Synthesis and In Vitro Antimycobacterial and Antibacterial Activity of 8-OMe Ciprofloxacin-Hydrozone/Azole Hybrids
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anti-MTB Activity
2.3. Antibacterial Activity
3. Experimental Section
3.1. General
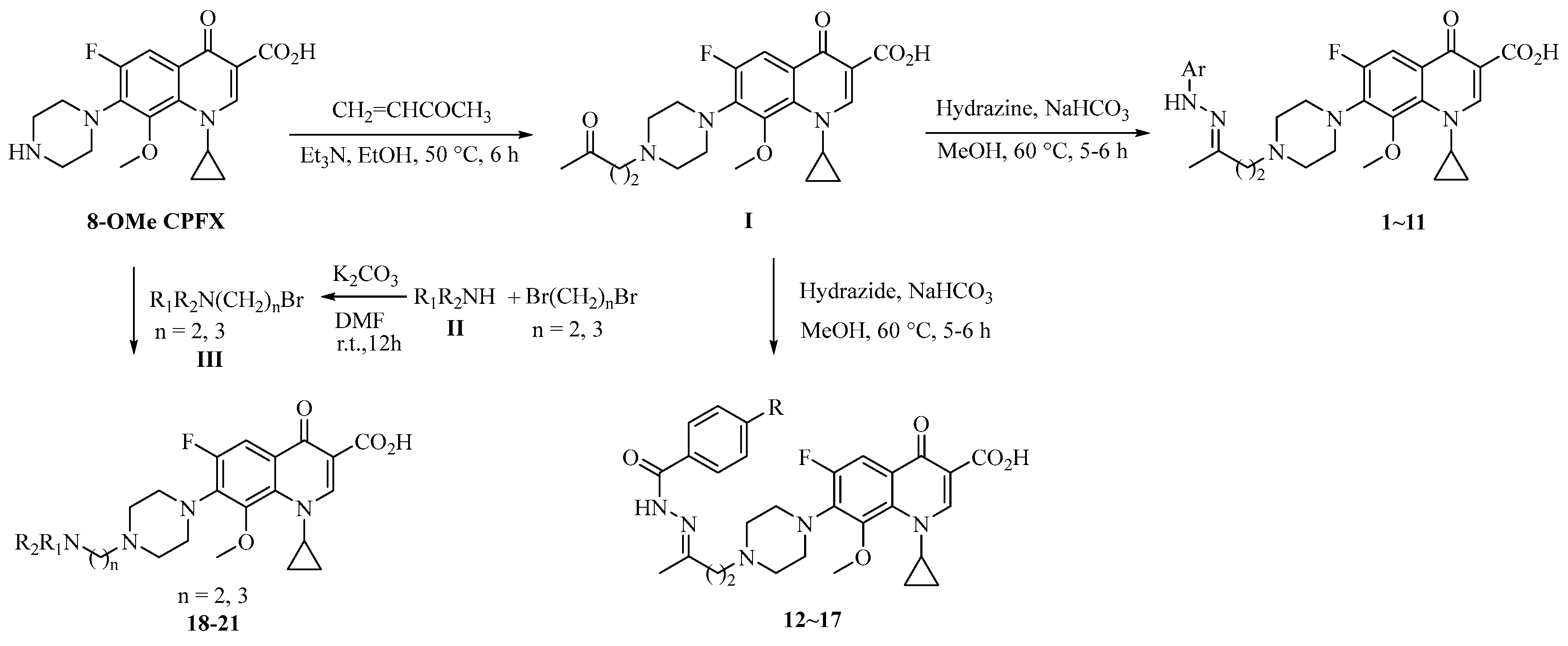
3.2. Synthesis
3.2.1. Method 1
3.2.2. Method 2
3.2.3. Method 3
3.3. Anti-MTB Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ball, P. Quinolone generations: Natural history or natural selection? J. Antimicrob. Chemother. 2000, 46, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gyoergy, C.; Jerzsele, A. Pradofloxacin, new generation fluoroquinolone in small animal practice. Magy. Allatorvosok. Lapja. 2012, 134, 289–296. [Google Scholar]
- Mitscher, L.A. Bacterial Topoisomerase Inhibitors: Quinolone and Pyridone Antibacterial Agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar] [CrossRef] [PubMed]
- Bisacchi, G.S. Origins of the Quinolone Class of Antibacterials: An Expanded “Discovery Story”. J. Med. Chem. 2015, 58, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Kerns, R.J.; Rybak, G.W.M.; Vaka, F.; Cha, R.; Grucz, R.G.; Diwadkar, V.U.; Ward, T.D. Piperazinyl-linked fluoroquinolone dimers possessing potent antibacterial activity against drug-resistant strains of Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2003, 13, 1745–1749. [Google Scholar] [CrossRef]
- Robinson, M.J.; Martin, B.A.; Gootz, T.D.; McGuirk, P.R.; Moynihan, M.; Sutcliffe, J.A.; Osheroff, N. Effects of quinolone derivatives on eukaryotic topoisomerase II. A novel mechanism for enhancement of enzyme-mediated DNA cleavage. J. Biol. Chem. 1991, 266, 14585–14592. [Google Scholar] [PubMed]
- Mather, R.; Karenchak, L.M.; Romanowski, E.G.; Kowalski, R.P. Fourth generation FQs: New weapons in the arsenal of ophthalmic antibiotics. Am. J. Ophthalmol. 2002, 133, 463–466. [Google Scholar] [CrossRef]
- Crofton, J.; Choculet, P.; Maher, D. Guidelines for the Management of Drug-Resistant Tuberculosis WHO/TB/96–210 (Rev. 1); World Health Organization: Geneva, France, 1997. [Google Scholar]
