by uhplc-esi-ms/ms and derivatization chemistry. Food Chem. 2013, 141, 2328–2333. [Google Scholar]
- Ma, C.; Gao, W.; Gao, Y.; Man, S.; Huang, L.; Liu, C. Identification of chemical constituents in extracts and rat plasma from fructus aurantii by uplc-pda-q-tof/ms. Phytochem. Anal. 2015, 22, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Chen, D.L.; Xu, Q.; Xue, X.Y.; Zhang, F.F.; Liang, X.M. Characterization of polymethoxylated flavones in fructus aurantii by liquid chromatography with atmospheric pressure chemical ionization combined with tandem mass spectrometry. J. Pharm. Biomed. 2007, 43, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Cacciola, F.; Bonaccorsi, I.; Dugo, P.; Mondello, L. Determination of flavanones in citrus juices by means of one- and two-dimensional liquid chromatography. J. Sep. Sci. 2015, 34, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Fanali, C.; Dugo, L.; Mondello, L. A nano-lc/uv method for the analysis of principal phenolic compounds in commercial citrus juices and evaluation of antioxidant potential. Electrophoresis 2014, 35, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Wojtanowski, K.K.; Mroczek, T. Study of a complex secondary metabolites with potent anti-radical activity by two dimensional tlc/hplc coupled to electrospray ionization time-of-flight mass spectrometry and bioautography. Anal. Chim. Acta 2018, 1029, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Ventura, J.; Castro, C.; Boone, V.; Rojas, R.; Ascacio-Valdés, J.; Martínez-Ávila, G. Uplc-esi-qtof-ms²-based identification and antioxidant activity assessment of phenolic compounds from red corn cob (Zea mays L.). Molecules 2018, 23, 1425. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Ghinea, I.; Furdui, B.; Dah-Nouvlessounon, D.; Raclea, R.; Costache, T.; Cucolea, I.; Urlan, F.; Dinica, R. Exploring new antioxidant and mineral compounds from wild-grown in danube delta biosphere. Molecules 2018, 23, 1247. [Google Scholar] [CrossRef] [PubMed]
- Chebrolu, K.K.; Jifon, J.; Patil, B.S. Modulation of flavanone and furocoumarin levels in grapefruits (citrus paradisi macfad.) by production and storage conditions. Food Chem. 2016, 196, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Bioactive phenolics and antioxidant propensity of flavedo extracts of mauritian citrus fruits: Potential prophylactic ingredients for functional foods application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Bai, F.; Nie, J.; Lu, S.; Lu, C.; Zhu, X.; Zhuo, L.; Lin, X. Didymin ameliorates hepatic injury through inhibition of mapk and nf-κb pathways by up-regulating rkip expression. Int. Immunopharm. 2017, 42, 130–138. [Google Scholar]
- Cassani, J.; Araujo, A.; Martínez-Vázquez, M.; Manjarrez, N.; Moreno, J.; Estrada-Reyes, R. Anxiolytic-like and antinociceptive effects of 2(s)-neoponcirin in mice. Molecules 2013, 18, 7584–7599. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Piscioneri, A.; Salerno, S.; Al-Fageeh, M.B.; Drioli, E.; De Bartolo, L. Neuroprotective effect of didymin on hydrogen peroxide-induced injury in the neuronal membrane system. Cells Tissues Organs 2014, 199, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int. J. Mol. Sci 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [PubMed]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ray, S.K. Synergistic anti-tumor actions of luteolin and silibinin prevented cell migration and invasion and induced apoptosis in glioblastoma snb19 cells and glioblastoma stem cells. Brain Res. 2015, 1629, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.; Besse, B. New windows open for immunotherapy in lung cancer. Nature 2018, 558, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H. A new standard of care for advanced lung cancer. N. Engl. J. Med. 2018, 378, 2135–2137. