The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri
Abstract
:1. Introduction
2. Results
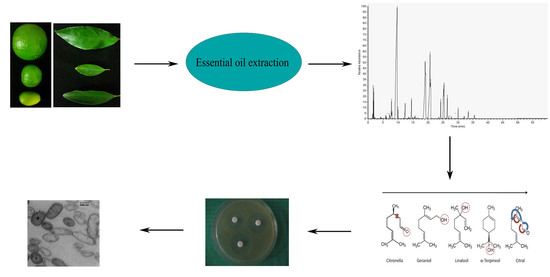
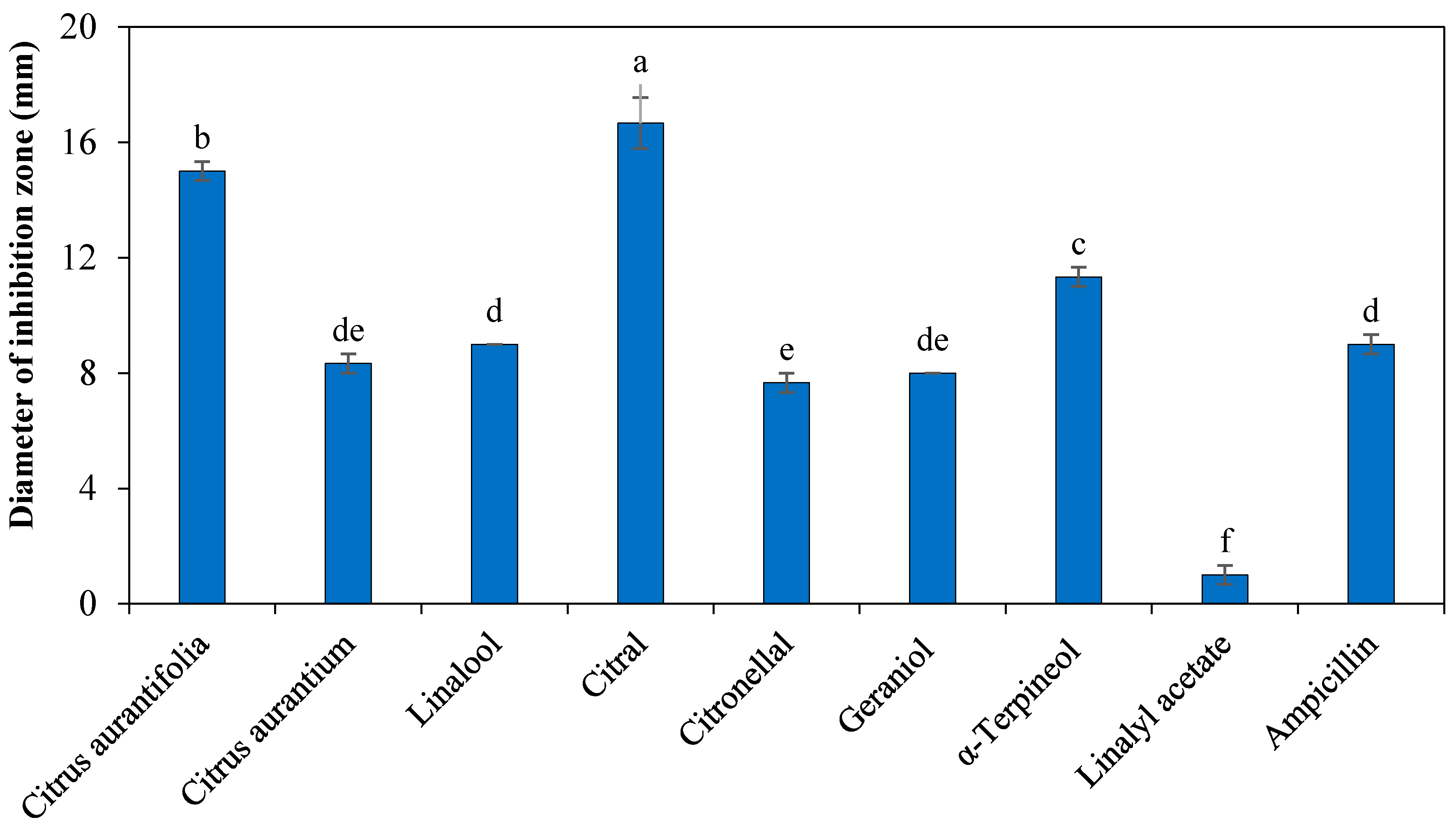
2.1. Antibacterial Activity Assays
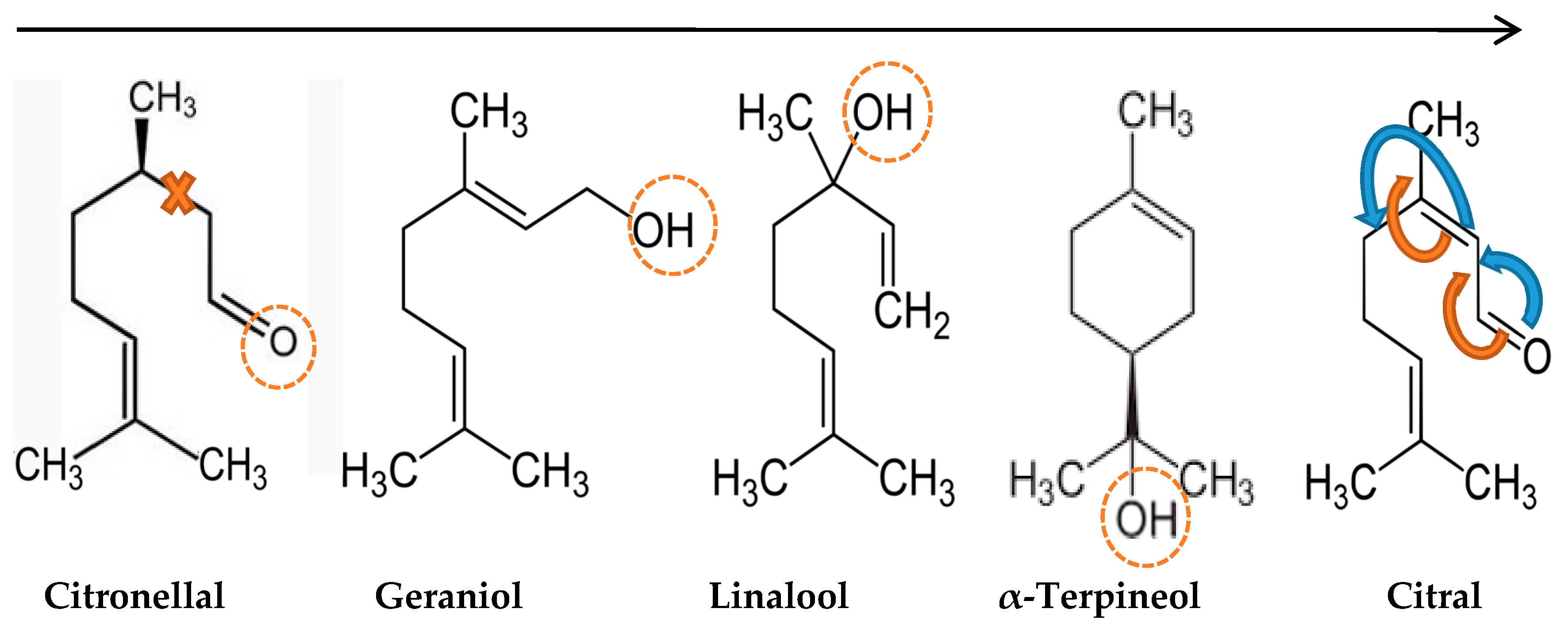
2.2. GC-MS Analysis
2.3. Determination of MIC and MBC
2.4. Synergist Assay
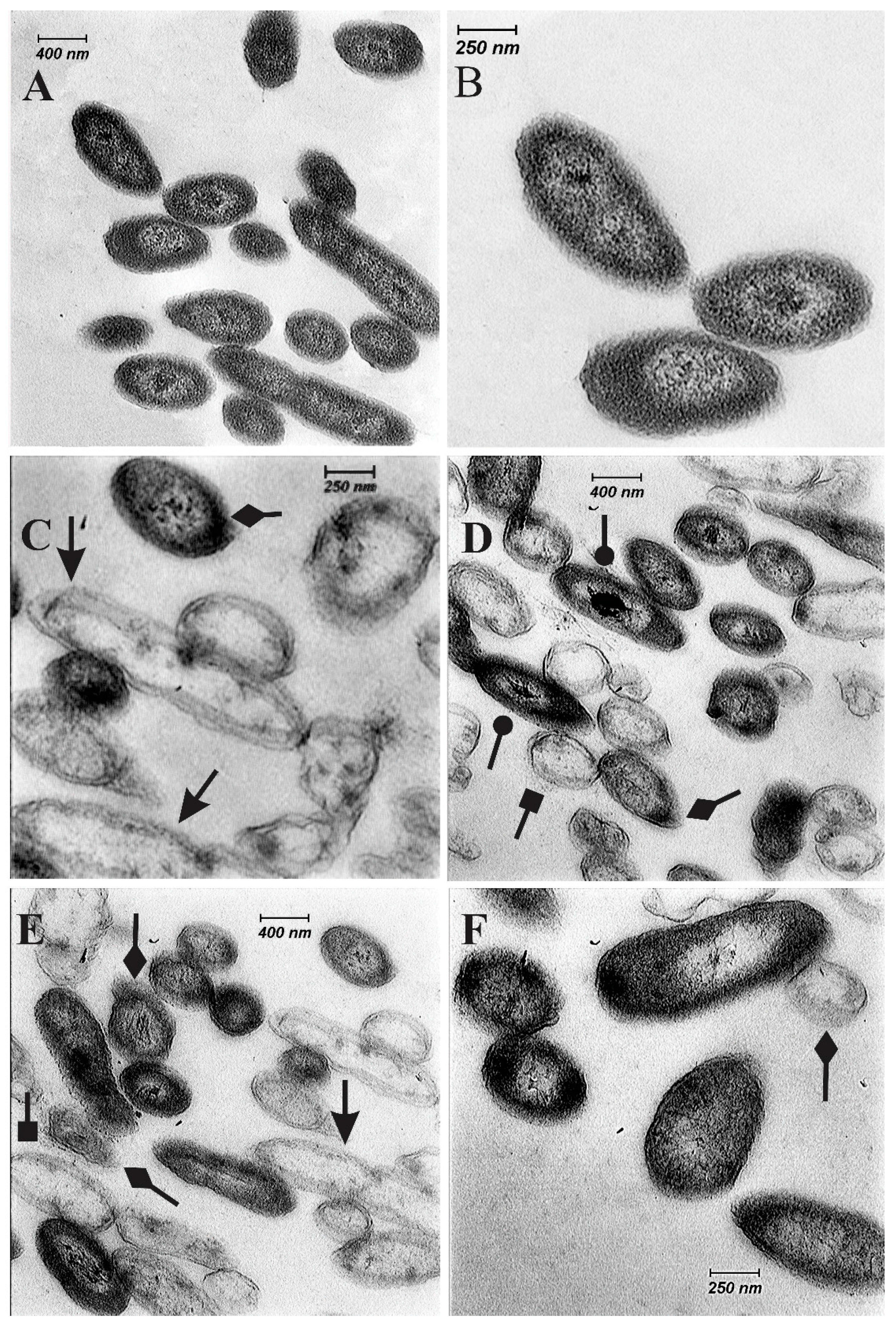
2.5. Ultrastructural Changes of KVXCC1
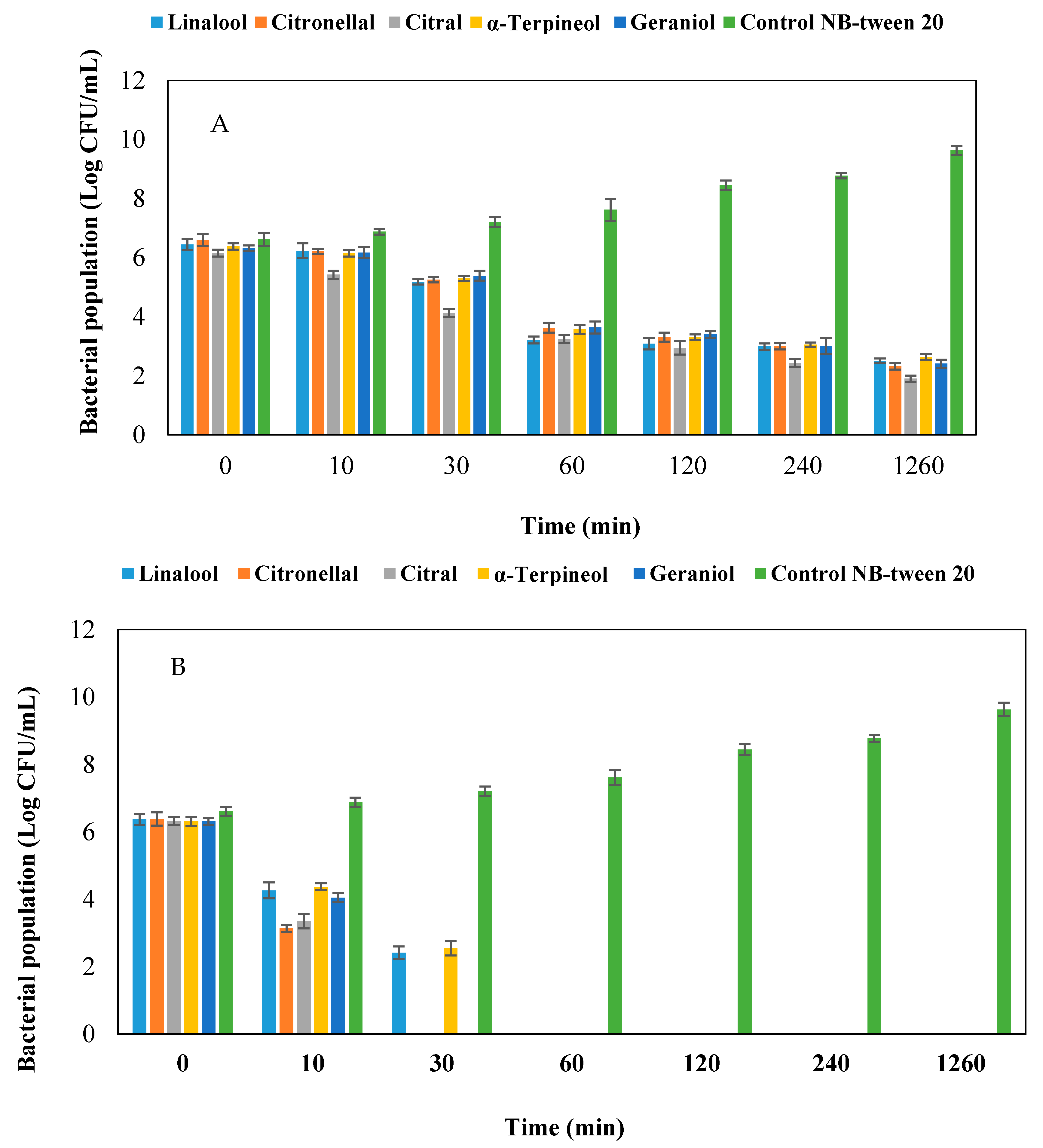
2.6. Time-Kill Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions, and Chemical Constituents
4.2. Essential Oils Extraction
4.3. Disk Diffusion Assay
4.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.5. GC-MS Analysis
4.6. Identification of Synergistic Effects between EOs Constituents
4.7. Time-Kill Assay
4.8. Preparation of Cells for TEM
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15. [Google Scholar] [PubMed]
- Pruvost, O.; Goodarzi, T.; Boyer, K.; Soltaninejad, H.; Escalon, A.; Alavi, S.; Javegny, S.; Boyer, C.; Cottyn, B.; Gagnevin, L. Genetic structure analysis of strains causing citrus canker in Iran reveals the presence of two different lineages of Xanthomonas citri pv. citri pathotype A. Plant Pathol. 2015, 64, 776–784. [Google Scholar] [CrossRef]
- Picchi, S.; Takita, M.; Coletta-Filho, H.; Machado, M.; Souza, A. N-acetylcysteine interferes with the biofilm formation, motility and epiphytic behaviour of Xanthomonas citri subsp. citri. Plant Pathol. 2015, 65, 561–569. [Google Scholar] [CrossRef]
- Leduc, A.; Traoré, Y.; Boyer, K.; Magne, M.; Grygiel, P.; Juhasz, C.; Boyer, C.; Guerin, F.; Wonni, I.; Ouedraogo, L. Bridgehead invasion of a monomorphic plant pathogenic bacterium: Xanthomonas citri pv. citri, an emerging citrus pathogen in Mali and Burkina Faso. Environ. Microbiol. 2015, 17, 4429–4442. [Google Scholar] [PubMed]
- Deng, Z.; Xu, L.; Li, D.; Long, G.; Liu, L.; Fang, F.; Shu, G. Screening citrus genotypes for resistance to canker disease (Xanthomonas axonopodis pv. citri). Plant Breed. 2010, 129, 341–345. [Google Scholar] [CrossRef]