- Ginsburg, A.S.; Grosset, J.H.; Bishai, W.R. FQs, tuberculosis, and resistance. Lancet Infect. Dis. 2003, 3, 432–442. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, S.; Gao, C.; Zhao, F.; Lv, Z.S.; Feng, L.S. Isatin hybrids and their anti-tuberculosis activity. Chin. Chem. Lett. 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Sriram, D.; Aubry, A.; Yogeeswaria, P.; Fisher, L.M. Gatifloxacin derivatives: Synthesis, antimycobacterial activities, and inhibition of Mycobacterium tuberculosis DNA gyrase. Bioorg. Med. Chem. Lett. 2006, 16, 2982–2985. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswaria, P.; Basha, J.S.; Radhaet, D.R.; Nagaraja, V. Synthesis and antimycobacterial evaluation of various 7-substituted ciprofloxacin derivatives. Bioorg. Med. Chem. 2005, 13, 5774–5778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.H.; Pine, R.; Domagala, J.; Drlica, K. Fluoroquinolone Action against Clinical Isolates of Mycobacterium tuberculosis: Effects of a C-8 Methoxyl Group on Survival in Liquid Media and in Human Macrophages. Antimicrob. Agents Chemother. 1999, 43, 661–666. [Google Scholar] [PubMed]
- Sanchez, J.P.; Gogliotti, R.D.; Domagala, J.M.; Gracheck, S.J.; Huband, M.D.; Sesnie, J.A.; Cohen, M.A.; Shapiro, M.A. The Synthesis, Structure-Activity, and Structure-Side Effect Relationships of a Series of 8-Alkoxy- and 5-Amino-8-alkoxyquinolone Antibacterial Agents. J. Med. Chem. 1995, 38, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Liu, M.L.; Zhang, S.; Chai, Y.; Wang, B.; Zhang, Y.B.; Lv, K.; Guan, Y.; Guo, H.Y.; Xiao, C.L. Synthesis and in vitro antimycobacterial activity of 8-OCH3 ciprofloxacin methylene and ethylene isatin derivatives. Eur. J. Med. Chem. 2011, 46, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Xu, Z.; Zhang, S.; Wu, X.; Ding, J.W.; Zao, S.L.; Feng, L.S. Recent developments of coumarin-containing derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 136, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Zhang, S.; Gao, C.; Zhao, F.; Ding, J.W.; Feng, L.S.; Lv, Z.S.; Xu, Z.; Wu, X. Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 133, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Determination of the Primary Molecular Target of 1,2,4-Triazole-Ciprofloxacin Hybrids. Molecules 2015, 26, 6254–6272. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskaya, V.; Belakhov, V.; Hainrichson, M.; Yaron, S.; Baasov, T. Design, Synthesis, and Evaluation of Novel Fluoroquinolone-Aminoglycoside Hybrid Antibiotics. J. Med. Chem. 2009, 52, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Singh, V.; Nath, G.; Awasthi, S.K.; Agarwal, A. Design, synthesis and biological evaluation of ciprofloxacin tethered bis-1,2,3-triazole conjugates as potent antibacterial agents. Eur. J. Med. Chem. 2016, 124, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kapron, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Staczek, P.; Swiatek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Search for factors affecting antibacterial activity and toxicity of 1,2,4-triazole-ciprofloxacin hybrids. Eur. J. Med. Chem. 2015, 97, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Rajtar, B.; Polz-Dacewicz, M. Synthesis and in vitro activity of 1,2,4-triazole-ciprofloxacin hybrids against drug-susceptible and drug-resistant bacteria. Eur. J. Med. Chem. 2013, 60, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Foroumadi, A.; Emami, S.; Hassanzadeh, A.; Rajaee, M.; Sokhanvar, K.; Moshafi, M.H.; Shafiee, A. Synthesis and antibacterial activity of N-(5-benzylthio-1,3,4-thiadiazol-2-yl) and N-(5-benzylsulfonyl-1,3,4-thiadiazol-2-yl)piperazinyl quinolone derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4488–4492. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Liu, M.L.; Lv, K.; Feng, L.S.; Li, S.J.; Sun, L.Y.; Wang, S.; Guo, H.Y. Synthesis and in vitro antibacterial activity of a series of novel gatifloxacin derivatives. Eur. J. Med. Chem. 2011, 46, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Liu, M.L.; Wang, B.; Chai, Y.; Hao, X.Q.; Meng, S.; Guo, H.Y. Synthesis and in vitro antimycobacterial activity of balofloxacin ethylene isatin derivatives. Eur. J. Med. Chem. 2010, 45, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Liu, M.L.; Wang, S.; Cai, Y.; Lv, K.; Shan, G.Z.; Cao, J.; Li, S.J.; Guo, H.Y. Synthesis of naphthyridone derivatives containing 8-alkoxyimino-1,6-dizaspiro[3.4]octane scaffolds. Tetrahedron 2011, 67, 8264–8270. [Google Scholar] [CrossRef]
- Feng, L.S.; Lv, K.; Liu, M.L.; Wang, S.; Zhao, J.; You, X.F.; Li, S.J.; Cao, J.; Guo, H.Y. Synthesis and in vitro antibacterial activity of gemifloxacin derivatives containing a substituted benzyloxime moiety. Eur. J. Med. Chem. 2012, 55, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Tan, Y.H.; Liu, M.L. Antibacterial activity of naphthyridone derivatives containing 8-alkoxyimino-1,6-dizaspiro[3.4]octane scaffolds. Asian J. Chem. 2014, 26, 3805–3807. [Google Scholar]