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Tan, S.J.; Lim, W.T.; Lim, C.T. An extracellular matrix-related prognostic and predictive indicator for early-stage non-small cell lung cancer. Nat. Commun. 2017, 8, 1734. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Spandidos, D.A. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma hepg2 cells via cyp1a-catalyzed metabolism, activation of jnk and erk and p53/p21 up-regulation. J. Nutr. Biochem. 2013, 24, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.-C.; Chiang, T.-H.; Wang, J.-S.; Lin, L.-J.; Chao, W.-C.; Chen, B.-H.; Lu, J.-F. Flavonoids from gynostemma pentaphyllum exhibit differential induction of cell cycle arrest in h460 and a549 cancer cells. Molecules 2014, 19, 17663–17681. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Golstein, P. The fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Teshiba, R.; Kawano, S.; Wang, L.L.; He, L.; Naranjo, A.; London, W.B.; Seeger, R.C.; Gastier-Foster, J.M.; Look, A.T.; Hogarty, M.D.; et al. Age-dependent prognostic effect by mitosis-karyorrhexis index in neuroblastoma: A report from the children’s oncology group. Pediatr. Dev. Pathol. 2014, 17, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bell, J.L.; Carter, D.; Gherardi, S.; Poulos, R.C.; Milazzo, G.; Wong, J.W.; Al-Awar, R.; Tee, A.E.; Liu, P.Y.; et al. Wdr5 supports an n-myc transcriptional complex that drives a protumorigenic gene expression signature in neuroblastoma. Cancer Res. 2015, 75, 5143–5154. [Google Scholar] [CrossRef] [PubMed]
- Varan, A.; Kesik, V.; Senocak, M.E.; Kale, G.; Akyuz, C.; Buyukpamukcu, M. The efficacy of delayed surgery in children with high-risk neuroblastoma. J. Cancer Res. Ther. 2015, 11, 268–271. [Google Scholar] [PubMed]
- Singhal, J.; Nagaprashantha, L.D.; Vatsyayan, R.; Awasthi, S.; Singhal, S.S. Didymin induces apoptosis by inhibiting n-myc and upregulating rkip in neuroblastoma. Cancer Prev. Res. (Phila.) 2012, 5, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Nieto Montesinos, R.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Delivery of p-glycoprotein substrates using chemosensitizers and nanotechnology for selective and efficient therapeutic outcomes. J. Control. Release 2012, 161, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Al-Mulla, F. Novel flavonoid didymin inhibits neuroblastomas—Letter. Cancer Prev. Res. (Phila.) 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ramirez, L.; Vodnala, S.K.; Nini, R. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 2018, 9, 1944. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the united states, 1970–2014. JAMA 2017, 318, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, J.; Zhang, Y.; He, Y.; Jiang, Q.; Yue, D.; Xu, X.; Gu, Z. Highly stable fluorinated nanocarriers with irgd for overcoming the stability dilemma and enhancing tumor penetration in an orthotopic breast cancer. ACS Appl. Mater. Interfaces 2016, 8, 28468–28479. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Hsieh, C.J.; Tsai, E.M.; Hung, J.Y.; Chang, W.A.; Hou, M.F.; Kuo, P.L. Didymin reverses phthalate ester-associated breast cancer aggravation in the breast cancer tumor microenvironment. Oncol. Lett. 2016, 11, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Vernekar, A.A.; Sinha, D.; Srivastava, S.; Paramasivam, P.U.; D’Silva, P.; Mugesh, G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 2014, 5, 5301. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, H. Anti-parkinson potential of silymarin: Mechanistic insight and therapeutic standing. Front. Pharmacol. 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Xu, R.; Vandiver, M.S.; Cha, J.Y.; Snowman, A.M.; Snyder, S.H. Cystathionine gamma-lyase deficiency mediates neurodegeneration in huntington’s disease. Nature 2014, 509, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Xu, Y.; Mueller, K.A.; Chen, X.; Granucci, E.; Paganoni, S.; Sadri-Vakili, G.; Schwarzschild, M.A. Urate mitigates oxidative stress and motor neuron toxicity of astrocytes derived from als-linked sod1(g93a) mutant mice. Mol. Cell. Neurosci. 