- Escalon, A.; Javegny, S.; Vernière, C.; Noël, L.D.; Vital, K.; Poussier, S.; Hajri, A.; Boureau, T.; Pruvost, O.; Arlat, M. Variations in type III effector repertoires, pathological phenotypes and host range of Xanthomonas citri pv. citri pathotypes. Mol. Plant Pathol. 2013, 14, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Imran, M.; Rasool, A.; Azeem, M.; Riaz, A.; Afzal, M. Evaluation of commercial citrus cultivars for resistance to citrus leaf miner and its management. J. Entomol. Zool. Stud. 2014, 2, 213–216. [Google Scholar]
- Ali, A.; Haider, M.S.; Hanif, S.; Akhtar, N. Assessment of the antibacterial activity of Cuscuta pedicellata Ledeb. Afr. J. Biotechnol. 2014, 13, 430–433. [Google Scholar]
- Chudasama, K.S.; Thaker, V.S. Screening of potential antimicrobial compounds against Xanthomonas campestris from 100 essential oils of aromatic plants used in India: An ecofriendly approach. Arch. Phytopathol. Plant Prot. 2012, 45, 783–795. [Google Scholar] [CrossRef]
- Machial, C. M.; Shikano, I.; Smirle, M.; Bradbury, R.; Isman, M.B. Evaluation of the toxicity of 17 essential oils against Choristoneura rosaceana (Lepidoptera: Tortricidae) and Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. sci. 2010, 66, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Samavi, S.; Hassanzadeh, N.; Faghihi, M.; Danesh, Y.R. Effects of thyme (zaatar) essential oil and some chemical compounds in the control of citrus bacterial canker in Iran. J. Plant Pathol. 2009, 91, 691–696. [Google Scholar]
- Lucas, G.C.; Alves, E.; Pereira, R.B.; Perina, F.J.; Souza, R.M.D. Antibacterial activity of essential oils on Xanthomonas vesicatoria and control of bacterial spot in tomato. Pesq. Agropec. Bras. 2012, 47, 351–359. [Google Scholar] [CrossRef]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. IBSPR 2014, 2, 1–7. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.T.; Moayyedi, M. Comparison of heat and mass transfer of different microwave-assisted extraction methods of essential oil from Citrus limon (Lisbon variety) peel. Food Sci. Nutr. 2015, 3, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Carvajal, A.; Arguello, H.; Martínez-Lobo, F.; Naharro, G.; Rubio, P. Antibacterial activity and mode of action of a commercial citrus fruit extract. J. Appl. Microbiol. 2013, 115, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009, 106, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Chutia, M.; Bhuyan, P.D.; Pathak, M.; Sarma, T.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT-Food Sci. Technol. 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Asnaashari, S.; Delazar, A.; Habibi, B.; Vasfi, R.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Essential Oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice. Phytother. Res. 2010, 24, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Monsef-Esfahani, H.R.; Amanzade, Y.; Alhani, Z.; Hajimehdipour, H.; Faramarzi, M.A. GC/MS analysis of Citrus aurantium L. hydrolate and its comparison with the commercial samples. Iran. J. Pharm. Res. 2004, 3, 177–179. [Google Scholar]
- Ammar, A.H.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Romdhane, M.; Zagrouba, F. Chemical composition and in vitro antimicrobial and antioxidant activities of Citrus aurantium L. flowers essential oil (Neroli oil). Pak. J. Biol. Sci. 2012, 15, 1034. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Bisignano, C.; Filocamo, A.; Grasso, E.; Occhiuto, F.; Spadaro, F. Antimicrobial activity and chemical composition of Citrus aurantifolia (Christm.) Swingle essential oil from Italian organic crops. J. Essent. Oil Res. 2014, 26, 400–408. [Google Scholar] [CrossRef]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s variation impact on Citrus aurantium leaves essential oil: Chemical composition and biological activities. J. Food Sci. 2012, 77, T173–T180. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Caltavuturo, L.; Cini, M.; Della Croce, C.; Maserti, B. Antibacterial and antioxidant activity of essential oils from Citrus spp. J. Essent. Oil Res. 2011, 23, 27–31. [Google Scholar] [CrossRef]
