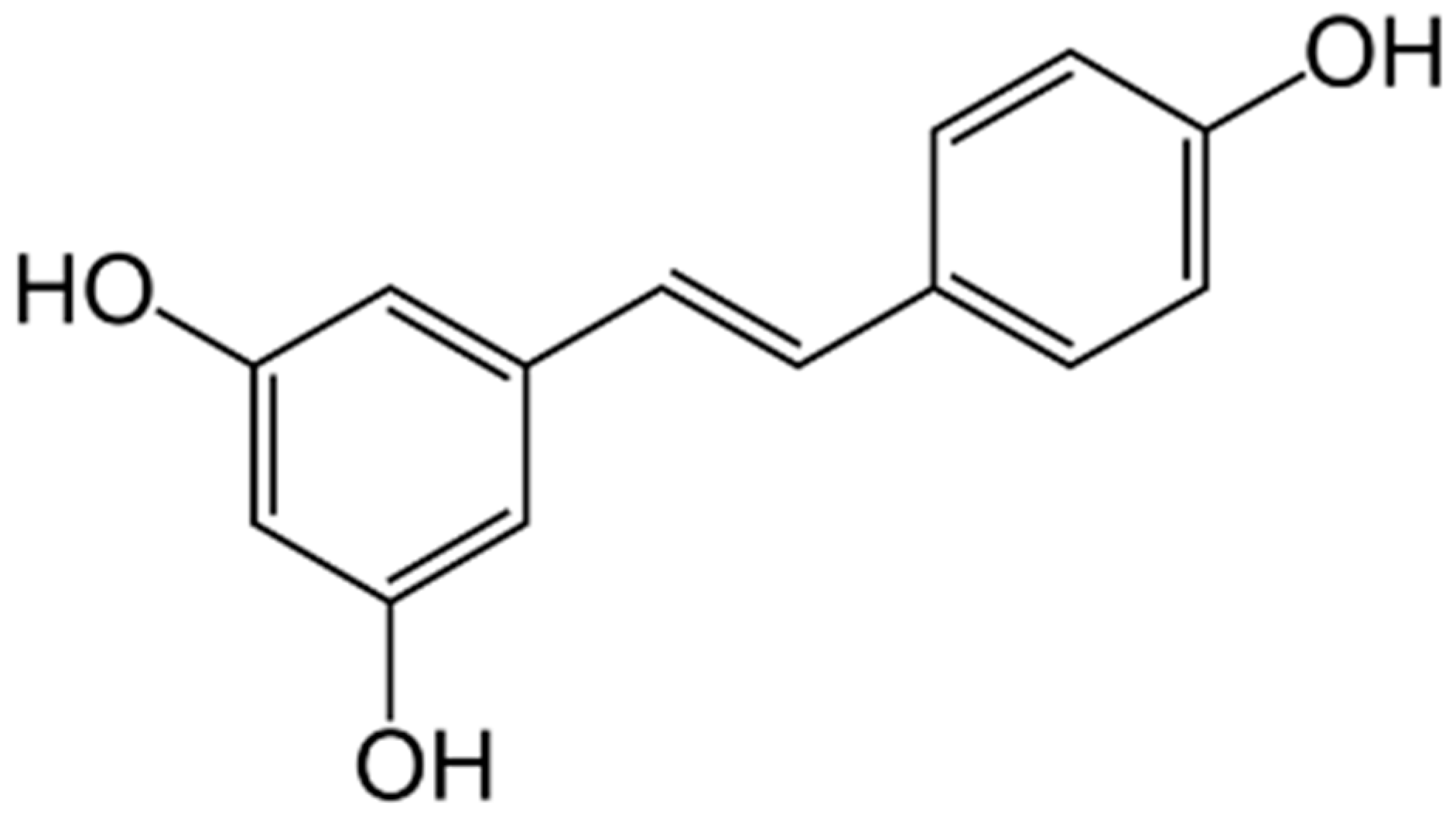
Resveratrol-Induced Effects on Body Fat Differ Depending on Feeding Conditions
Abstract
1. Introduction
2. RSV Effects in Body Weight and Adipose Tissue Weight under Overfeeding Conditions in Preclinical Studies
3. RSV Effects in Body Weight and Adipose Tissue Weight under Normal Feeding Conditions in Preclinical Studies