2018, 92, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeong, J.M.; Kim, S.J.; Seo, W.; Kim, M.H.; Choi, W.M.; Yoo, W.; Lee, J.H.; Shim, Y.R.; Yi, H.S.; et al. Pro-inflammatory hepatic macrophages generate ros through nadph oxidase 2 via endocytosis of monomeric tlr4-md2 complex. Nat. Commun. 2017, 8, 2247. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Lu, S.Y.; Sivasubramaniyam, T.; Revelo, X.S.; Cai, E.P.; Luk, C.T.; Schroer, S.A.; Patel, P.; Kim, R.H.; Bombardier, E.; et al. Dj-1 links muscle ros production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 2015, 6, 7415. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive oxygen species signalling in the diabetic heart: Emerging prospect for therapeutic targeting. Heart (Br. Card. Soc.) 2018, 104, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yoon, Y.S.; Lee, S.J. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death Dis. 2018, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Martínez-Vázquez, M.; Gallegos-Solís, A.; Heinze, G.; Moreno, J. Depressant effects of clinopodium mexicanum benth. Govaerts (lamiaceae) on the central nervous system. J. Ethnopharmacol. 2010, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.K.; Jun, W.; Lee, J. Mechanism of er stress and inflammation for hepatic insulin resistance in obesity. Ann. Nutr. Metab. 2015, 67, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Kirpich, I.; Ma, Z.; Wang, C.; Zhang, M.; Suttles, J.; McClain, C.; Feng, W. Lactobacillus rhamnosus gg reduces hepatic tnfalpha production and inflammation in chronic alcohol-induced liver injury. J. Nutr. Biochem. 2013, 24, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; McLeod, D.; Kelaeng, K.S.; Mangia, A.; Berg, T.; Thabet, K.; Irving, W.L.; Dore, G.J.; Sheridan, D.; Grønbæk, H.; et al. IFN-λ3, not IFN-λ4, likely mediates IFNL3–IFNL4 haplotype–dependent hepatic inflammation and fibrosis. Nat. Genet. 2017, 49, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.; Ozeke, O. Association of prediabetes with higher coronary atherosclerotic burden among patients with first diagnosed acute coronary syndrome. Angiology 2018. [Google Scholar] [CrossRef]
- Castellano, I.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Pipino, C. Anti-inflammatory activity of marine ovothiol a in an in vitro model of endothelial dysfunction induced by hyperglycemia. Oxid. Med. Cell. Longev. 2018, 2018, 2087373. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Kirtikar, S.; Himangshu, S.; Ashish, S.; Kota, V.R. Didymin prevents hyperglycemia-induced human umbilical endothelial cells dysfunction and death. Biochem. Pharmacol. 2018, 152, 1–10. [Google Scholar]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Cent. Eur. J. Biol. 2017, 7, 108–123. [Google Scholar]
- Sanchez-Cano, C.; Romero-Canelón, I.; Geraki, K.; Sadler, P.J. Microfocus x-ray fluorescence mapping of tumour penetration by an organo-osmium anticancer complex. J. Inorg. Biochem. 2018, 185, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, L.; Wang, C.; Wang, X.; Xu, Y.; Yu, H.; Wu, P.; Li, S.; Han, L.; Gunatilaka, A.; et al. Methylglucosylation of aromatic amino and phenolic moieties of drug-like biosynthons by combinatorial biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4980–E4989. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Tao, X.; Tian, B.; Tang, Y.; Shao, Y.; Kou, L.; Gou, J.; Li, X.; Yin, T.; Tang, X. Improved oral bioavailability of core–shell structured beads by redispersion of the shell-forming nanoparticles: Preparation, characterization and in vivo studies. Colloid Surf. B 2014, 113, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Keck, C.M. Flavonoid nanocrystals produced by artcrystal®-technology. Int. J. Pharm. 