- Shimada, T.; Endo, T.; Fujii, H.; Rodríguez, A.; Peña, L.; Omura, M. Characterization of three linalool synthase genes from Citrus unshiu Marc. and analysis of linalool-mediated resistance against Xanthomonas citri subsp. citri and Penicilium italicum in citrus leaves and fruits. Plant Sci. 2014, 229, 154–166. [Google Scholar] [PubMed]
- Taiwo, S.; Oyekanmi, B.; Adesiji, Y.; Opaleye, O.; Adeyeba, O. In vitro antimicrobial activity of crude extracts of Citrus aurantifolia Linn and Tithonia diversifolia Poaceae on clinical bacterial isolates. Int. J. Trop. Med. 2007, 2, 113–117. [Google Scholar]
- Belda-Galbis, C.M.; Leufvén, A.; Martínez, A.; Rodrigo, D. Quantitative assessment of citral antimicrobial potential at different temperatures. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 1257–1264. [Google Scholar]
- Somolinos, M.; García, D.; Condón, S.; Mackey, B.; Pagán, R. Inactivation of Escherichia coli by citral. J. Appl. Microbiol. 2010, 108, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Silva-Angulo, A.B.; Zanini, S.F.; Rosenthal, A.; Rodrigo, D.; Klein, G.; Martínez, A. Comparative Study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes cells. PLoS ONE 2015, 10, e0114026. [Google Scholar] [CrossRef] [PubMed]
- Rasoul, M.; Marei, G.; Abdelgaleil, S. Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. Afr. J. Microbiol. Res. 2012, 6, 3667–3672. [Google Scholar]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Belda-Galbis, C.M.; Pina-Pérez, M.C.; Leufvén, A.; Martínez, A.; Rodrigo, D. Impact assessment of carvacrol and citral effect on Escherichia coli K12 and Listeria innocua growth. Food Control 2013, 33, 536–544. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.A.; Bezerra, A.P.D.B.; Sousa, J.P.D.; Guerra, F.Q.S.; Lima, E.D.O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Saddiq, A.A.; Khayyat, S.A. Chemical and antimicrobial studies of monoterpene: Citral. Pest. Biochem. Physiol. 2010, 98, 89–93. [Google Scholar] [CrossRef]
