Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene—An In Vitro Study
Abstract
:1. Introduction
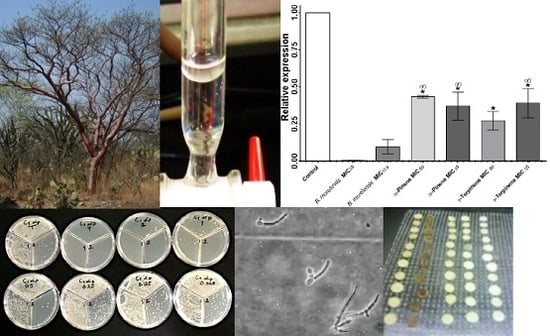
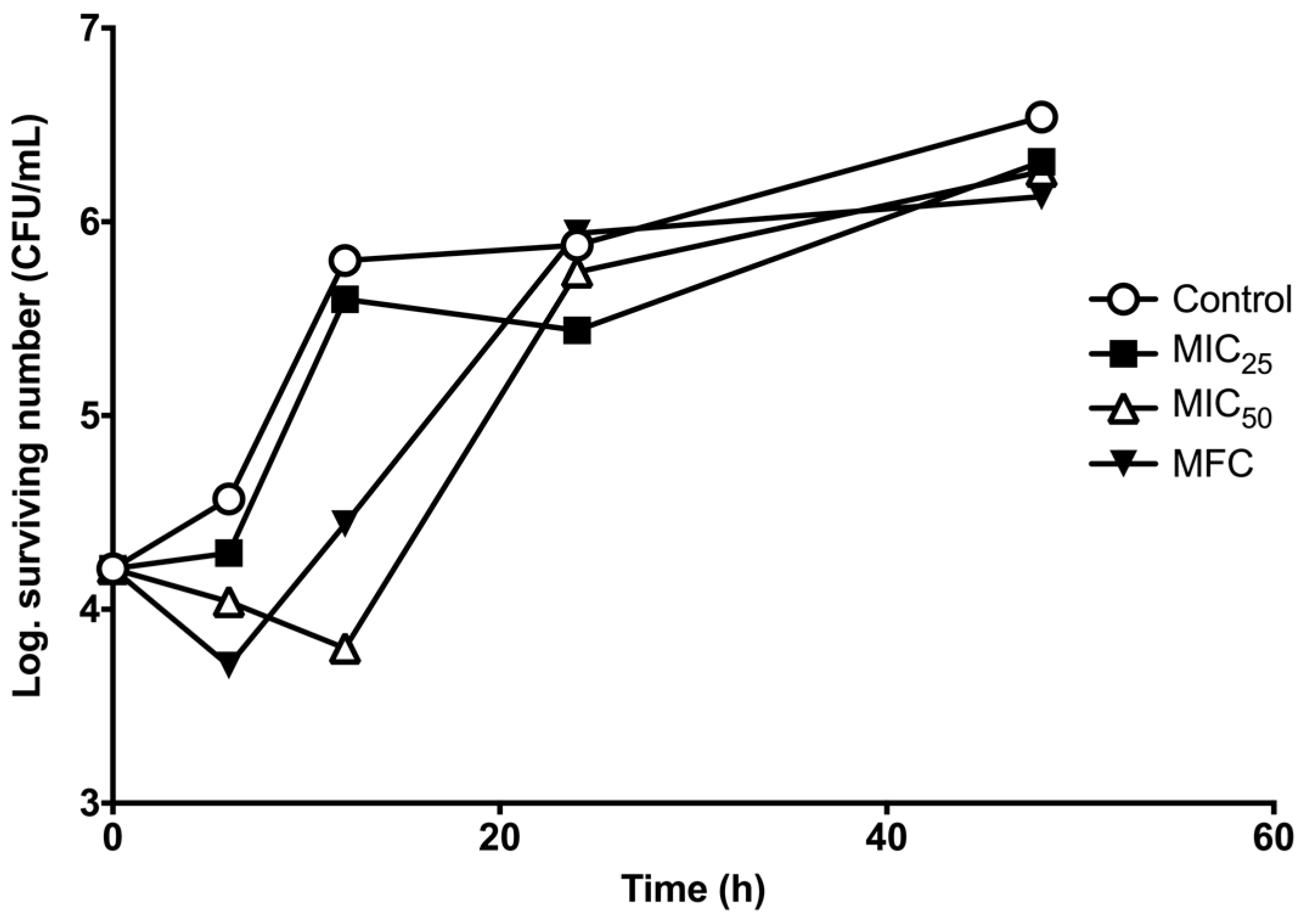
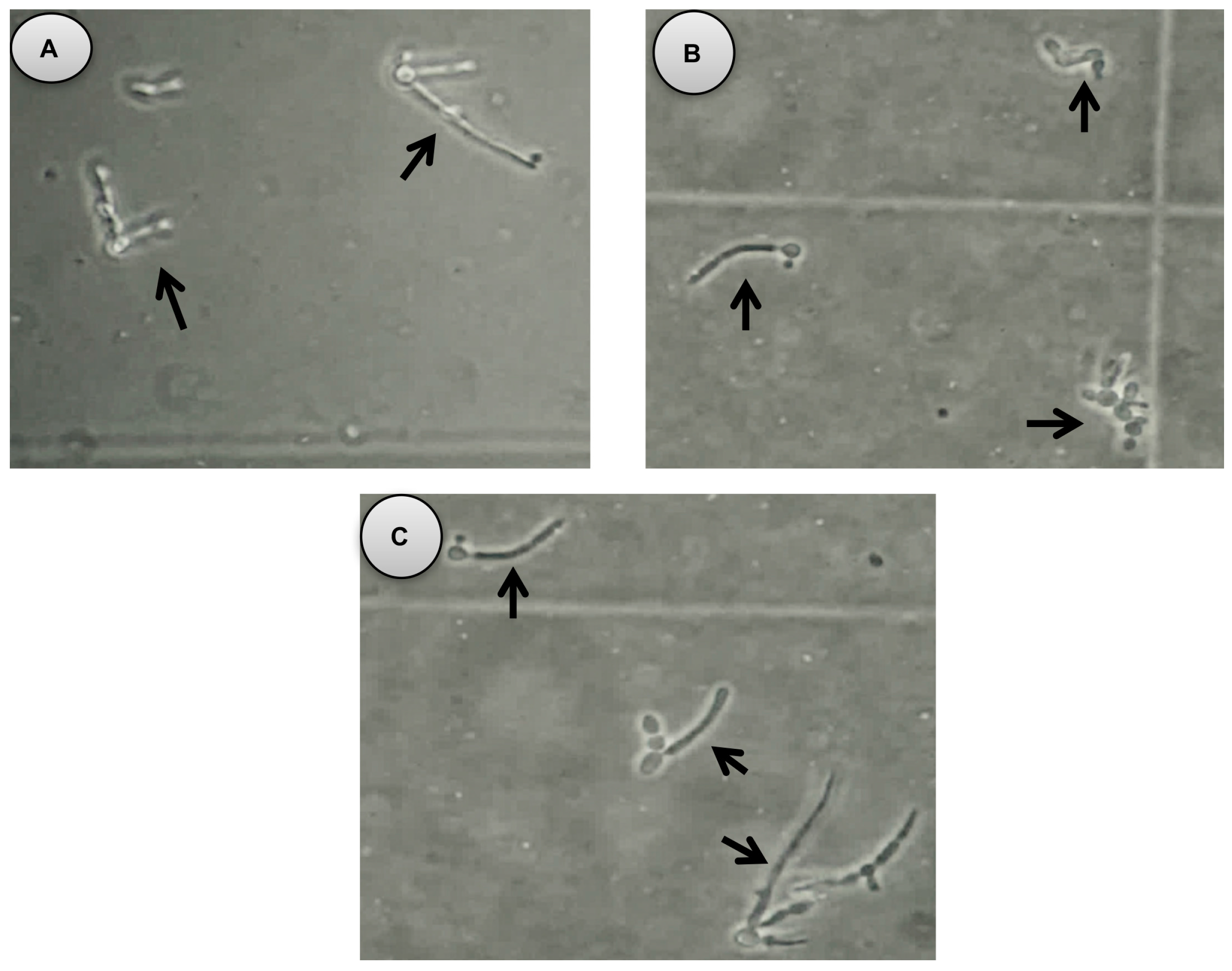
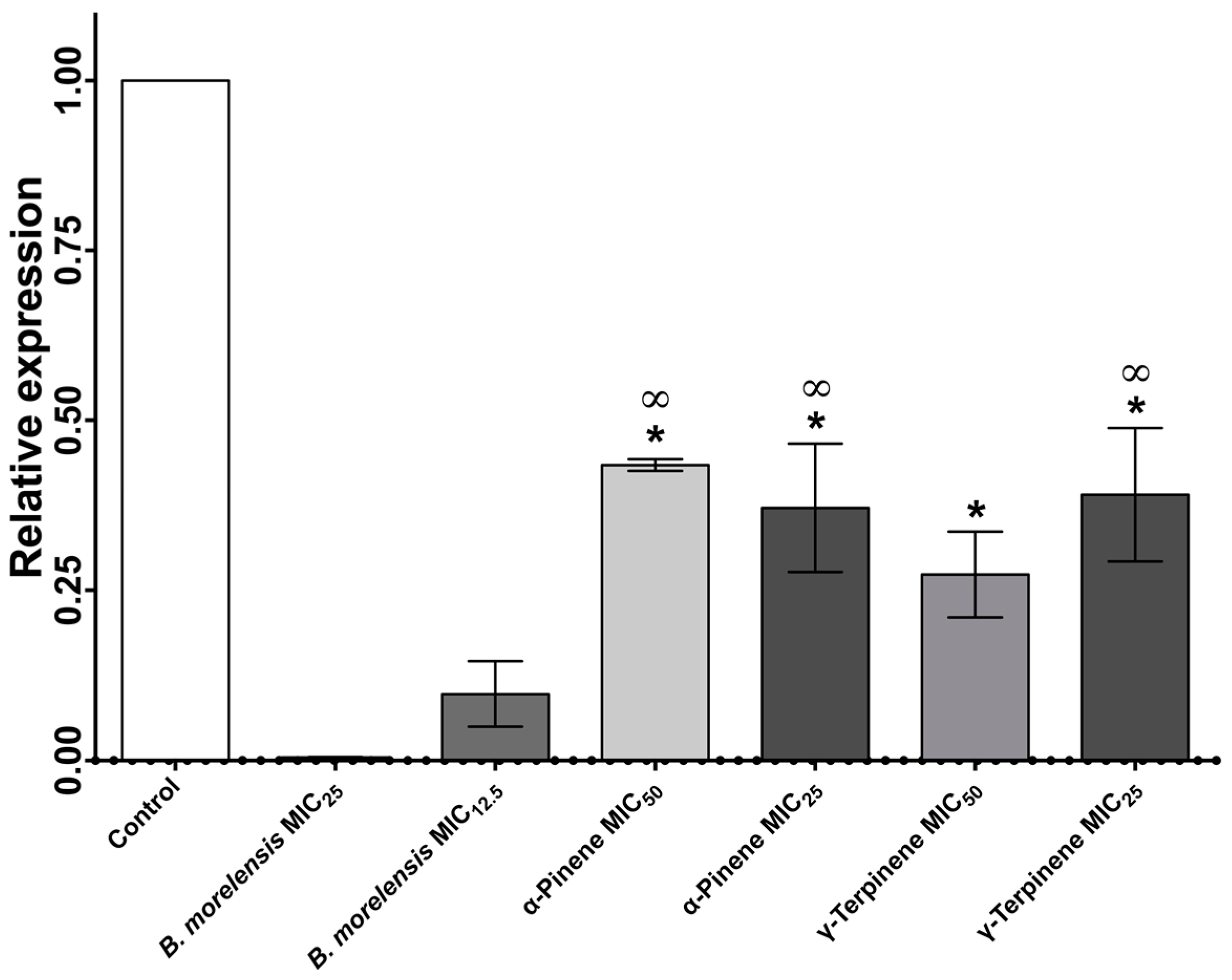
2. Results
Anti-Candida Assay
3. Discussion
4. Material and Methods
4.1. Collection and Identification of B. morelensis
4.2. Essential Oil Extraction
4.3. Essential Oil Chemical Composition
4.4. Fungal Strains
4.5. Anti-Candida Assays
4.6. Survival Curve Assay
4.7. Germ Tube Formation Assay
4.8. Cell Wall Integrity
4.9. RNA Extraction and cDNA Synthesis
4.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ascioglu, S.; Rex, J.H.; de Pauw, B.; Bennett, J.E.; Bille, J.; Crokaert, F.; Denning, D.W.; Donnelly, J.P.; Edwards, J.E.; Erjavec, Z.; et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clin. Infect. Dis. 2002, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kibbler, C. Defining invasive fungal infections in neutropenic or stem cell transplant patients. J. Antimicrob. Chemother. 2005, 56 (Suppl. 1), i12–i16. [Google Scholar] [CrossRef] [PubMed]
