Neuroprotective Role of Phytochemicals
Abstract
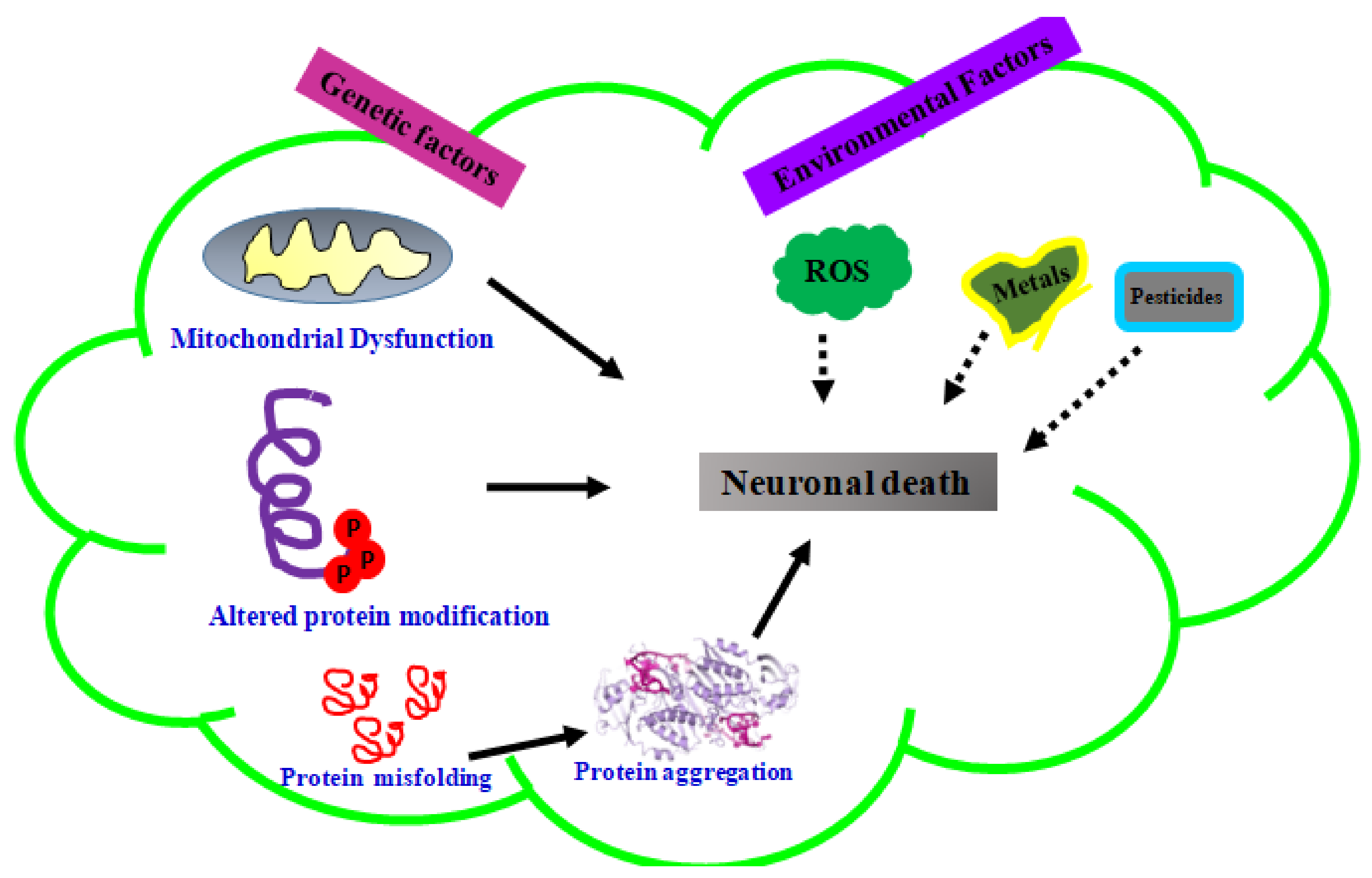
1. Introduction
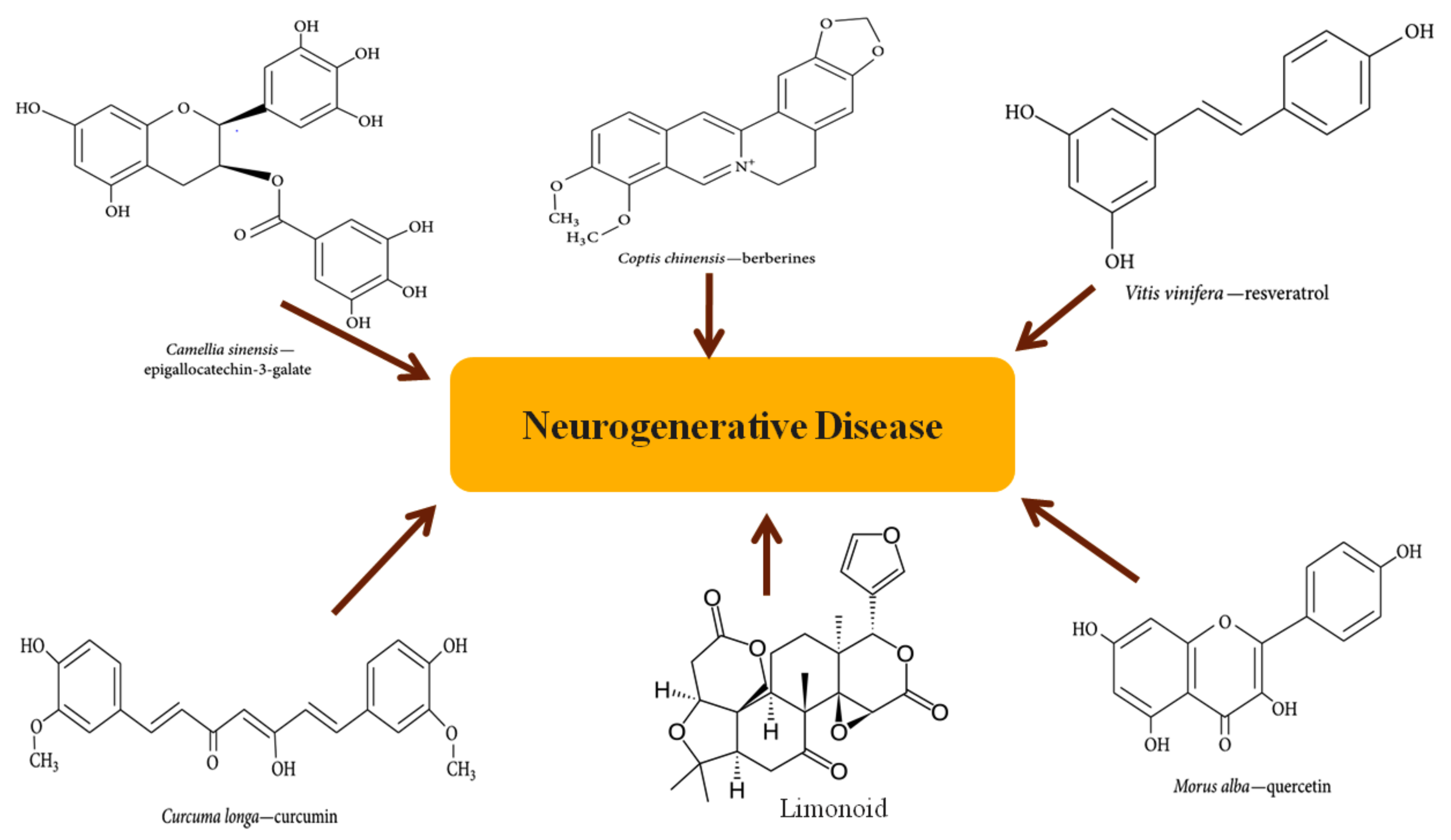
2. Overview of Phytochemicals
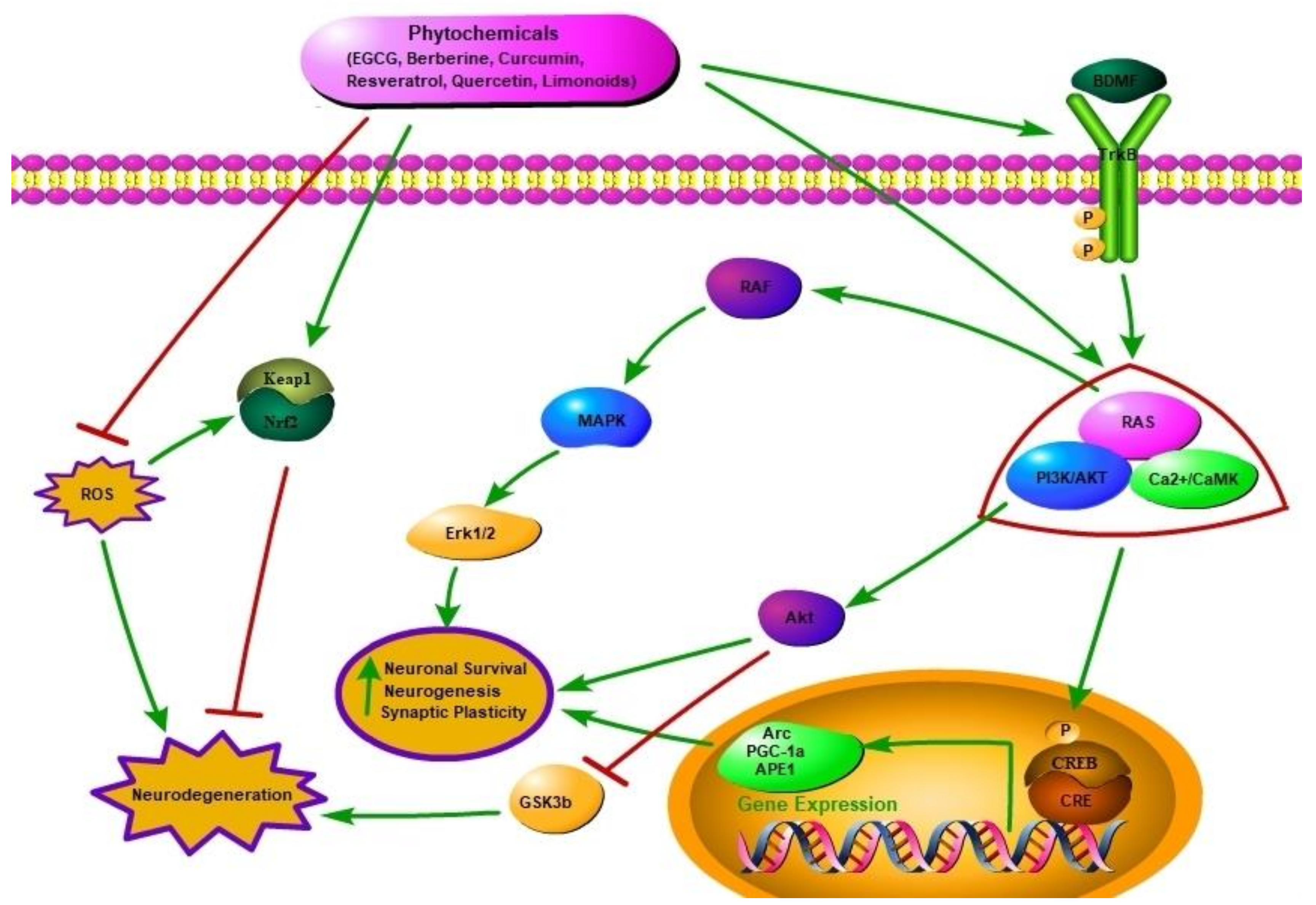
2.1. Neuroprotective Potential of Phytochemicals
2.1.1. Epigallocatechin-3-Galate
2.1.2. Berberine
2.1.3. Curcumin
2.1.4. Resveratrol
2.1.5. Quercetin
2.1.6. Limonoids
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Helman, A.M.; Murphy, M.P. Vascular cognitive impairment: Modeling a critical neurologic disease in vitro and in vivo. Biochim. Biophys. Acta 2016, 1862, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Kohl, Z.; Gage, F.H. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 2011, 33, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. Effect of lifestyle, aging, and phytochemicals on the onset of neurological disorders. In Phytochemicals, Signal Transduction, and Neurological Disorders; Springer: New York, NY, USA, 2012; pp. 1–29. [Google Scholar]
- Marini, A.M.; Jiang, X.; Wu, X.; Tian, F.; Zhu, D.; Okagaki, P.; Lipsky, R.H. Role of brain-derived neurotrophic factor and NF-kappab in neuronal plasticity and survival: From genes to phenotype. Restor. Neurol. Neurosci. 2004, 22, 121–130. [Google Scholar] [PubMed]
- Barbacid, M. The Trk family of neurotrophin receptors. J. Neurobiol. 1994, 25, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. BioMed. Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Ryu, J.K.; Taghibiglou, C.; Ge, Y.; Chan, A.W.; Liu, L.D.; Lu, J.; McLarnon, J.G.; Wang, Y.T. Long-term potentiation promotes proliferation/survival and neuronal differentiation of neural stem/progenitor cells. PLoS ONE 2013, 8, e76860. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- Dawbarn, D.; Allen, S.J. Neurotrophins and neurodegeneration. Neuropathol. Appl. Neurobiol. 2003, 29, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Sampson, E.L.; Blanchard, M.R.; Jones, L.; Tookman, A.; King, M. Dementia in the acute hospital: Prospective cohort study of prevalence and mortality. Br. J. Psychiatry 2009, 195, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Sonntag, K.C.; Andersson, T.; Bjorklund, L.M.; Park, J.J.; Kim, D.W.; Kang, U.J.; Isacson, O.; Kim, K.S. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur. J. Neurosci. 2002, 16, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Jelic, V. Long-term treatment of Alzheimer disease: Efficacy and safety of acetylcholinesterase inhibitors. Alzheimer Dis. Assoc. Disord. 2004, 18 (Suppl. 1), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.D.; Mucke, L. 100 years and counting: Prospects for defeating Alzheimer’s disease. Science 2006, 314, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bemis, M.; Desilets, A.R. Role of medium chain triglycerides (Axona®) in the treatment of mild to moderate Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement.® 2014, 29, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ondo, W.; Hunter, C.; Almaguer, M.; Jankovic, J. A novel sublingual apomorphine treatment for patients with fluctuating Parkinson’s disease. Mov. Disord. 1999, 14, 664–668. [Google Scholar] [CrossRef]
- Jankovic, J. Levodopa strengths and weaknesses. Neurology 2002, 58, S19–S32. [Google Scholar] [CrossRef] [PubMed]
- Kaakkola, S.; Gordin, A.; Mannisto, P.T. General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase. Gen. Pharmacol. 1994, 25, 813–824. [Google Scholar] [CrossRef]
- Jankovic, J.; Watts, R.L.; Martin, W.; Boroojerdi, B. Transdermal rotigotine: Double-blind, placebo-controlled trial in Parkinson disease. Arch. Neurol. 2007, 64, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Korczyn, A.D.; Brunt, E.R.; Larsen, J.P.; Nagy, Z.; Poewe, W.H.; Ruggieri, S. A 3-year randomized trial of ropinirole and bromocriptine in early Parkinson’s disease. The 053 study group. Neurology 1999, 53, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, W. The treatment strategies for neurodegenerative diseases by integrative medicine. Integr. Med. Int. 2014, 1, 223–225. [Google Scholar] [CrossRef]
- Mizuno, Y. Recent research progress in and future perspective on treatment of Parkinson’s disease. Integr. Med. Int. 2014, 1, 67–79. [Google Scholar] [CrossRef]
- Somani, S.J.; Modi, K.P.; Majumdar, A.S.; Sadarani, B.N. Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother. Res. 2015, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M. Screening of radical scavenging activity and polyphenol content of Bulgarian plant species. Pharmacogn. Res. 2011, 3, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; De Galarreta, C.M.; Cuadrado, A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Liu, D. Phytochemical genistein in the regulation of vascular function: New insights. Curr. Med. Chem. 2007, 14, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an alzheimer’s disease mouse model. J. Alzheimers Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging and inhibition of abeta42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem 2016, 199, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z.; et al. (-)-epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 49, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Park, C.S.; Kim, D.J.; Cho, M.H.; Jin, B.K.; Pie, J.E.; Chung, W.G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Koh, S.H.; Kim, S.H.; Kwon, H.; Park, Y.; Kim, K.S.; Song, C.W.; Kim, J.; Kim, M.H.; Yu, H.J.; Henkel, J.S.; et al. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res. Mol. Brain Res. 2003, 118, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, P.; Rajashree, K.; Priya, L.B.; Padma, V.V. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, M.; Jing, X.; Shi, H.; Ren, M.; Lou, H. (-)-epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem. Res. 2014, 39, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Srividhya, R.; Jyothilakshmi, V.; Arulmathi, K.; Senthilkumaran, V.; Kalaiselvi, P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (-)-epigallocatechin-3-gallate. Int J. Dev. Neurosci. 2008, 26, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Castellano-Gonzalez, G.; Pichaud, N.; Ballard, J.W.; Bessede, A.; Marcal, H.; Guillemin, G.J. Epigallocatechin-3-gallate induces oxidative phosphorylation by activating cytochrome c oxidase in human cultured neurons and astrocytes. Oncotarget 2016, 7, 7426–7440. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, L.; Zhang, H.; Diao, X.; Zhao, S.; Zhou, W. Reduction in autophagy by (-)-epigallocatechin-3-gallate (EGCG): A potential mechanism of prevention of mitochondrial dysfunction after subarachnoid hemorrhage. Mol. Neurobiol. 2017, 54, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, L.; Marquez-Valadez, B.; Gomez-Sanchez, A.; Silva-Lucero, M.D.; Torres-Perez, M.; Tellez-Ballesteros, R.I.; Ichwan, M.; Meraz-Rios, M.A.; Kempermann, G.; Ramirez-Rodriguez, G.B. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience 2016, 322, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Amit, T.; Youdim, M.B.; Mandel, S. Involvement of protein kinase c activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002, 277, 30574–30580. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, Y.G.; Fang, D.; Le, W.D. (-)-epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004, 78, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Chung Wei, J.; Huang, H.C.; Chen, W.J.; Huang, C.N.; Peng, C.H.; Lin, C.L. Epigallocatechin gallate attenuates amyloid beta-induced inflammation and neurotoxicity in EOC 13.31 microglia. Eur. J. Pharmacol. 