- Miladinović, D.; Ilić, B.; Kocić, B.; Miladinović, M. An in vitro antibacterial study of savory essential oil and geraniol in combination with standard antimicrobials. Nat. Prod. Commun. 2014, 9, 1629–1632. [Google Scholar] [PubMed]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014, 45, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Gelaw, T. K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Rezaee, M.-B.; Jaimand, K.; Alinezhad, S.; Saberi, R.; Yoshinari, T. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control 2009, 20, 1018–1024. [Google Scholar] [CrossRef]
- Dongmo, P.J.; Tatsadjieu, L.; Tchinda, E.; Kuate, J.; Amvam, P.; Menut, C. Essential oils of Citrus aurantifolia from Cameroon and their antifungal activity against Phaeoramularia angolensis. Afr. J. Agric. Res. 2009, 4, 354–358. [Google Scholar]
- Babazadeh Darjazi, B. Comparison of leaf components of sweet orange and sour orange (Citrus sp.). Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1558–1568. [Google Scholar]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- Caccioni, D.R.; Guizzardi, M.; Biondi, D.M.; Renda, A.; Ruberto, G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int. J. Food Microbiol. 1998, 43, 73–79. [Google Scholar] [CrossRef]
- Rahimi, A.; Hashemi, P.; Talei, G.R.; Borzuei, M.; Ghiasvand, A.R. Comparative analyses of the volatile components of Citrus aurantium L. flowers using ultrasonic-assisted headspace SPME and hydrodistillation combined with GC-MS and evaluation of their antimicrobial activities. Anal. Bioanal. Chem. 2014, 1, 83–91. [Google Scholar]