4. RSV Effects in Body Weight and Adipose Tissue Weight under Energy Restriction Conditions in Preclinical Studies
5. RSV Effects in Body Weight and Body Fat Weight in Clinical Studies
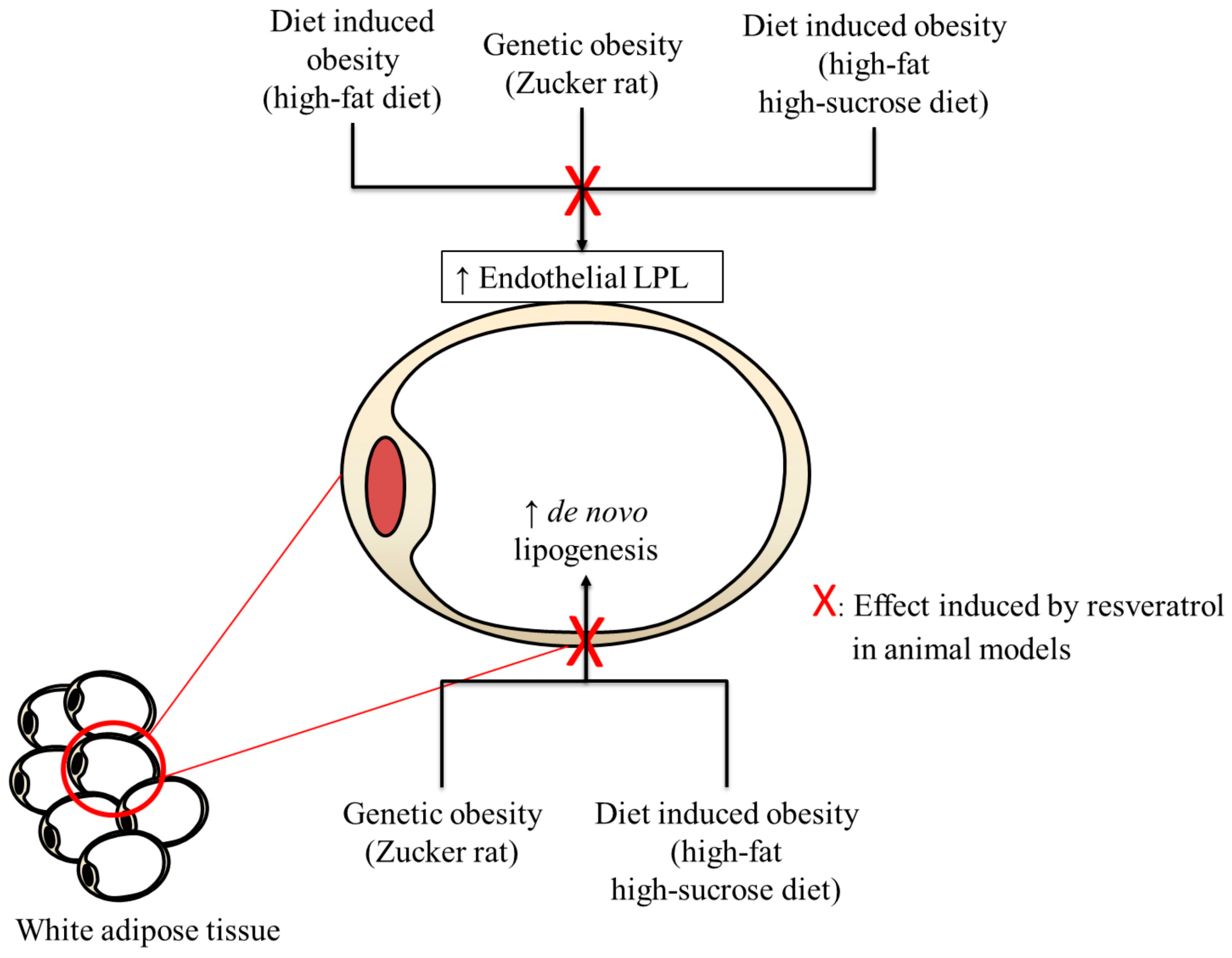
6. Metabolic Background
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barness, L.A. Obesity in children. Fetal Pediatr. Pathol. 2007, 26, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 25 November 2017).