- Haghdoost, N.; Salehi, T.; Khosravi, A.; Sharifzadeh, A. Antifungal activity and influence of propolis against germ tube formation as a critical virulence attribute by clinical isolates of Candida albicans. J. Mycol. Méd. 2016, 26, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, J.L.; Kaufman, P.D. Chromatin-mediated Candida albicans virulence. Biochim. Biophys. Acta 2012, 1819, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zorić, N.; Kopjar, N.; Bobnjarić, I.; Horvat, I.; Tomić, S.; Kosalec, I. Antifungal Activity of Oleuropein against Candida albicans—The In Vitro Study. Molecules 2016, 21, 1631. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.A.; Bendel, C.M.; McClellan, M.; Hauser, M.; Becker, J.M.; Berman, J.; Hostetter, M.K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 1998, 279, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Kinneberg, K.M.; Bendel, C.M.; Jechorek, R.P.; Cebelinski, E.A.; Gale, C.A.; Berman, J.G.; Erlandsen, S.L.; Hostetter, M.K.; Wells, C.L. Effect of INT1 gene on Candida albicans murine intestinal colonization. J. Surg. Res. 1999, 87, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Verki, N.; Shapoorzadeh, A.; Razzaghi-Abyaneh, M.; Atyabi, S.-M.; Shams-Ghahfarokhi, M.; Jahanshiri, Z.; Gholami-Shabani, M. Cold atmospheric plasma inhibits the growth of Candida albicans by affecting ergosterol biosynthesis and suppresses the fungal virulence factors in vitro. Photodiagnosis Photodyn. Ther. 2016, 13, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Mir, A.P.B.; Barghi, V.G.; Shams-Ghahfarokhi, M. Eradication of C. albicans and T. rubrum with photoactivated indocyanine green, Citrus aurantifolia essential oil and fluconazole. Photodiagnosis Photodyn. Ther. 2015, 12, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumpetis, T.; Manolakaki, D.; Velmahos, G.; Chang, Y.; Alam, H.B.; De Moya, M.M.; Sailhamer, E.A.; Mylonakis, E. Candida infection and colonization among non-trauma emergency surgery patients. Virulence 2010, 1, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Alina, C.-M.C.; Rocío, R.-L.; Aurelio, R.-M.M.; Margarita, C.-M.M.; Angélica, R.-G.; Rubén, J.-A. Chemical Composition and In vivo Anti-inflammatory Activity of Bursera morelensis Ramírez Essential Oil. J. Essent. Oil Bear. Plants 2014, 17, 758–768. [Google Scholar] [CrossRef]
- Canales-Martinez, M.; Rivera-Yañez, C.; Salas-Oropeza, J.; Lopez, H.; Jimenez-Estrada, M.; Rosas-Lopez, R.; Duran, D.; Flores, C.; Hernandez, L.; Rodriguez-Monroy, M. Antimicrobial activity of Bursera morelensis ramírez essential oil. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 74–82. [Google Scholar]
- Khanna, A.; Rizvi, F.; Chander, R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J. Ethnopharmacol. 2002, 82, 19–22. [Google Scholar] [CrossRef]
- López-Hernández, L.R. Propiedades Medicinales y Determinación de los Compuestos del aceite Esencial de Bursera morelensis Ramirez. Bachelor’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2011. Available online: http://132.248.9.195/ptd2013/Presenciales/0704321/Index.html (accessed on 20 November 2017).