- Quijano, C.E.; Pino, J.A. Volatile compounds of kumquat (Fortunella margarita (Lour.) Swingle) leaf oil. J. Essent. Oil Res. 2009, 21, 194–196. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Hawary, S.S.; Mohammed, M.M.; Farid, M.A.; Abdel-Wahed, N.A.; Ali, M.A.; El-Abd, E.A. Chemical composition, antiviral against avian Influenza (H5N1) virus and antimicrobial activities of the essential oils of the leaves and fruits of Fortunella margarita, Lour. Swingle, growing in Egypt. J. Appl. Pharm. Sci. 2015, 5, 006–012. [Google Scholar]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, M.; Li, H.; Chen, C.; Wang, J.; Zhang, Y. A study on the molecular mechanism of resistance to amicarthiazol in Xanthomonas campestris pv. citri. Pest. Manag. Sci. 2006, 62, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Krishnan, T.; Chan, K.-G.; Lim, S. Antibacterial mode of action of Cinnamomum verum bark essential oil, Alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli Strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Charles, D.J.; Simon, J.E. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J. Am. Soc. Hortic. Sci. 1990, 115, 458–462. [Google Scholar]
- Militello, M.; Settanni, L.; Aleo, A.; Mammina, C.; Moschetti, G.; Giammanco, G.; Blàzquez, M.A.; Carrubba, A. Chemical composition and antibacterial potential of Artemisia arborescens L. essential oil. Curr. Microbiol. 2011, 62, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Lapenda, J.; Silva, P.; Vicalvi, M.; Sena, K.; Nascimento, S. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2015, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Tawaha, K.A.; Hudaib, M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Gerits, E.; Blommaert, E.; Lippell, A.; O’Neill, A.J.; Weytjens, B.; De Maeyer, D.; Fierro, A.C.; Marchal, K.; Marchand, A.; Chaltin, P. Elucidation of the mode of action of a new antibacterial compound active against Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE 2016, 11, e0155139. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| No. | Citrus aurantifolia | Citrus aurantium | Fortunella sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RI | Constituent | Percent | RI | Constituent | Percent | RI | Constituent | Percent | |