2016, 770, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Perkins, I.; van Olphen, A.; Burdash, N.; Klein, T.W.; Friedman, H. Epigallocatechin gallate modulates cytokine production by bone marrow-derived dendritic cells stimulated with lipopolysaccharide or muramyldipeptide, or infected with legionella pneumophila. Exp. Biol. Med. (Maywood) 2005, 230, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Durairajan, S.S.; Liu, L.F.; Lu, J.H.; Chen, L.L.; Yuan, Q.; Chung, S.K.; Huang, L.; Li, X.S.; Huang, J.D.; Li, M. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging 2012, 33, 2903–2919. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.N.S.; Frozza, R.L.; Hoppe, J.B.; de Melo Menezes, B.; Salbego, C.G. Berberine was neuroprotective against an in vitro model of brain ischemia: Survival and apoptosis pathways involved. Brain Res. 2014, 1557, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Matsumoto, K.; Murakami, Y.; Hori, H.; Zhao, Q.; Obi, R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: Involvement of b-cell lymphoma 2 phosphorylation suppression. Biol. Pharm. Bull. 2009, 32, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qian, Z.; Pan, L.; Li, H.; Zhu, H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiol. Hung. 2012, 99, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.H.; Choi, H.S.; Shin, K.S.; Lee, B.K.; Lee, C.K.; Hwang, B.Y.; Lim, S.C.; Lee, M.K. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in pc12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010, 486, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Iwata, N.; Yoshikawa, A.; Aizaki, Y.; Ishiura, S.; Saido, T.C.; Maruyama, K. Berberine alters the processing of Alzheimer’s amyloid precursor protein to decrease abeta secretion. Biochem. Biophys. Res. Commun. 2007, 352, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.Y.; Chen, C.S.; Wu, S.N.; Jong, Y.J.; Lo, Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.N.; Aboutaleb, N.; Souri, F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J. Chem. Neuroanat. 2018, 87, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cho, K.H.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Shin, D.H.; Yang, H.J. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int. J. Mol. Med. 2014, 33, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Choi, H.S.; Zhao, T.T.; Suh, K.H.; Kwon, I.H.; Choi, S.O.; Lee, M.K. Neurotoxic effects of berberine on long-term L-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch. Pharm Res. 2013, 36, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Kim, Y.J.; Choi, H.R.; Jeon, J.W. Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of phosphoinositide 3-kinase/protein kinase b signaling pathway. Int. Neurourol. J. 2014, 18, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kysenius, K.; Brunello, C.A.; Huttunen, H.J. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. PLoS ONE 2014, 9, e107129. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Chu, Y.K.; Lee, H.; Ahn, B.H.; Park, J.H.; Kim, M.J.; Lee, S.; Ryoo, H.S.; Jang, J.H.; Lee, S.R.; et al. Effects of berberine on hippocampal neuronal damage and matrix metalloproteinase-9 activity following transient global cerebral ischemia. J. Neurosci. Res. 2012, 90, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.J.; Yoon, Y.S.; et al. Effects of curcumin (curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and creb signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, H.; Amini, A.; Taheri, S.; Sajadi, E.; Shafikhani, S.; Schuger, L.A.; Reddy, V.B.; Ghoreishi, S.K.; Pouriran, R.; Chien, S.; et al. The effect of combined photobiomodulation and curcumin on skin wound healing in type I diabetes in rats. J. Photochem. Photobiol. B 2018, 181, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.H.; Chen, A.P.; Mehta, J.L. Curcumin inhibits Ox-LDL-Activated Hepatic Stellate cells in vitro by suppressing gene expression of lectin-like oxidized-LDL receptor via activation of peroxisome proliferator-activated receptor-gamma. Gastroenterology 2008, 134, A-779. [Google Scholar] [CrossRef]
- El-Bahr, S.M. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin b1. Phytother. Res. 2015, 29, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, B.S.; Lee, K.G.; Choi, C.Y.; Jang, S.S.; Kim, Y.H.; Lee, S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Q.; Song, S.L.; Li, J.; Liang, T. Neuroprotective effect of curcumin on hippocampal injury in 6-ohda-induced Parkinson’s disease rat. Pathol. Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Gao, Z.; Xie, K.; Zhang, Q.; Jiang, H.; Pang, Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014, 271, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, J.B.; Coradini, K.; Frozza, R.L.; Oliveira, C.M.; Meneghetti, A.B.; Bernardi, A.; Pires, E.S.; Beck, R.C.R.; Salbego, C.G. Free and nanoencapsulated curcumin suppress beta-amyloid-induced cognitive impairments in rats: Involvement of BDNF and Akt/GSK-3 beta signaling pathway. Neurobiol. Learn. Mem. 2013, 106, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Canovi, M.; Zona, C.; Aurilia, D.; Niarakis, A.; La