- Tscherner, M.; Schwarzmüller, T.; Kuchler, K. Pathogenesis and antifungal drug resistance of the human fungal pathogen Candida glabrata. Pharmaceuticals 2011, 4, 169–186. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Mann, C.; Markham, J.; Bell, H.; Gustafson, J.; Warmington, J.; Wyllie, S. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.O.; de Araújo G. Oliveira, R.; de O. Lima, E.; de Souza, E.L.; Farias, N.P.; de Fátima Navarro, D. Inhibitory effect of some phytochemicals in the growth of yeasts potentially causing opportunistic infections. Revista Brasileira Ciências Farmacêuticas 2005, 41, 199–203. [Google Scholar] [CrossRef]
- Tangarife-Castaño, V.; Correa-Royero, J.; Zapata-Londoño, B.; Durán, C.; Stanshenko, E.; Mesa-Arango, A.C. Anti-Candida albicans activity, cytotoxicity and interaction with antifungal drugs of essential oils and extracts from aromatic and medicinal plants. Infectio 2011, 15, 160–167. [Google Scholar] [CrossRef]
- Carson, C.; Hammer, K.; Riley, T. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.; Riley, T. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Tsuruoka, T.; Watanabe, M.; Takeo, K.; Akao, M.; Nishiyama, Y.; Yamaguchi, H. Inhibitory effect of essential oils on apical growth of Aspergillus fumigatus by vapour contact Hemmung des apikalen Wachstums von Aspergillus fumigatus durch Dämpfe ätherischer Ole. Mycoses 2000, 43, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Hasan, M.; Takahashi, J.; Murata, Y.; Kitagawa, E.; Kodama, O.; Iwahashi, H. Response of Saccharomyces cerevisiae to a monoterpene: Evaluation of antifungal potential by DNA microarray analysis. J. Antimicrob. Chemother. 2004, 54, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Cantón, E.; Pemán, J. Curvas de letalidad en antifúngicos. Rev. Iberoam. Micol. 1999, 16, 82–85. [Google Scholar] [PubMed]
- Mai, A.; Rotili, D.; Massa, S.; Brosch, G.; Simonetti, G.; Passariello, C.; Palamara, A.T. Discovery of uracil-based histone deacetylase inhibitors able to reduce acquired antifungal resistance and trailing growth in Candida albicans. Bioorg. Med. Chem. Lett. 2007, 17, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.M.; Zuluaga, A. Nuevos antimicóticos y su uso en dermatología. Med. Cutan. Iber. Lat. Am. 2004, 32, 231–242. [Google Scholar]
- Cambi, A.; Netea, M.G.; Mora-Montes, H.M.; Gow, N.A.; Hato, S.V.; Lowman, D.W.; Kullberg, B.-J.; Torensma, R.; Williams, D.L.; Figdor, C.G. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J. Biol. Chem. 2008, 283, 20590–20599. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-C.; Joosten, L.A.; Kullberg, B.-J.; Netea, M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012, 80, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; de Mello, J.C.P.; Cortez, D.A.G.; Dias Filho, B.P.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Yañez, C. Actividad Anti-Candida de Cuatro Plantas Medicinales. Master’s thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2013. [Google Scholar]
- Kumar, R.; Shukla, P. Amphotericin B resistance leads to enhanced proteinase and phospholipase activity and reduced germ tube formation in Candida albicans. Fungal Biol. 2010, 114, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liesche, J.; Marek, M.; Günther-Pomorski, T. Cell wall staining with Trypan blue enables quantitative analysis of morphological changes in yeast cells. Front. Microbiol. 2015, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, M.K. Integrin-like proteins in Candida spp. and other microorganisms. Fungal Genet. Biol. 1999, 28, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.; Gerami-Nejad, M.; McClellan, M.; Vandoninck, S.; Longtine, M.S.; Berman, J. Candida albicans Int1p interacts with the septin ring in yeast and hyphal cells. Mol. Biol. Cell 2001, 12, 3538–3549. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011, 19, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canales, M.; Hernandez, T.; Caballero, J.; Romo de Vivar, A.; Avila, G.; Duran, A.; Lira, R. Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlan, Puebla, Mexico. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Fernández, B.M.N. Análisis de la dinámica de comunidades vegetales con relación a la evolución del paisaje, en la zona semiárida de Coxcatlán, Puebla; UNAM: DF Mexico, Mexico, 1999; Available online: http://132.248.9.195/pd1999/274775/Index.html (accessed on 20 November 2017).