| 1 | 935 | α-pinene | 0.3 | 973 | sabinene | 2.1 | 952 | camphene | 0.2 |
| 2 | 978 | β-pinene | 3.4 | 978 | β-pinene | 1.7 | 1100 | linalool | 0.6 |
| 3 | 1028 | limonene | 47.2 | 992 | β-myrcene | 2.3 | 1168 | borneol | 0.9 |
| 4 | 1039 | cis-β-ocimene | 0.5 | 1028 | limonene | 2.3 | 1188 | α-terpineol | 0.4 |
| 5 | 1100 | linalool | 6.7 | 1042 | cis-β-ocimene | 2.3 | 1259 | trans-geraniol | 0.1 |
| 6 | 1133 | cis-limonene oxide | 0.2 | 1086 | α-terpinolene | 0.2 | 1335 | δ-elemene | 9.4 |
| 7 | 1142 | trans-limonene oxide | 0.1 | 1100 | linalool | 25.9 | 1351 | α-cubebene | 0.6 |
| 8 | 1154 | citronellal | 4.9 | 1188 | α-terpineol | 9.6 | 1377 | α-copaene | 1 |
| 9 | 1201 | (4Z)-decanal | 0.1 | 1250 | geraniol | 1.7 | 1392 | β-elemene | 5 |
| 10 | 1227 | nerol | 0.1 | 1257 | linalyl acetate | 43.7 | 1417 | trans-caryophyllene | 7 |
| 11 | 1250 | geraniol | 9.8 | 1367 | neryl acetate | 0.8 | 1440 | β-gurjunene | 6 |
| 12 | 1341 | citral | 5.2 | 1386 | geranyl acetate | 2.5 | 1445 | aromadendrene | 0.3 |
| 13 | 1357 | citronellyl acetate | 0.3 | 1417 | trans-caryophyllene | 0.1 | 1448 | α-humulene | 2.5 |
| 14 | 1367 | neryl acetate | 2.1 | 1448 | α-humulene | 0.2 | 1481 | germacrene d | 17.4 |
| 15 | 1386 | geranyl acetate | 9.3 | 1508 | α-farnesene | 0.1 | 1487 | β-selinene | 1.6 |
| 16 | 1417 | trans-caryophyllene | 3.9 | 1725 | farnesol | 0.1 | 1492 | δ-selinene | 0.5 |
| 17 | 1419 | cis-α-bergamotene | 0.3 | 1490 | valencene | 7.3 | |||
| 18 | 1448 | α-humulene | 0.4 | 1507 | (E)-α-farnesene | 0.4 | |||
| 19 | 1458 | (E)-β-farnesene | 0.7 | 1509 | ϒ-cadinene | 2 | |||
| 20 | 1582 | caryophyllene oxide | 0.8 | 1519 | δ-cadinene | 1.9 | |||
| 21 | 1677 | (Z)-nerolidyl acetate | 0.1 | 1549 | elemol | 7.9 | |||
| 22 | 1725 | (2E,6Z)-farnesol | 0.1 | 1553 | germacrene B | 8.5 | |||