2015, 482, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Fatma, S.; Talegaonkar, S.; Iqbal, Z.; Panda, A.K.; Negi, L.M.; Goswami, D.G.; Tariq, M. Novel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by p-glycoprotein modulation: An in vitro, ex vivo and in vivo investigations. Drug Deliv. 2016, 23, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Qin, X.S.; Yang, Q.Y.; Luo, Z.G.; Xiao, Z.G.; Peng, X.C. Comparative structural characterization of spiral dextrin inclusion complexes with vitamin e or soy isoflavone. J. Agric. Food Chem. 2017, 65, 8744–8753. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
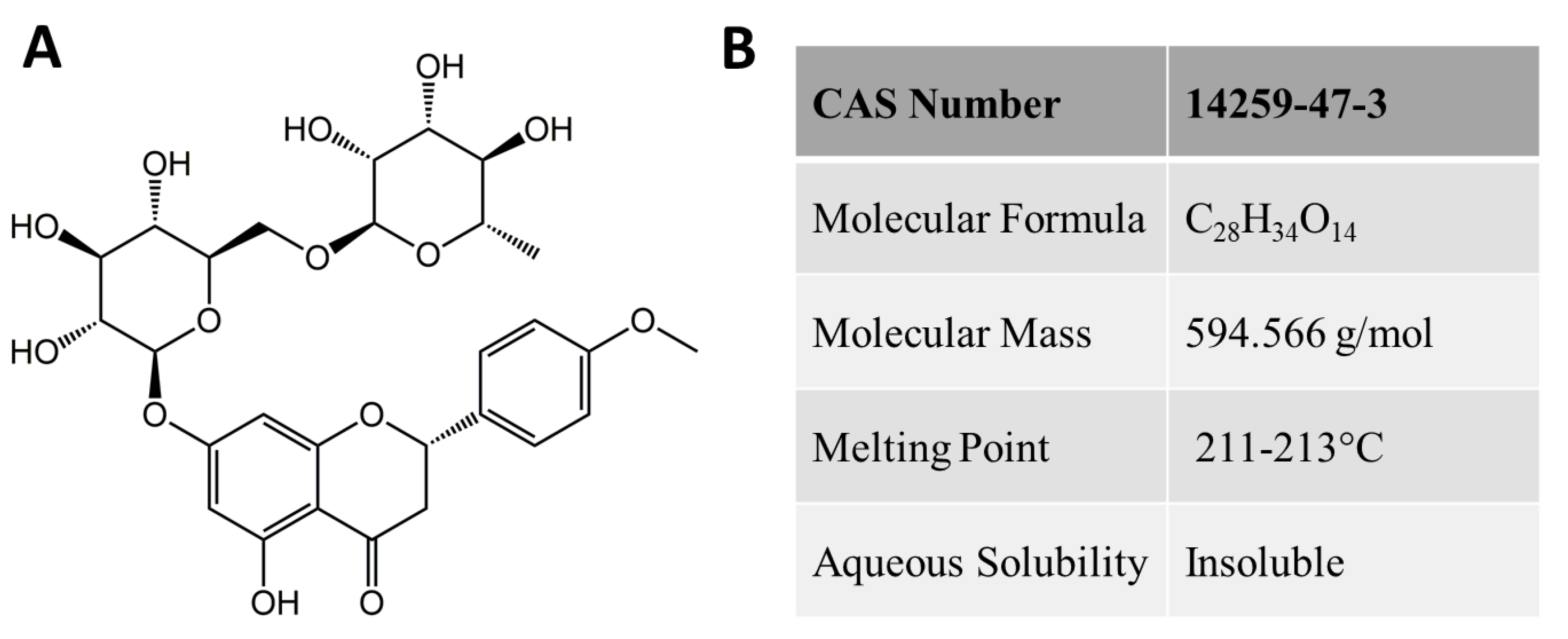

| Didymin is a Flavonoid Glycoside Commonly Found in Citrus Fruits | |
|---|---|
| Source | Orange [20] |
| Grapefruit [21,38] | |
| Mandarin [39] | |
| Bergamot [19] | |
| Other citrus [17,26,27] | |
| Origanum Vulgare [40] | |
| Clinopodium [22,41] etc. | |
| HPLC is the Preferred Method for Separating and Detecting Citrus Flavonoids | |
| Extraction and Detection Method | MS-HPLC [28,29] |
| Ultra-HPLC (UHPLC) [30,31,32] | |
| Comprehensive multidimensional LC methods [33] | |
| RP-HPLC and photodiode array detection [23] | |
| Nano-LC/UV-Vis apparatus [34] | |
| UAE [35] | |
| UPLC-ESI-QTOF-MS/MS [36] | |
| Disease | Mechanism Studies | Ref. |
|---|---|---|
| Lung cancer | The primary pathway of apoptosis induced by didymin is the Fas/Fas ligand apoptotic system, which does not mediate p53 and p21/WAF1. | [17] |
| Neuroblastoma | Inhibition of N-Myc transcription, up-regulated RKIP and down-regulated PI13K, Akt and vimentin. | [56] |
| Downregulation of cyclin D1, cyclin B1, CDK4, CD31, Ki67, and N-Myc also enhance the anti-tumor effect of didymin. | ||
| Breast cancer | Didymin can effectively inhibit phthalate-mediated invasion, migration, and proliferation of breast cancer cells. | [62] |
| Neurodegenerative disease | Removing excess ROS or inhibiting its production by antioxidant molecules could effectively maintain cell redox homeostasis and prevent oxidative damage. | [42] |
| Effectively inhibits apoptosis and activates antioxidant defense enzymes. | ||
| Sleeplessness | GABAergic system participation in the anxiolytic actions of didymin. Didymin could exert its anxiolytic-like effect through the interaction with the GABAA receptors. | [41] |
| Hepatic diseases | Didymin has antioxidant activity, scavenges free radicals, and regulates MAPK and NF-κB signaling pathways. | [19] |
| Cardiovascular complications | Didymin prevented HG-induced (ROS) and the production of lipid peroxidation product malondialdehyde and prevented HG-induced monocyte-endothelial cell adhesion, ICAM-1 and VCAM-1 expression, and NF-κB activation. | [78] |
| Didymin inhibits the release of various inflammatory cytokines and chemokines from HG-treated HUVECs. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Lin, M.-T.; Zhu, Y.-D.; Xu, H.-L.; Zhao, Y.-Z. Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin. Molecules 2018, 23, 2547. https://doi.org/10.3390/molecules23102547
Yao Q, Lin M-T, Zhu Y-D, Xu H-L, Zhao Y-Z. Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin. Molecules. 2018; 23(10):2547. https://doi.org/10.3390/molecules23102547
Chicago/Turabian StyleYao, Qing, Meng-Ting Lin, Yin-Di Zhu, He-Lin Xu, and Ying-Zheng Zhao. 2018. "Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin" Molecules 23, no. 10: 2547. https://doi.org/10.3390/molecules23102547
APA StyleYao, Q., Lin, M.-T., Zhu, Y.-D., Xu, H.-L., & Zhao, Y.-Z. (2018). Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin. Molecules, 23(10), 2547. https://doi.org/10.3390/molecules23102547