- Chung, K.W.; Kim, D.H.; Park, M.H.; Choi, Y.J.; Kim, N.D.; Lee, J.; Yu, B.P.; Chung, H.Y. Recent advances in calorie restriction research on aging. Exp. Gerontol. 2013, 48, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Arias, E.B.; Sequea, D.A.; Cartee, G.D. Preventing the calorie restriction-induced increase in insulin-stimulated Akt2 phosphorylation eliminates calorie restriction’s effect on glucose uptake in skeletal muscle. Biochim. Biophys. Acta 2012, 1822, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P. Caloric restriction: Is mammalian life extension linked to p53? Aging 2012, 4, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. Caloric restriction, sirt1 and longevity. Trends Endocrinol. Metab. 2009, 20, 325–331. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size, 1935. Nutrition 1989, 5, 155–171. [Google Scholar] [PubMed]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vasc. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech. Ageing Dev. 2010, 131, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Carboneau, B.A.; Krzysik-Walker, S.M.; de Cabo, R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012, 11, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie 2017, 134, 62–70. [Google Scholar] [CrossRef] [PubMed]
- West, D.B.; Boozer, C.N.; Moody, D.L.; Atkinson, R.L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. 1992, 262, R1025–R1032. [Google Scholar] [PubMed]
- Eckel, R.H. Obesity: Mechanisms and Clinical Management; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Nascimento-Sales, M.; Fredo-da-Costa, I.; Mendes, A.C.B.; Melo, S.; Ravache, T.T.; Gomez, T.G.B.; Gaisler-Silva, F.; Ribeiro, M.O.; Santos, A.R.; Carneiro-Ramos, M.S.; et al. Is the FVB/N mouse strain truly resistant to diet-induced obesity? Physiol. Rep. 2017, 5, e13271. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, E.; Luostarinen, M.; Martonen, E.; Finckenberg, P.; Kovalainen, M.; Huotari, A.; Herzig, K.H.; Lecklin, A.; Mervaala, E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and fatty liver formation. J. Nutr. Metab. 2011, 2011, 525094. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Jung, U.J.; Choi, M.S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hong, H.J.; Guan, J.; Kim, D.G.; Yang, E.J.; Koh, G.; Park, D.; Han, C.H.; Lee, Y.J.; Lee, D.H. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: In vitro and in vivo experiments in rodents. Metabolism 2012, 61, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, J.; Li, X.; Zhou, Q.; Bai, J.; Shi, Y.; Le, G. Resveratrol prevents suppression of regulatory t-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr. Res. 2013, 33, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Gomez-Zorita, S.; Gupta, R.; Grès, S.; Rancoule, C.; Cadoudal, T.; Mercader, J.; Gomez, A.; Bertrand, C.; Iffiu-Soltész, Z. Combination of low dose of the anti-adipogenic agents resveratrol and phenelzine in drinking water is not sufficient to prevent obesity in very-high-fat diet-fed mice. Eur. J. Nutr. 2014, 53, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; de la Fuente, S.; Fonteriz, R.I.; Moreno, A.; Alvarez, J. Effects of long-term feeding of the polyphenols resveratrol and kaempferol in obese mice. PLoS ONE 2014, 9, e112825. [Google Scholar] [CrossRef] [PubMed]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; González, A.; et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Chen, L.L.; Xiao, F.X.; Sun, H.; Ding, H.C.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating amp-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Larsen, J.; Hamilton-Dutoit, S.; Clasen, B.F.; Jessen, N.; Paulsen, S.K.; Kjær, T.N.; Richelsen, B.; Pedersen, S.B. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr. Res. 2012, 32, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.L.; de Pinho, L.; Andrade, J.M.; Paraíso, A.F.; Lula, J.F.; Macedo, S.M.; Feltenberger, J.D.; Guimarães, A.L.; de Paula, A.M.; Santos, S.H. Distinct metabolic effects of resveratrol on lipogenesis markers in mice adipose tissue treated with high-polyunsaturated fat and high-protein diets. Life Sci. 2016, 153, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Lee, S.A.; Choi, M.S. Antiobesity and vasoprotective effects of resveratrol in apoE-deficient mice. J. Med. Food 2014, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Macarulla, M.T.; Alberdi, G.; Gomez, S.; Tueros, I.; Bald, C.; Rodriguez, V.M.; Matinez, J.A.; Portillo, M.P. Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J. Physiol. Biochem. 2009, 65, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Martínez-Castaño, M.G.; Gómez-Zorita, S.; Miranda, J.; Martínez, J.A.; Portillo, M.P. The combination of resveratrol and conjugated linoleic acid is not useful in preventing obesity. J. Physiol. Biochem. 2011, 67, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Miranda, J.; Macarulla, M.T.; Aguirre, L.; Fernández-Quintela, A.; Andres-Lacueva, C.; Urpi-Sarda, M.; Portillo, M.P. The combination of resveratrol and conjugated linoleic acid attenuates the individual effects of these molecules on triacylglycerol metabolism in adipose tissue. Eur. J. Nutr. 2014, 53, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Milton-Laskibar, I.; Aguirre, L.; Macarulla, M.T.; Etxeberria, U.; Milagro, F.I.; Martínez, J.A.; Contreras, J.; Portillo, M.P. Comparative effects of energy restriction and resveratrol intake on glycemic control improvement. Biofactors 2017, 43, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Lasa, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition 2013, 29, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M. The obese zucker rat: A choice for fat metabolism 1968–1988: Twenty years of research on the insights of the zucker mutation. Prog. Lipid Res. 1989, 28, 53–66. [Google Scholar] [CrossRef]
- Aleixandre, A.; Miguel, M. Zucker rats as an experimental model for the study of various diseases. Endocrinol. Nutr. Organo Soc. Esp. Endocrinol. Nutr. 2008, 55, 217–222. [Google Scholar]
- Joseph, A.M.; Malamo, A.G.; Silvestre, J.; Wawrzyniak, N.; Carey-Love, S.; Nguyen, L.M.; Dutta, D.; Xu, J.; Leeuwenburgh, C.; Adhihetty, P.J. Short-term caloric restriction, resveratrol, or combined treatment regimens initiated in late-life alter mitochondrial protein expression profiles in a fiber-type specific manner in aged animals. Exp. Gerontol. 2013, 48, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Macarulla, M.T.; Portillo, M.P.; Rodriguez, V.M. Resveratrol does not increase body fat loss induced by energy restriction. J. Physiol. Biochem. 2014, 70, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Fernandez-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Carpéné, C.; Fernández, M.; Aguirre, L.; Milton-Laskibar, I.; Contreras, J.; Portillo, M.P. Anti-obesity effects of resveratrol: Comparison between animal models and humans. J. Physiol. Biochem. 2016, 73, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.; Whitby, S.J.; Mellor, D.; Thomas, J.; McKune, A.; Roach, P.D.; Naumovski, N. The effects of resveratrol supplementation in overweight and obese humans: A systematic review of randomized trials. Metab. Syndr. Relat. Disord. 2016, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.; Schechtman, K.B.; Gu, C.; Kunz, I.; Rossi Fanelli, F.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-Zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore-Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2092–2103. [Google Scholar] [CrossRef] [PubMed]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stødkilde-Jørgensen, H.; Jessen, N.; Jørgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No beneficial effects of resveratrol on the metabolic syndrome: A randomized placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.C.; Cleary, M.P. Metabolic measurements among homozygous (fa/fa) obese, heterozygous (fa/fa) lean and homozygous (fa/fa) lean zucker rat pups at 17 days of age. J. Nutr. 1994, 124, 1230–1237. [Google Scholar] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicque, L.; Bindels, L.B.; Pachikian, B.D.; Sohet, F.M.; et al. Inulin-type fructans with prebiotic properties counteract gpr43 overexpression and pparγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Miyahara, H.; Takeo, J.; Katayama, M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol. Metab. Syndr. 2012, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Rodriguez, V.M.; Miranda, J.; Macarulla, M.T.; Arias, N.; Andres-Lacueva, C.; Portillo, M.P. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr. Metab. 2011, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Schweiger, M.; Kotzbeck, P.; Churruca, I.; Simon, E.; Zechner, R.; Portillo, M.P. Resveratrol regulates lipolysis via adipose triglyceride lipase. J. Nutr. Biochem. 2012, 23, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.S. Physiological mechanisms in the adaptive response of metabolic rates to energy restriction. Nutr. Res. Rev. 1990, 3, 49–74. [Google Scholar] [CrossRef] [PubMed]