| 23 | 1563 | (E)-nerolidol | 1.5 | ||||||
| 24 | 1581 | spathulenol | 1.2 | ||||||
| 25 | 1590 | globulol | 2.1 | ||||||
| 26 | 1624 | 10-epi-γ-eudesmol | 1.3 | ||||||
| 27 | 1649 | β-eudesmol | 2.5 | ||||||
| 28 | 1693 | α-eudesmol | 6.7 | ||||||
| total | 96.5 | 95.6 | 96.8 | ||||||
| Essential Oil/Compound | MIC mg/mL | MBC mg/mL |
|---|---|---|
| Citrus aurantifolia | 0.5 b | 0.9 b |
| Citrus aurantium | 1.3 g | 2 g |
| α-terpineol | 0.625 c | 1.1 c |
| citral | 0.375 a | 0.725 a |
| citronellal | 1 f | 1.41 f |
| geraniol | 0.9 e | 1.325 e |
| linalool | 0.85 d | 1.225 d |
| linalyl acetate | 8.5 h | 14.5 h |
| Compound | FIC Index | Activity |
|---|---|---|
| α-terpineol-citral | 0.44 | Synergistic |
| α-terpineol-citronellal | 1 | Indifferent |
| α-terpineol-geraniol | 0.625 | Additive |
| α-terpineol-linalool | 1 | Indifferent |
| citral-citronellal | 0.5 | Synergistic |
| citral-geraniol | 0.313 | Synergistic |
| citral-linalool | 0.625 | Additive |
| citronellal-geraniol | 0.625 | Additive |
| citronellal-linalool | 0.5 | Synergistic |
| geraniol-linalool | 0.625 | Additive |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzaei-Najafgholi, H.; Tarighi, S.; Golmohammadi, M.; Taheri, P. The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri. Molecules 2017, 22, 591. https://doi.org/10.3390/molecules22040591
Mirzaei-Najafgholi H, Tarighi S, Golmohammadi M, Taheri P. The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri. Molecules. 2017; 22(4):591. https://doi.org/10.3390/molecules22040591
Chicago/Turabian StyleMirzaei-Najafgholi, Hossein, Saeed Tarighi, Morteza Golmohammadi, and Parissa Taheri. 2017. "The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri" Molecules 22, no. 4: 591. https://doi.org/10.3390/molecules22040591
APA StyleMirzaei-Najafgholi, H., Tarighi, S., Golmohammadi, M., & Taheri, P. (2017). The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri. Molecules, 22(4), 591. https://doi.org/10.3390/molecules22040591