- Lu, Y.; Zheng, M.; Wang, B.; Fu, L.; Zhao, W.; Li, P.; Xu, J.; Zhu, H.; Jin, H.; Yin, D.; et al. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob. Agents Chemother. 2011, 55, 5185–5193. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
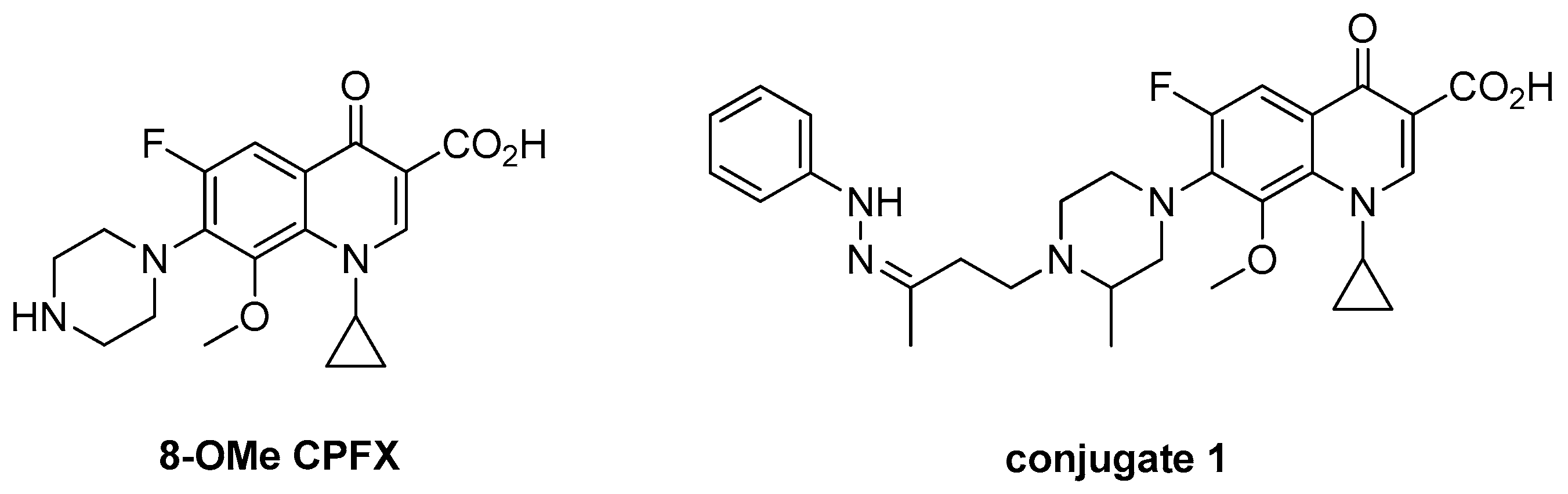
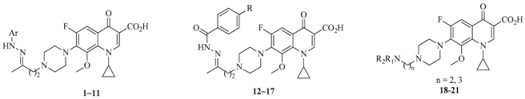
| Compound. | Ar, R or R1R2N (n) | Clog P a | MIC (μM) |
|---|---|---|---|
| 1 |  | 2.45 | 0.398 |
| 2 |  | 2.04 | 0.434 |
| 3 | 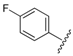 | 2.26 | 0.215 |
| 4 | 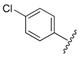 | 2.82 | 0.433 |
| 5 | 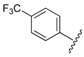 | 3.12 | 0.394 |
| 6 | 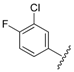 | 3.03 | 0.396 |
| 7 | 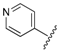 | 0.99 | 1.78 |
| 8 | 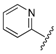 | 0.99 | 0.372 |
| 9 | 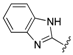 | 1.91 | 0.434 |
| 10 | 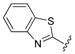 | 2.47 | 0.313 |
| 11 | 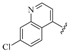 | 3.15 | 0.266 |
| 12 | -H | 0.99 | 0.226 |
| 13 | -OCH3 | 1.27 | 0.214 |
| 14 | -OH | 0.66 | 0.210 |
| 15 | -F | 1.34 | 0.266 |
| 16 | -Cl | 1.90 | 0.272 |
| 17 | -NO2 | 1.16 | 0.294 |
| 18 |  (2) (2) | −0.13 | 1.72 |
| 19 | 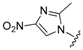 (3) (3) | −0.01 | 60.3 |
| 20 | 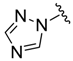 (2) (2) | −1.16 | 4.10 |
| 21 | 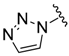 (2) (2) | 0.88 | 14.2 |
| CPFX | −0.72 | 1.30 | |
| MXFX | −0.08 | 0.289 | |
| INH | 0.336 |
| Compound | MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| MSSE | MRSE | MSSA | MRSA | E.fa.1 | E.fa.2 | E.fm.1 | E.fm.2 | |
| 1 | 0.25 | 64 | 0.25 | 0.25 | 1 | 1 | 64 | 64 |
| 2 | 0.25 | >64 | 0.125 | 0.125 | 2 | 2 | >64 | >64 |
| 3 | 0.125 | 32 | 0.125 | 0.125 | 1 | 1 | 32 | 32 |
| 4 | 0.125 | 32 | 0.06 | 0.06 | 1 | 1 | 64 | 64 |
| 5 | 0.25 | 32 | 0.125 | 0.125 | 1 | 1 | 64 | 64 |
| 6 | 0.125 | 32 | 0.125 | 0.125 | 0.5 | 0.5 | 64 | 16 |
| 7 | 2 | >64 | 2 | 2 | 8 | 8 | >64 | >64 |
| 8 | 0.125 | 32 | 0.125 | 0.125 | 1 | 1 | 64 | 64 |
| 9 | 1 | 64 | 1 | 1 | 2 | 2 | 128 | 128 |