- Vanden, B.; Vlietinck, A. Screening methods for antibacterial and antiviral agents from higher plants. In Methods in Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; London Academic Press: London, UK, 1991. [Google Scholar]
- Lim, C.S.Y.; Wong, W.F.; Rosli, R.; Ng, K.P.; Seow, H.F.; Chong, P.P. 2-dodecanol (decyl methyl carbinol) inhibits hyphal formation and SIR2 expression in C. albicans. J. Basic Microbiol. 2009, 49, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
Sample Availability: Samples of the essential oil and compounds are available from the authors. |


| Components | Retention Time | Abundance (%) |
|---|---|---|
| Thujane | 4.924 | 0.15 |
| α-Pinene | 5.052 | 2.85 |
| α-Phellandrene | 5.453 | 1.58 |
| β-Pinene | 5.541 | 2.30 |
| β-Phellandrene | 5.814 | 18.27 |
| γ-Terpinene | 6.078 | 65.46 |
| Isobutylbenzene | 7.714 | 0.32 |
| Cyclohexane | 8.339 | 0.44 |
| 4-butan-2-yl-2,3-dihydrofuran | 8.531 | 0.39 |
| 2-Acetylcyclopentanone | 8.651 | 0.46 |
| Caryophyllene | 9.357 | 5.13 |
| α-Caryophyllene | 9.597 | 0.27 |
| Caryophyllene oxide | 10.479 | 0.34 |
| Strains | B. morelensis | α-Pinene | γ-Terpinene | Positive Control (Nystatin) |
|---|---|---|---|---|
| C. albicans 1 | 10.0 ± 1.00 | 9.7 ± 0.58 | 7.0 ± 0.00 | 18.0 ± 1.00 |
| C. albicans 14065 | 11.8 ± 0.76 | 11.3 ± 0.58 | 6.33 ± 0.58 | 19.67 ± 0.50 |
| C. albicans 32354 | 11.3 ± 0.58 | 11.3 ± 1.15 | 7.0 ± 0.00 | 22.0 ± 2.00 |
| C. albicans CDBB-L-1003 | 11.2 ± 0.29 | 8.3 ± 0.58 | 6.0 ± 0.00 | 22.0 ± 1.00 |
| Strains | B. morelensis | α-Pinene | γ-Terpinene | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFC | MIC75 | MIC50 | MIC25 | MFC | MIC75 | MIC50 | MIC25 | MFC | MIC75 | MIC50 | MIC25 | |
| C. albicans 1 | 2.0 | 0.25 | 0.125 | 0.062 | 4.0 | 1.0 | 0.5 | 0.125 | 16.0 | 12.0 | 6.0 | 4.0 |
| C. albicans 14065 | 2.0 | 1.0 | 0.5 | 0.125 | 4.0 | 2.0 | 0.5 | 0.125 | 16.0 | 14.0 | 10.0 | 8.0 |
| C. albicans 32354 | 2.0 | 0.5 | 0.125 | 0.062 | 4.0 | 1.0 | 0.5 | 0.065 | 16.0 | 10.0 | 6.0 | 2.0 |
| C. albicans CDBB-L-1003 | 2.0 | 0.25 | 0.125 | 0.062 | 2.0 | 1.0 | 0.5 | 0.25 | 12.0 | 10.0 | 8.0 | 6.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Yañez, C.R.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Fariña, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene—An In Vitro Study. Molecules 2017, 22, 2095. https://doi.org/10.3390/molecules22122095
Rivera-Yañez CR, Terrazas LI, Jimenez-Estrada M, Campos JE, Flores-Ortiz CM, Hernandez LB, Cruz-Sanchez T, Garrido-Fariña GI, Rodriguez-Monroy MA, Canales-Martinez MM. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene—An In Vitro Study. Molecules. 2017; 22(12):2095. https://doi.org/10.3390/molecules22122095
Chicago/Turabian StyleRivera-Yañez, C. Rebeca, L. Ignacio Terrazas, Manuel Jimenez-Estrada, Jorge E. Campos, Cesar M. Flores-Ortiz, Luis B. Hernandez, Tonatiuh Cruz-Sanchez, German I. Garrido-Fariña, Marco A. Rodriguez-Monroy, and M. Margarita Canales-Martinez. 2017. "Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene—An In Vitro Study" Molecules 22, no. 12: 2095. https://doi.org/10.3390/molecules22122095
APA StyleRivera-Yañez, C. R., Terrazas, L. I., Jimenez-Estrada, M., Campos, J. E., Flores-Ortiz, C. M., Hernandez, L. B., Cruz-Sanchez, T., Garrido-Fariña, G. I., Rodriguez-Monroy, M. A., & Canales-Martinez, M. M. (2017). Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene—An In Vitro Study. Molecules, 22(12), 2095. https://doi.org/10.3390/molecules22122095