Ferla, B.; Salmona, M.; Nicotra, F.; Gobbi, M.; Antimisiaris, S.G. Curcumin-decorated nanoliposomes with very high affinity for amyloid-beta 1-42 peptide. Biomaterials 2011, 32, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Xiong, T.T.; Zhou, J.; He, H.J.; Wu, D.D.; Du, X.W.; Li, X.Y.; Xu, B. Enzymatic formation of curcumin in vitro and in vivo. Nano Res. 2018, 11, 3453–3461. [Google Scholar] [CrossRef]
- Zhang, C.; Browne, A.; Child, D.; Tanzi, R.E. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J. Biol. Chem. 2010, 285, 28472–28480. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Lue, L.F. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem. Int. 2001, 39, 333–340. [Google Scholar] [CrossRef]
- He, G.L.; Luo, Z.; Yang, J.; Shen, T.T.; Chen, Y.; Yang, X.S. Curcumin ameliorates the reduction effect of PGE(2) on fibrillar beta-amyloid peptide (1-42)-induced microglial phagocytosis through the inhibition of EP2-PKA signaling in N9 microglial cells. PLoS ONE 2016, 11, e0147721. [Google Scholar] [CrossRef]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-ohda model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zheng, W.; Xin, N.; Chi, Z.H.; Wang, N.Q.; Nie, Y.X.; Feng, W.Y.; Wang, Z.Y. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuv. Res. 2010, 13, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Caesar, I.; Jonson, M.; Nilsson, K.P.R.; Thor, S.; Hammarstrom, P. Curcumin promotes a-beta fibrillation and reduces neurotoxicity in transgenic drosophila. PLoS ONE 2012, 7, e31424. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.S.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, A.S.; Carroll, R.T.; Bishayee, A.; Novotny, N.A.; Geldenhuys, W.J.; Van der Schyf, C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drug 2012, 21, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S.A.; Hu, W.; Kim, P.; Miller, S.A.; Chu, T.; Harris-White, M.E.; Cole, G.M. Phenolic anti-inflammatory antioxidant reversal of a beta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2001, 22, 993–1005. [Google Scholar] [CrossRef]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. 2013, 68, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Munch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Anastacio, J.R.; Netto, C.A.; Castro, C.C.; Sanches, E.F.; Ferreira, D.C.; Noschang, C.; Krolow, R.; Dalmaz, C.; Pagnussat, A. Resveratrol treatment has neuroprotective effects and prevents cognitive impairment after chronic cerebral hypoperfusion. Neurol. Res. 2014, 36, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tan, M.S.; Yu, J.T.; Tan, L. Resveratrol as a therapeutic agent for Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 350516. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Y.; Wang, J.H.; Xu, M.; Wang, Y.; Zhang, M.; Zhou, Y.Y. Resveratrol improves cognitive impairment by regulating apoptosis and synaptic plasticity in streptozotocin-induced diabetic rats. Cell. Physiol. Biochem. 2016, 40, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Albani, D.; Polito, L.; Batelli, S.; De Mauro, S.; Fracasso, C.; Martelli, G.; Colombo, L.; Manzoni, C.; Salmona, M.; Caccia, S.; et al. The sirt1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009, 110, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Yazir, Y.; Utkan, T.; Gacar, N.; Aricioglu, F. Resveratrol exerts anti-inflammatory and neuroprotective effects to prevent memory deficits in rats exposed to chronic unpredictable mild stress. Physiol. Behav. 2015, 138, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Li, X.Q.; Zhu, J.X.; Xie, W.J.; Le, W.D.; Fan, Z.; Jankovic, J.; Pan, T.H. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Huang, H.M.; Hsieh, S.J.; Jeng, K.C.G.; Kuo, J.S. Resveratrol inhibits interleukin-6 production in cortical mixed glial cells under hypoxia/hypoglycemia followed by reoxygenation. J. Neuroimmunol. 2001, 112, 28–34. [Google Scholar] [CrossRef]
- Chen, C.Y.; Jang, J.H.; Li, M.H.; Surh, Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005, 331, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Schools, G.P.; Lei, T.; Wang, W.; Kimelberg, H.K.; Zhou, M. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Exp. Neurol. 2008, 212, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, Y.F.; Wu, Q.; Liu, J.; Shi, J.S. Resveratrol promotes neurotrophic factor release from astroglia. Exp. Biol. Med. (Maywood) 2012, 237, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.S.; Zhou, H.; Wilson, B.; Hong, J.S.; Gao, H.M. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.; Lindsay, C.B.; Quintanilla, R.A.; Carvajal, F.J.; Cerpa, W.; Inestrosa, N.C. Quercetin exerts differential neuroprotective effects against H2O2 and a beta aggregates in hippocampal neurons: The role of mitochondria. Mol. Neurobiol. 2017, 54, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Padma, V.V.; Baskaran, R.; Roopesh, R.S.; Poornima, P. Quercetin attenuates lindane induced oxidative stress in wistar rats. Mol. Biol. Rep. 2012, 39, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; D’Onofrio, G.