| Author | Animal Model | Diet | Increase in Dietary Fat (vs. Control) | Lower Body Weight | Lower Fat Weight |
|---|---|---|---|---|---|
| Lagouge et al., 2006 [29] | C57BL/6J mice | High-fat diet (40% fat) | 31.6% | Yes | Visceral Yes Subcutaneous Yes |
| Baur et al., 2006 [16] | C57BL/6NIA mice | High-fat diet (60% fat) | 40% | No | No |
| Kim et al., 2011 [18] | C57BL/6J mice | High-fat diet (40% fat) | 28% | Yes | Visceral Yes Subcutaneous N/A |
| Kang et al., 2012 [20] | C57BL/6N mice | High-fat diet (58% fat) | 45% | No | N/A |
| Cho et al., 2012 [19] | C57BL/6J mice | High-fat diet (40% fat) | 29% | Yes | Visceral Yes Subcutaneous N/A |
| Wang et al., 2013 [21] | C57BL/6J mice | High-fat diet (44% fat) | 31% | No | N/A |
| Jeon et al., 2014 [30] | Homozygous apoE-deficient mice | Atherogenic diet (20% fat) | No control diet fed group | Yes | Visceral Yes Subcutaneous N/A |
| Qiao et al., 2014 [22] | Kunming mice | High-fat diet (50% fat) | 40% | Yes | Yes Location data N/A |
| Carpéné et al., 2014 [23] | C57BL/6J wild-typemice | Very-high-fat diet (60% fat) | 50% | No | No |
| Montero et al., 2014 [24] | C57BL mice | High-fat diet (60% fat) | 50% | Yes | N/A |
| Shang et al., 2008 [26] | Wistar rats | High-fat diet (59% fat) | STD diet composition N/A | Yes | Visceral Yes Subcutaneous N/A |
| Macarulla et al., 2009 [31] | Sprague-Dawley rats | Obesogenic diet (45% fat) | No control diet fed group | No | Visceral Yes Subcutaneous Yes |
| Arias et al., 2011 [32] | Wistar rats | Obesogenic diet (45% fat) | No control diet fed group | Yes | Visceral Yes Subcutaneous No |
| Poulsen et al., 2012 [27] | Wistar rats | High-fat diet (60% fat) | 50% | No | Visceral No Subcutaneous N/A |
| Arias et al., 2014 [33] | Wistar rats | Obesogenic diet (45% fat) | No control diet fed group | No | Visceral No Subcutaneous No |
| Author | Animal Model | Diet | Lower Body Weight | Lower Fat Weight |
|---|---|---|---|---|
| Lagouge et al., 2006 [29] | C57BL/6J mice | Chow diet (8% lipid, 19% protein, 73% carbohydrate) | No | N/A |
| Mendes et al., 2016 [28] | FVB/N mice | Standard diet (12% lipid, 23% protein, 65% carbohydrate) | No | No |
| Milton-Laskibar et al., 2017 [35] | Wistar rats | Standard diet (16% lipid, 20% protein, 64% carbohydrates) | No | No |
| Author | Animal Model | Diet | Energy Restriction Degree (vs. Control) | Lower Body Weight | Lower Fat Weight |
|---|---|---|---|---|---|
| Joseph et al., 2013 [40] | Fischer 344 × Brown Norway Hybrid rats | Standard maintenance diet (10% lipid, 14% protein, 76% carbohydrate) | 20% | No | No |
| Alberdi et al., 2014 [41] | Wistar rats | Standard diet (16% lipid, 20% protein, 64% carbohydrate) | 25% | No | No |
| Milton-Laskibar et al., 2017 [35] | Wistar rats | Standard diet (16% lipid, 20% protein, 64% carbohydrate) | 15% | No | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milton-Laskibar, I.; Gómez-Zorita, S.; Aguirre, L.; Fernández-Quintela, A.; González, M.; Portillo, M.P. Resveratrol-Induced Effects on Body Fat Differ Depending on Feeding Conditions. Molecules 2017, 22, 2091. https://doi.org/10.3390/molecules22122091
Milton-Laskibar I, Gómez-Zorita S, Aguirre L, Fernández-Quintela A, González M, Portillo MP. Resveratrol-Induced Effects on Body Fat Differ Depending on Feeding Conditions. Molecules. 2017; 22(12):2091. https://doi.org/10.3390/molecules22122091
Chicago/Turabian StyleMilton-Laskibar, Iñaki, Saioa Gómez-Zorita, Leixuri Aguirre, Alfredo Fernández-Quintela, Marcela González, and María P. Portillo. 2017. "Resveratrol-Induced Effects on Body Fat Differ Depending on Feeding Conditions" Molecules 22, no. 12: 2091. https://doi.org/10.3390/molecules22122091
APA StyleMilton-Laskibar, I., Gómez-Zorita, S., Aguirre, L., Fernández-Quintela, A., González, M., & Portillo, M. P. (2017). Resveratrol-Induced Effects on Body Fat Differ Depending on Feeding Conditions. Molecules, 22(12), 2091. https://doi.org/10.3390/molecules22122091









