Structural Characterization of Polysaccharides from Dendrobium officinale and Their Effects on Apoptosis of HeLa Cell Line
Abstract
:1. Introduction
2. Results
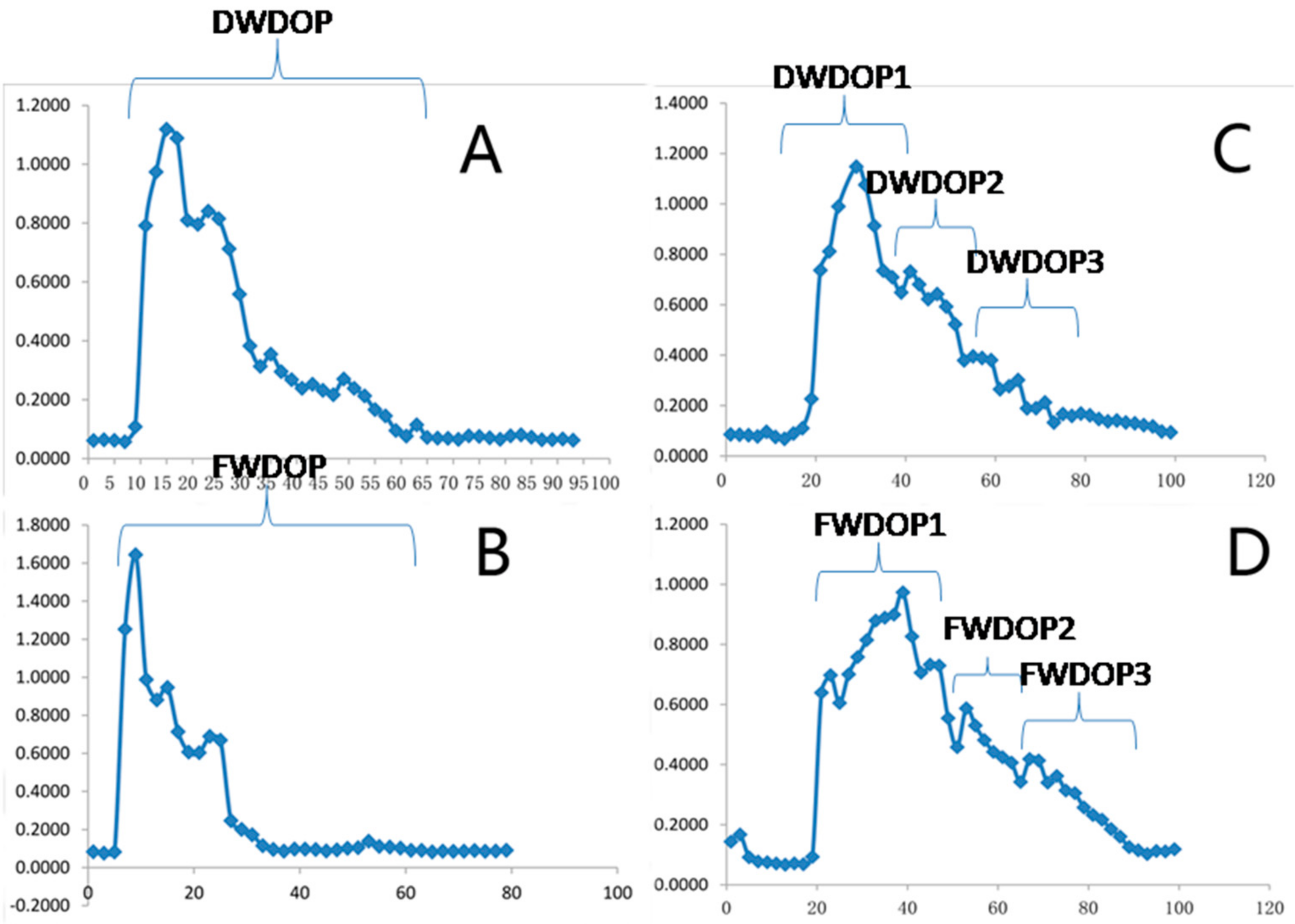
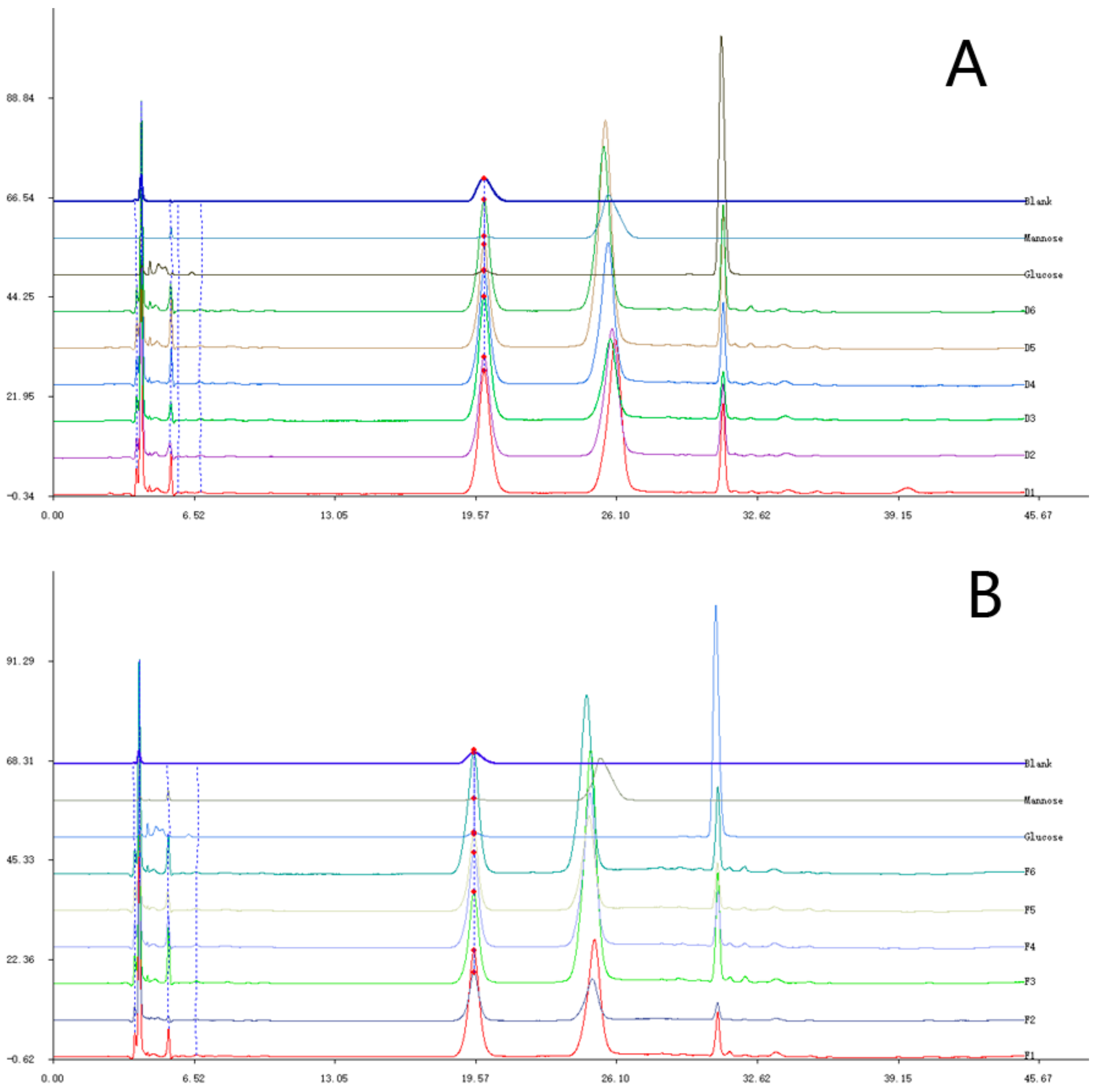
2.1. Elution Graph of the Purified Polysaccharide Fractions (PPFs)
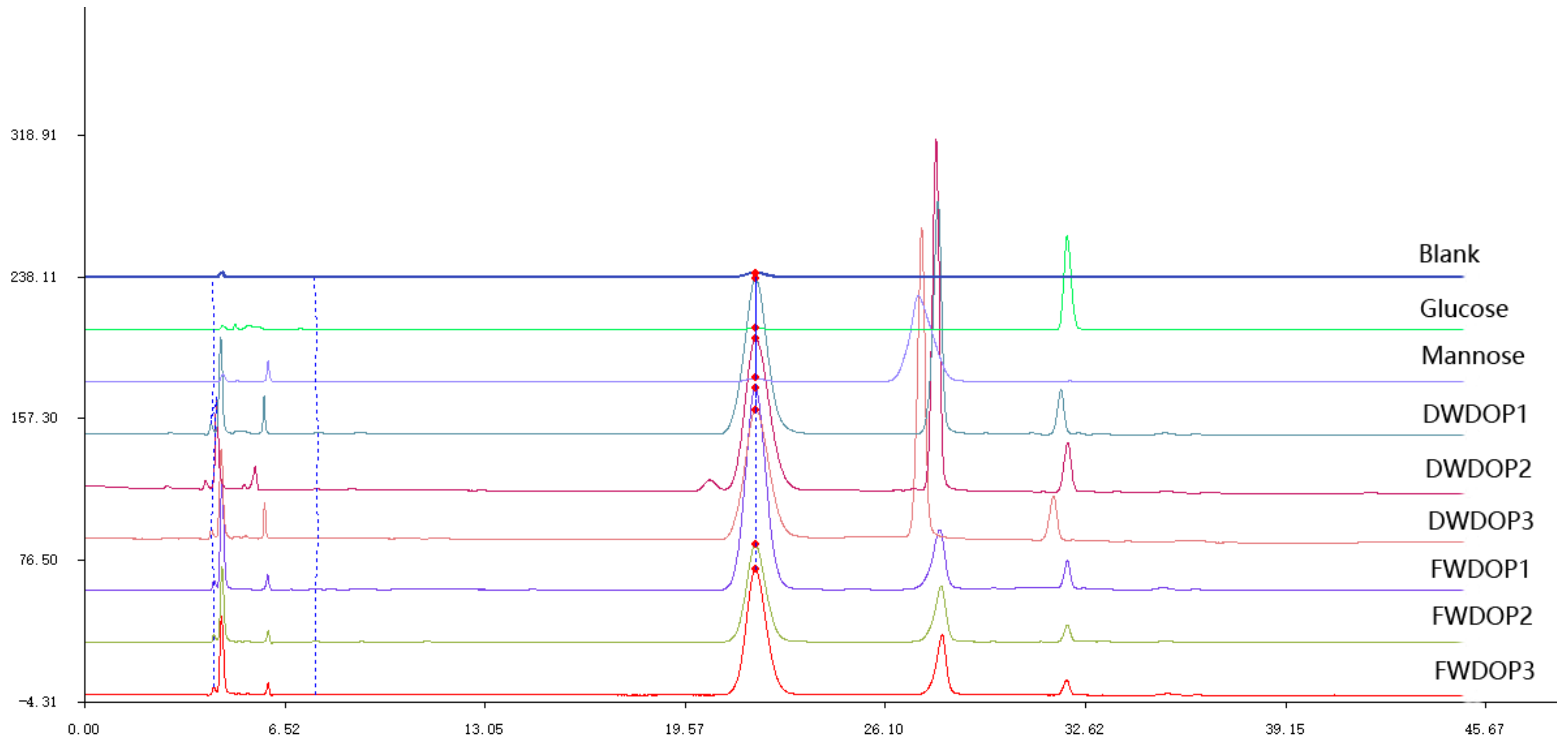
2.2. Molecular Weight and Monosaccharide Composition of the PPFs
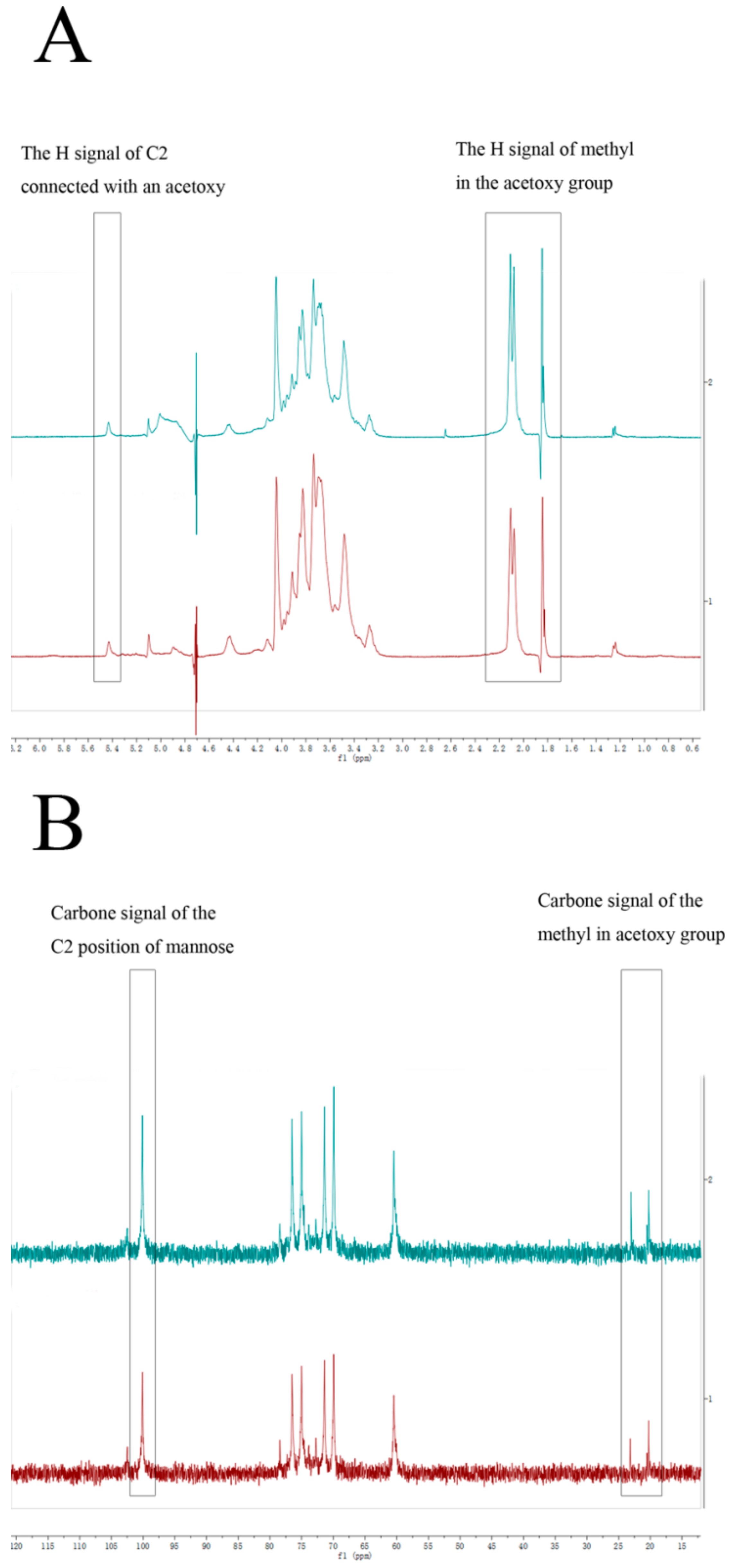
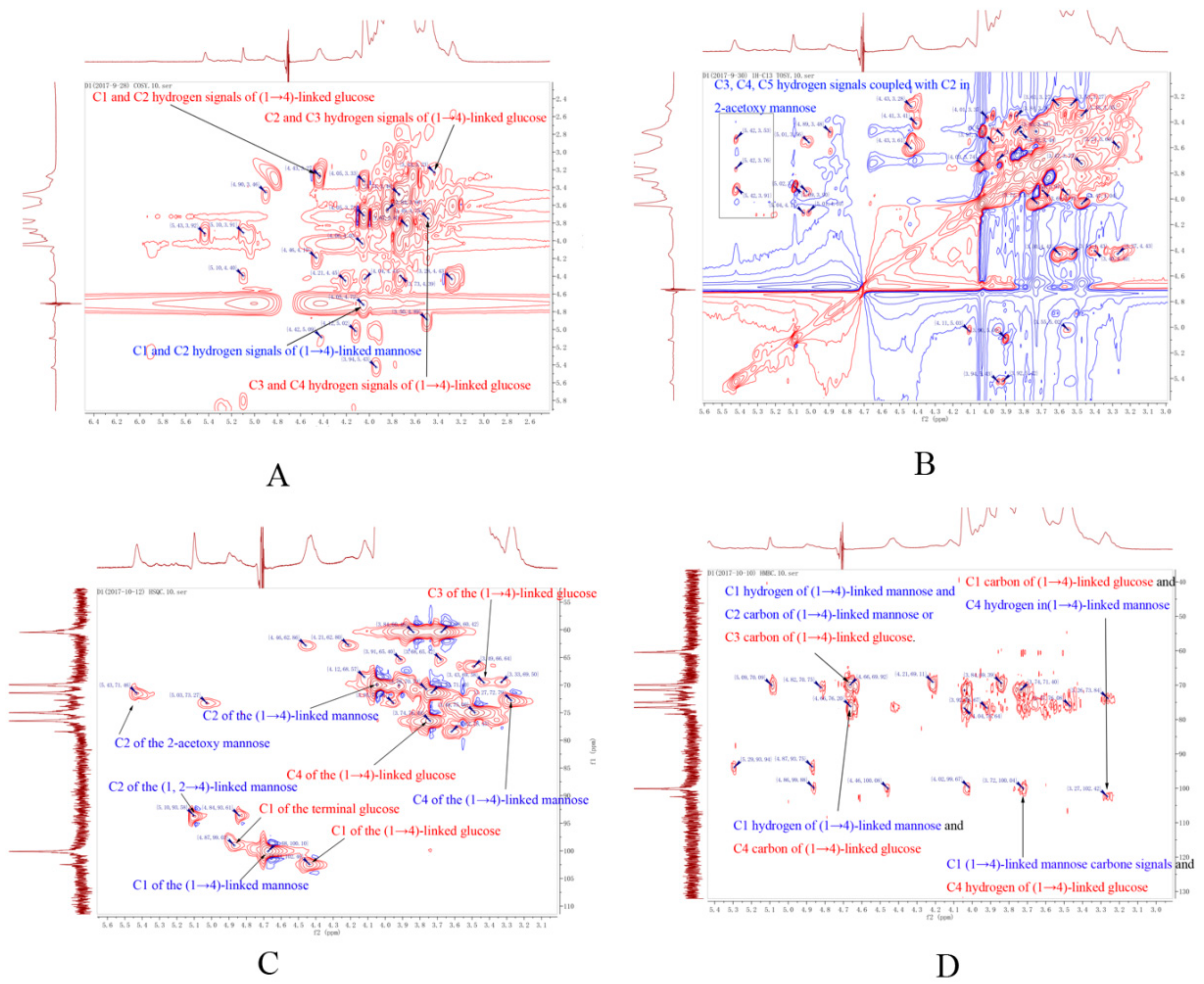
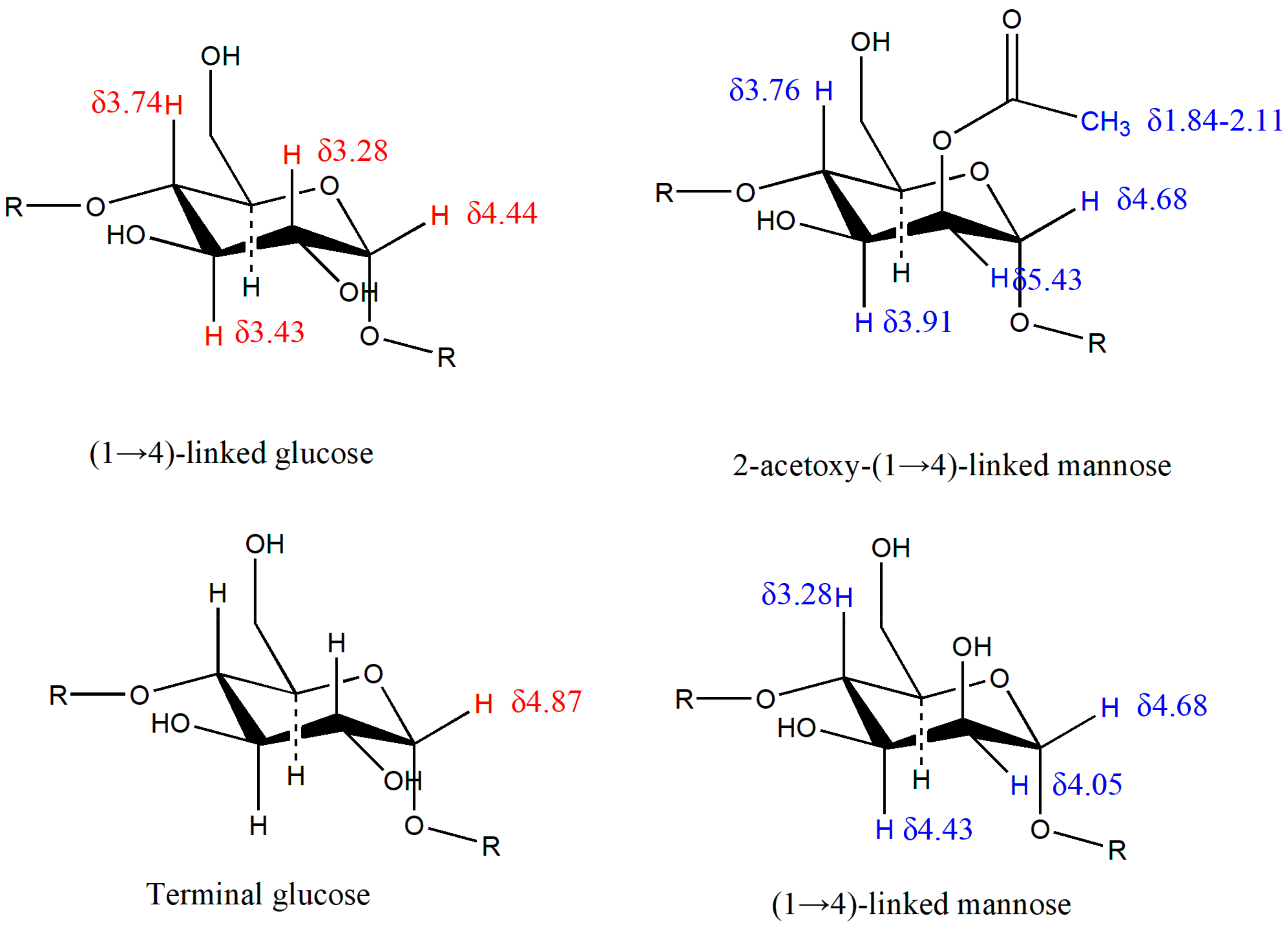
2.3. Structure of DWDOP1 and FWDOP1
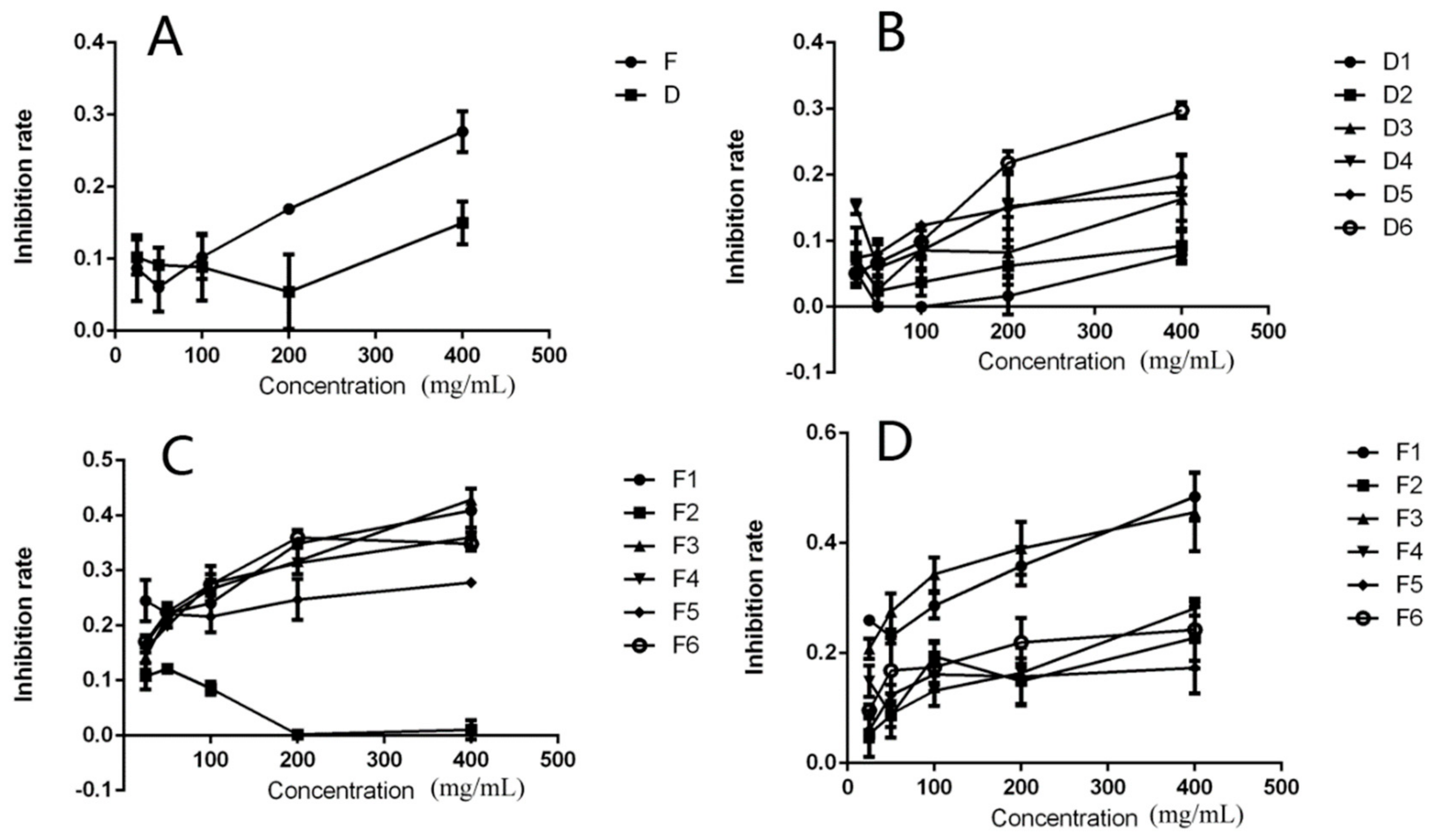
2.4. Effects of the DPFs on HeLa Cell Proliferation
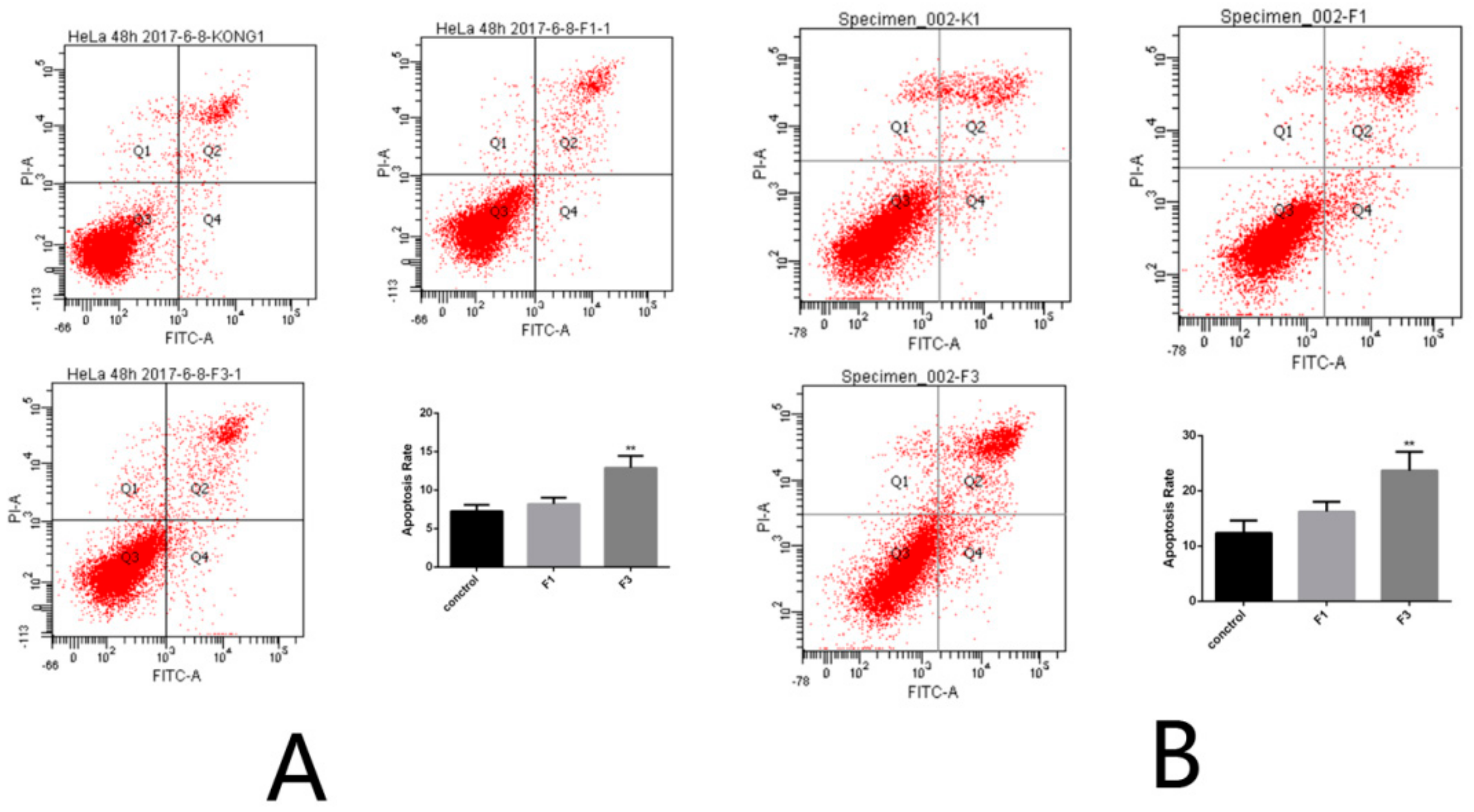
2.5. Apoptosis Rate of HeLa Cells after Treatment with F1 and F3 for 24 h and 48 h
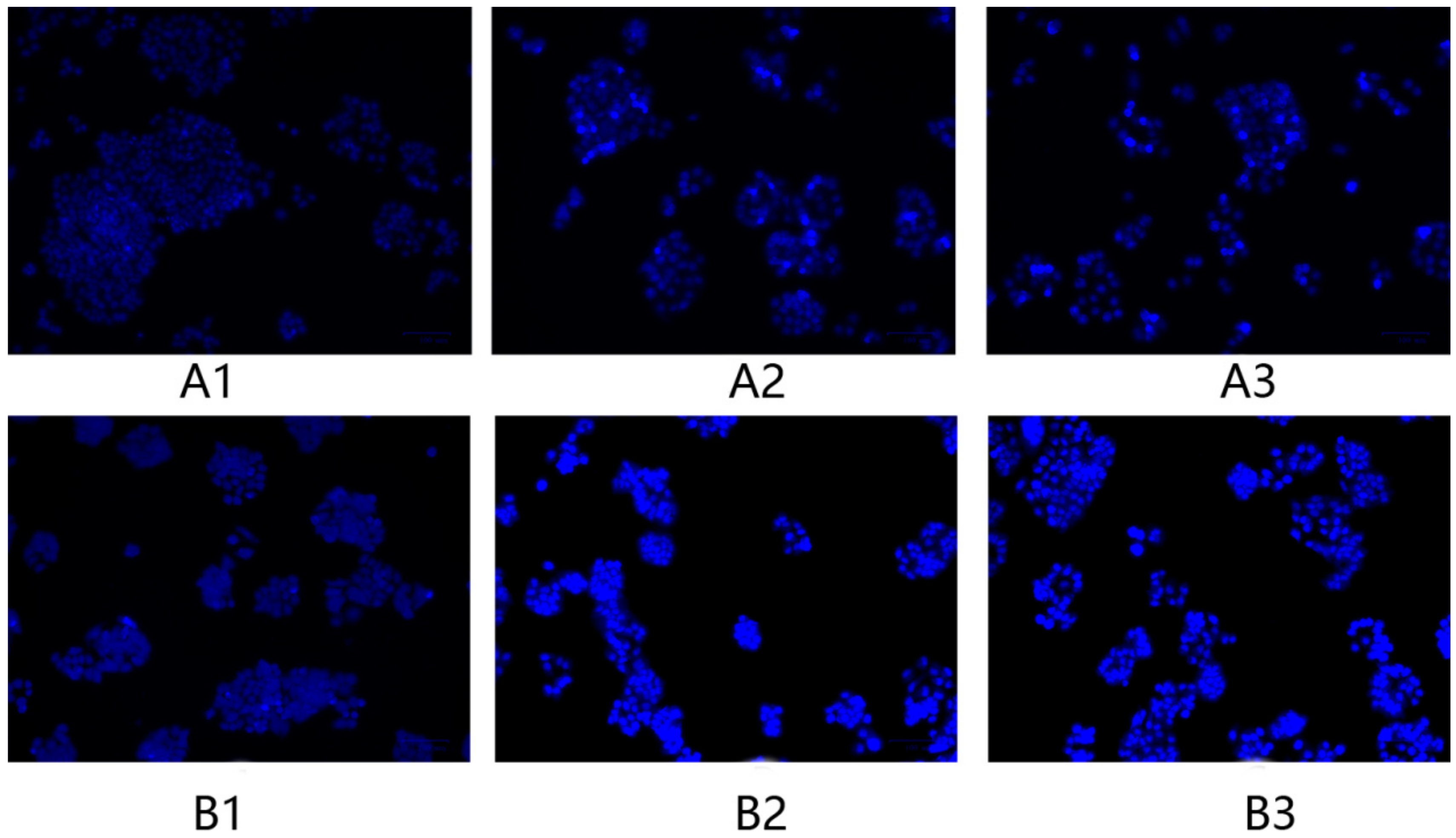
2.6. Morphology Changes in HeLa Cells after Treatment with F1 and F3 for 24 h and 48 h
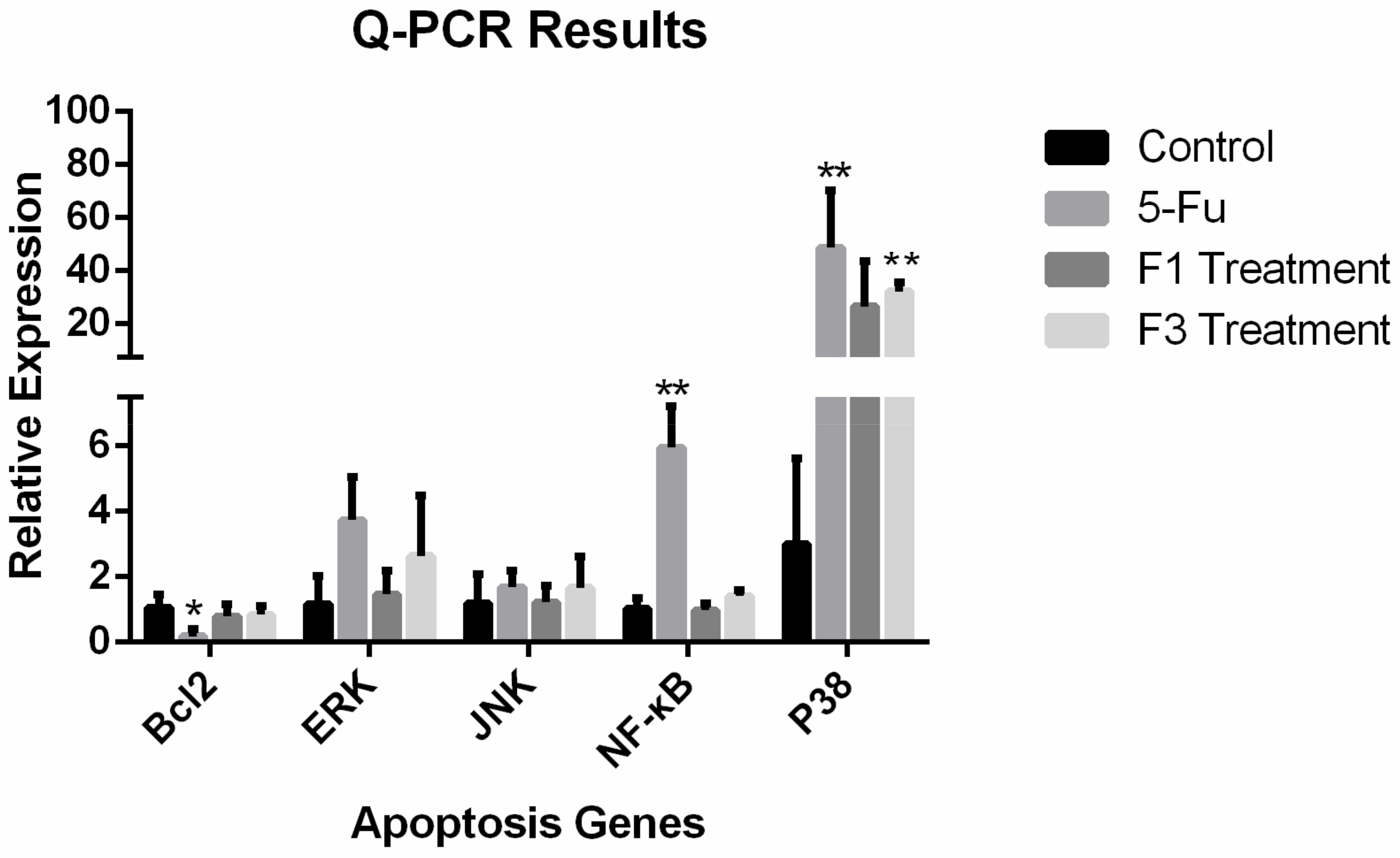
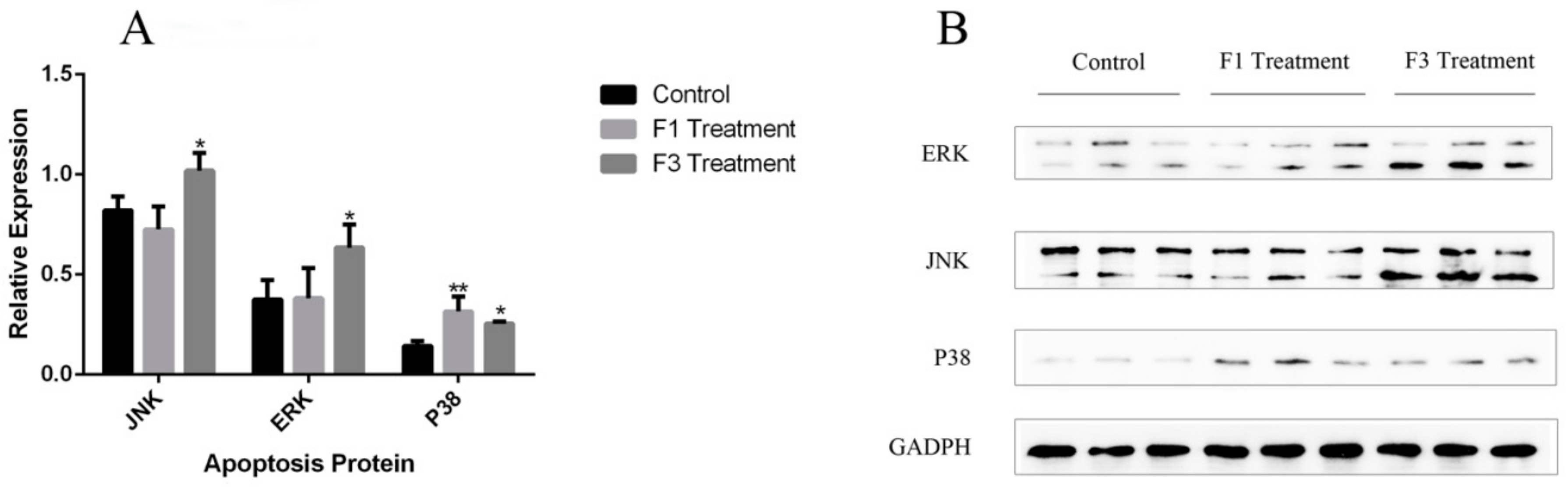
2.7. Relative Expression of Apoptosis Genes and Proteins
3. Discussion