| 10 | 0.5 | 64 | 0.25 | 0.5 | 1 | 1 | 64 | 64 |
| 11 | 1 | 128 | 0.5 | 0.5 | 4 | 4 | >128 | >128 |
| 12 | 0.125 | 64 | 0.125 | 0.125 | 0.5 | 0.5 | 64 | 64 |
| 13 | 0.125 | 64 | 0.125 | 0.25 | 1 | 1 | 64 | 64 |
| 14 | 0.06 | 64 | 0.125 | 0.06 | 0.5 | 0.5 | 64 | 64 |
| 15 | 0.125 | 64 | 0.125 | 0.125 | 0.5 | 0.5 | >64 | >64 |
| 16 | 0.125 | 32 | 0.125 | 0.125 | 1 | 1 | 32 | 32 |
| 17 | 0.125 | 128 | 0.25 | 0.25 | 1 | 1 | 128 | 128 |
| 18 | 1 | >128 | 1 | 1 | 2 | 2 | >128 | >128 |
| 19 | 32 | >64 | 32 | 32 | >64 | >64 | >64 | >64 |
| 20 | 4 | >128 | 4 | 4 | 16 | 16 | >128 | >128 |
| 21 | 2 | >128 | 4 | 4 | 16 | 16 | >128 | >128 |
| Conjugate-1 | 0.06 a | 0.06 a | 0.125 a | 0.25 a | ND | ND | 0.5 a | 0.5 a |
| CPFX | 0.125 | 64 | 0.25 | 0.25 | 0.5 | 0.5 | >128 | >128 |
| LVFX | 0.125 | 32 | 0.125 | 0.125 | 1 | 0.5 | 32 | 32 |
| Compound | MIC (μg/mL) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E.co.1 | E.co.2 | K.p.1 | K.p.2 | P.a. | A.c. | E.c. | E.a. | S.m.1 | M.m. | P.r. | P.v. | P.m. | S.m.2 | C.f. | |
| 1 | 0.06 | 64 | 2 | 0.25 | 2 | 1 | 0.06 | 0.5 | 0.5 | ≤0.03 | 0.06 | 0.06 | 0.25 | 4 | 0.25 |
| 2 | 0.125 | >64 | 8 | 2 | 8 | 2 | 0.25 | 0.5 | 2 | 0.125 | 0.06 | 0.06 | 0.5 | 16 | 0.25 |
| 3 | 0.06 | 32 | 4 | 0.25 | 1 | 1 | 0.06 | 0.25 | 0.5 | 0.06 | 0.06 | 0.06 | 0.25 | 8 | 0.125 |
| 4 | 0.06 | 32 | 4 | 0.25 | 4 | 1 | 0.125 | 0.25 | 0.5 | 0.06 | 0.06 | 0.06 | 0.5 | 4 | 1.125 |
| 5 | 0.125 | >64 | 8 | 0.5 | 4 | 1 | 0.125 | 0.25 | 1 | 0.125 | 0.125 | 0.125 | 0.5 | 8 | 0.25 |
| 6 | 0.06 | 32 | 4 | 0.25 | 4 | 0.5 | 0.06 | 0.25 | 1 | ≤0.03 | ≤0.03 | 0.06 | 0.25 | 4 | 0.125 |
| 7 | 1 | >64 | 64 | 4 | 32 | 16 | 2 | 8 | 8 | 1 | 1 | 1 | 8 | 32 | 2 |
| 8 | ≤0.03 | 32 | 4 | 0.25 | 2 | 0.25 | 0.06 | 0.25 | 0.25 | ≤0.03 | ≤0.03 | ≤0.03 | 0.25 | 2 | 0.06 |
| 9 | 0.5 | >128 | 16 | 1 | 16 | 4 | 0.5 | 1 | 2 | 0.5 | 0.25 | 0.25 | 2 | 16 | 1 |
| 10 | 0.125 | 128 | 4 | 0.5 | 4 | 2 | 0.25 | 0.5 | 1 | 0.06 | 0.06 | 0.06 | 0.125 | 16 | 0.125 |
| 11 | 0.5 | >128 | 64 | 4 | 16 | 8 | 0.5 | 2 | 4 | 0.5 | 0.25 | 0.5 | 1 | 32 | 1 |
| 12 | ≤0.03 | 32 | 2 | 0.06 | 2 | 0.5 | 0.06 | 0.125 | 0.25 | 0.06 | ≤0.03 | ≤0.03 | 0.125 | 2 | 0.06 |
| 13 | 0.06 | 16 | 2 | 0.25 | 8 | 0.5 | 0.06 | 0.25 | 0.5 | ≤0.03 | ≤0.03 | ≤0.03 | 0.25 | 4 | 0.125 |