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Neuroprotective effects of quercetin: From chemistry to medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Abin-Carriquiry, J.A.; Arredondo, F.; Blasina, F.; Echeverry, C.; Martinez, M.; Rivera, F.; Vaamonde, L. Quercetin in brain diseases: Potential and limits. Neurochem. Int. 2015, 89, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Quessy, P.; Martinoli, M.G. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 2009, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.C.G.E.I.; Puerta, E.; Suarez-Santiago, J.E.; Santos-Magalhaes, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Tsamo, A.; Langat, M.K.; Nkounga, P.; Waffo, A.F.K.; Nkengfack, A.E.; Mulholland, D.A. Limonoids from the West African Trichilia welwitschii (Meliaceae). Biochem. Syst. Ecol. 2013, 50, 368–370. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.K.; Ge, R.; Liang, J.Y.; Li, Q.S.; Min, Z.D. Novel NGF-potentiating limonoids from the fruits of Melia toosendan. Fitoterapia 2013, 90, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.H.; Min, Z.D.; Ip, N.Y. Melia toosendan regulates PC12 cell differentiation via the activation of protein kinase A and extracellular signal-regulated kinases. Neurosignals 2004, 13, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.C.; Kim, S.H.; Kim, M.J.; Yang, H.J.; Rhyu, M.R.; Park, J.H. Limonin, a component of dictamni radicis cortex, inhibits eugenol-induced calcium and cAMP Levels and PKA/CREB signaling pathway in non-neuronal 3T3-L1 cells. Molecules 2015, 20, 22128–22136. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velmurugan, B.K.; Rathinasamy, B.; Lohanathan, B.P.; Thiyagarajan, V.; Weng, C.-F. Neuroprotective Role of Phytochemicals. Molecules 2018, 23, 2485. https://doi.org/10.3390/molecules23102485
Velmurugan BK, Rathinasamy B, Lohanathan BP, Thiyagarajan V, Weng C-F. Neuroprotective Role of Phytochemicals. Molecules. 2018; 23(10):2485. https://doi.org/10.3390/molecules23102485
Chicago/Turabian StyleVelmurugan, Bharath Kumar, Baskaran Rathinasamy, Bharathi Priya Lohanathan, Varadharajan Thiyagarajan, and Ching-Feng Weng. 2018. "Neuroprotective Role of Phytochemicals" Molecules 23, no. 10: 2485. https://doi.org/10.3390/molecules23102485
APA StyleVelmurugan, B. K., Rathinasamy, B., Lohanathan, B. P., Thiyagarajan, V., & Weng, C.-F. (2018). Neuroprotective Role of Phytochemicals. Molecules, 23(10), 2485. https://doi.org/10.3390/molecules23102485