4. Methods and Reagents
4.1. Materials and Reagents
4.2. Extraction of Dendrobium Officinale Polysaccharides
4.3. Purification of Polysaccharides
4.4. Oxidative Degradation of Crude Polysaccharides
4.5. Detection of the Molecular Weight of PPF and DPF
4.6. Detection of Monosaccharide Composition and Ratio of PPF and DPF
4.7. NMR Spectrometer Analysis of PPFs
4.8. MTT Assay of HeLa Cells Treated with CP and DPF
4.9. Detection of the Apoptosis Rate of HeLa Cells
4.10. Morphological Observation of HeLa Cells
4.11. Q-PCR Assay of the Expression of Apoptosis-Related Genes
4.12. Western-Blotting Assay of MAPK Protein
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Wing Sze, S.C.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L.; Huang, X.J.; Nie, S.P.; Xie, M.Y.; Phillips, G.O.; Cui, S.W. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part III–Immunomodulatory activity in vitro. Bioact. Carbohydr. Diet. Fibre 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Huang, X.J.; Nie, S.P.; Cai, H.L.; Zhang, G.Y.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part VI. Protective effects against oxidative stress in immunosuppressed mice. Food Res. Int. 2015, 72, 168–173. [Google Scholar] [CrossRef]
- Xiang, L.; Stephen Sze, C.W.; Ng, T.B.; Tong, Y.; Shaw, P.C.; Sydney Tang, C.W.; Kalin Zhang, Y.B. Polysaccharides of Dendrobium officinale inhibit TNF-α-induced apoptosis in A-253 cell line. Inflamm. Res. 2013, 62, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, J.; Chung, W.; Sze, S.C.; Li, H.; Lao, L.; Zhang, Y. Polysaccharides of Dendrobium officinale induce aquaporin 5 translocation by activating M3 muscarinic receptors. Planta Med. 2015, 81, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Liu, X.; Guo, H.; Zhang, H.; Zhu, J.; Ren, F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods 2012, 4, 294–301. [Google Scholar] [CrossRef]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Douglas Goff, H.; Wang, Q. A review of isolation process, structural characteristics, and bioactivities of water-soluble polysaccharides from Dendrobium plants. Bioact. Carbohydr. Diet. Fibre 2013, 1, 131–147. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Chien, C.; Liao, S.K.; Liao, S.F.; Hung, W.T.; Yang, W.B.; Lin, C.C.; Cheng, T.J.; Chang, C.C.; Fang, J.M.; et al. Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 2008, 16, 6054–6068. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, Y.; Su, J.; Ye, Q.; Jia, Z. Isolation and structural characterization of a neutral polysaccharide from the stems of Dendrobium densiflorum. Int. J. Biol. Macromol. 2012, 50, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Xiao, J.J.; Zha, X.Q.; Pan, L.H.; Asghar, M.N.; Luo, J.P. Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohydr. Polym. 2014, 106, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Sun, Y.D.; Wang, Z.H.; Ye, Q.S. Composition analysis and anti-proliferation activity of polysaccharides from Dendrobium chrysotoxum. Int. J. Biol. Macromol. 2013, 62, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.C.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Structural characterization and antioxidant activity of a low-molecular polysaccharide from Dendrobium huoshanense. Fitoterapia 2013, 91, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Luo, J.P.; Zha, X.Q. Structural features of a pectic polysaccharide from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 81, 1–7. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.L.; Yue, R.Q.; Ko, C.H.; Hu, J.M.; Liu, J.; Ho, H.M.; Yi, T.; Zhao, Z.Z.; Zhou, J.; et al. A novel and rapid HPGPC-based strategy for quality control of saccharide-dominant herbal materials: Dendrobium officinale, a case study. Anal. Bioanal. Chem. 2014, 406, 6409–6417. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Prasad, K.N.; Jiang, Y. Structure identification of a polysaccharide purified from litchi (Litchi chinensis Sonn.) pulp. Carbohydr. Polym. 2016, 137, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.Q.; Lu, C.Q.; Cui, S.H.; Pan, L.H.; Zhang, H.L.; Wang, J.H.; Luo, J.P. Structural identification and immunostimulating activity of a Laminaria japonica polysaccharide. Int. J. Biol. Macromol. 