| 14 | ≤0.03 | 32 | 2 | 0.125 | 1 | 0.5 | 0.06 | 0.25 | 0.25 | ≤0.03 | ≤0.03 | ≤0.03 | 0.25 | 2 | 0.06 |
| 15 | 0.06 | 64 | 2 | 0.25 | 2 | 0.5 | ≤0.03 | 0.25 | 0.25 | ≤0.03 | 0.06 | ≤0.03 | 0.25 | 2 | 0.06 |
| 16 | ≤0.03 | 32 | 4 | 0.25 | 2 | 0.5 | 0.06 | 0.125 | 0.5 | ≤0.03 | ≤0.03 | 0.06 | 0.5 | 0.5 | 0.125 |
| 17 | 0.06 | 64 | 2 | 0.125 | 4 | 0.5 | ≤0.03 | 0.25 | 0.25 | 0.06 | ≤0.03 | ≤0.03 | 0.25 | 4 | 0.06 |
| 18 | 0.125 | 128 | 8 | 2 | 8 | 2 | 0.125 | 0.5 | 1 | 0.125 | 0.06 | 0.06 | 1 | 16 | 0.25 |
| 19 | 8 | >64 | >64 | 32 | >64 | >64 | 8 | 32 | 64 | 8 | 4 | 4 | 32 | >64 | 16 |
| 20 | 0.5 | >128 | 32 | 4 | 64 | 16 | 1 | 4 | 8 | 1 | 0.5 | 0.5 | 4 | 64 | 2 |
| 21 | 1 | >128 | 64 | 2 | 64 | 16 | 1 | 2 | 8 | 0.5 | 0.5 | 0.5 | 2 | 64 | 1 |
| Conjugate-1 | 0.25 a | 1 a | 0.5 a | 1 a | 0.5 a | ND | 0.25 a | ND | ND | ND | ND | ND | ND | ND | ND |
| CPFX | ≤0.03 | 16 | 0.5 | ≤0.03 | 0.25 | 0.5 | ≤0.03 | ≤0.03 | 0.06 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | 4 | ≤0.03 |
| LVFX | ≤0.03 | 16 | 0.5 | ≤0.03 | 1 | 0.125 | ≤0.03 | 0.06 | 0.125 | ≤0.03 | ≤0.03 | ≤0.03 | 0.06 | 1 | ≤0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhang, S.; Feng, L.-S.; Li, X.-N.; Huang, G.-C.; Chai, Y.; Lv, Z.-S.; Guo, H.-Y.; Liu, M.-L. Synthesis and In Vitro Antimycobacterial and Antibacterial Activity of 8-OMe Ciprofloxacin-Hydrozone/Azole Hybrids. Molecules 2017, 22, 1171. https://doi.org/10.3390/molecules22071171
Xu Z, Zhang S, Feng L-S, Li X-N, Huang G-C, Chai Y, Lv Z-S, Guo H-Y, Liu M-L. Synthesis and In Vitro Antimycobacterial and Antibacterial Activity of 8-OMe Ciprofloxacin-Hydrozone/Azole Hybrids. Molecules. 2017; 22(7):1171. https://doi.org/10.3390/molecules22071171
Chicago/Turabian StyleXu, Zhi, Shu Zhang, Lian-Shun Feng, Xiao-Ning Li, Guo-Cheng Huang, Yun Chai, Zao-Sheng Lv, Hui-Yuan Guo, and Ming-Liang Liu. 2017. "Synthesis and In Vitro Antimycobacterial and Antibacterial Activity of 8-OMe Ciprofloxacin-Hydrozone/Azole Hybrids" Molecules 22, no. 7: 1171. https://doi.org/10.3390/molecules22071171
APA StyleXu, Z., Zhang, S., Feng, L.-S., Li, X.-N., Huang, G.-C., Chai, Y., Lv, Z.-S., Guo, H.-Y., & Liu, M.-L. (2017). Synthesis and In Vitro Antimycobacterial and Antibacterial Activity of 8-OMe Ciprofloxacin-Hydrozone/Azole Hybrids. Molecules, 22(7), 1171. https://doi.org/10.3390/molecules22071171