2015, 78, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.Q.; Luo, J.P.; Luo, S.Z.; Jiang, S.T. Structure identification of a new immunostimulating polysaccharide from the stems of Dendrobium huoshanense. Carbohydr. Polym. 2007, 69, 86–93. [Google Scholar] [CrossRef]
- Hua, Y.F.; Zhang, M.; Fu, C.X.; Chen, Z.H.; Chan, G.Y. Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr. Res. 2004, 339, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part I. Extraction, purification, and partial structural characterization. Bioact. Carbohydr. Diet. Fibre 2014, 4, 74–83. [Google Scholar] [CrossRef]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part II. Fine structures of O-acetylated residues. Carbohydr. Polym. 2015, 117, 422–433. [Google Scholar] [CrossRef] [PubMed]
- He, T.B.; Huang, Y.P.; Yang, L.; Liu, T.T.; Gong, W.Y.; Wang, X.J.; Sheng, J.; Hu, J.M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Smoum, R.; Rubinstein, A.; Srebnik, M. Combined 1H, 13C and 11B-NMR and mass spectral assignments of boronate complexes of d-(+)-glucose, d-(+)-mannose, methyl-α-d-glucopyranoside, methyl-β-d-galactopyranoside and methyl-α-d-mannopyranoside. Magn. Reson. Chem. 2003, 41, 1015–1020. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr. Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xue, C.; Chang, Y.; Hu, Y.; Xu, X.; Ge, L.; Liu, G. Structure and rheological characteristics of fucoidan from sea cucumber Apostichopus japonicus. Food Chem. 2015, 180, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhao, M.; Qi, H. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohydr. Polym. 2014, 112, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The sample of plant Dendrobium officinale is available from the corresponding author Gang Wei and Zhouxi Lei. |
| Order | Vitamin C (mL) | H2O2 (mL) | FeCl2∙4H2O (mL) | CP | Water (mL) |
|---|---|---|---|---|---|
| 1 | 0.5 | 0.05 | 0.05 | 30 | 19.4 |
| 2 | 1 | 0.1 | 0.1 | 30 | 18.8 |
| 3 | 2 | 0.2 | 0.2 | 30 | 17.6 |
| 4 | 4 | 0.4 | 0.4 | 30 | 15.2 |
| 5 | 5 | 0.5 | 0.5 | 30 | 14 |
| 6 | 6 | 0.6 | 0.6 | 30 | 12.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Ren, Z.; Zhang, X.; Xing, S.; Tao, S.; Liu, C.; Wei, G.; Yuan, Y.; Lei, Z. Structural Characterization of Polysaccharides from Dendrobium officinale and Their Effects on Apoptosis of HeLa Cell Line. Molecules 2018, 23, 2484. https://doi.org/10.3390/molecules23102484
Yu W, Ren Z, Zhang X, Xing S, Tao S, Liu C, Wei G, Yuan Y, Lei Z. Structural Characterization of Polysaccharides from Dendrobium officinale and Their Effects on Apoptosis of HeLa Cell Line. Molecules. 2018; 23(10):2484. https://doi.org/10.3390/molecules23102484
Chicago/Turabian StyleYu, Wenxia, Zhiyao Ren, Xiaofeng Zhang, Shangping Xing, Shengchang Tao, Chenxing Liu, Gang Wei, Yuan Yuan, and Zhouxi Lei. 2018. "Structural Characterization of Polysaccharides from Dendrobium officinale and Their Effects on Apoptosis of HeLa Cell Line" Molecules 23, no. 10: 2484. https://doi.org/10.3390/molecules23102484
APA StyleYu, W., Ren, Z., Zhang, X., Xing, S., Tao, S., Liu, C., Wei, G., Yuan, Y., & Lei, Z. (2018). Structural Characterization of Polysaccharides from Dendrobium officinale and Their Effects on Apoptosis of HeLa Cell Line. Molecules, 23(10), 2484. https://doi.org/10.3390/molecules23102484






