Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity
Abstract
:1. Introduction
2. Results
2.1. Isolation and Structural Identification of Hydroxyphenylalkanes and Diarylheptanoids from A. melegueta
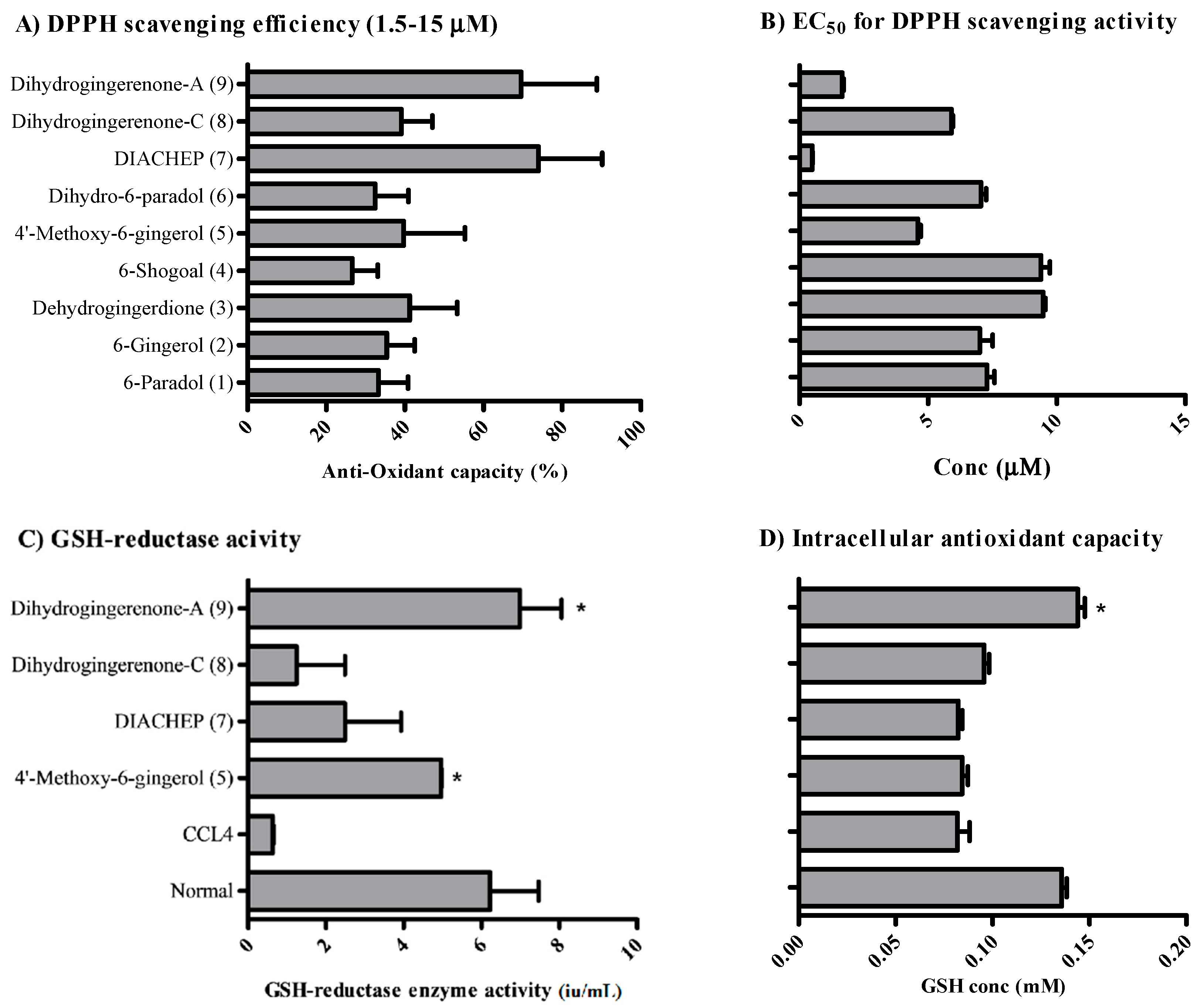
2.2. Anti-Oxidant Activity of Isolated Hydroxyphenylalkanes and Diarylheptanoids
2.3. Cytotoxicity Assessment of Hydroxyphenylalkanes and Diarylheptanoids
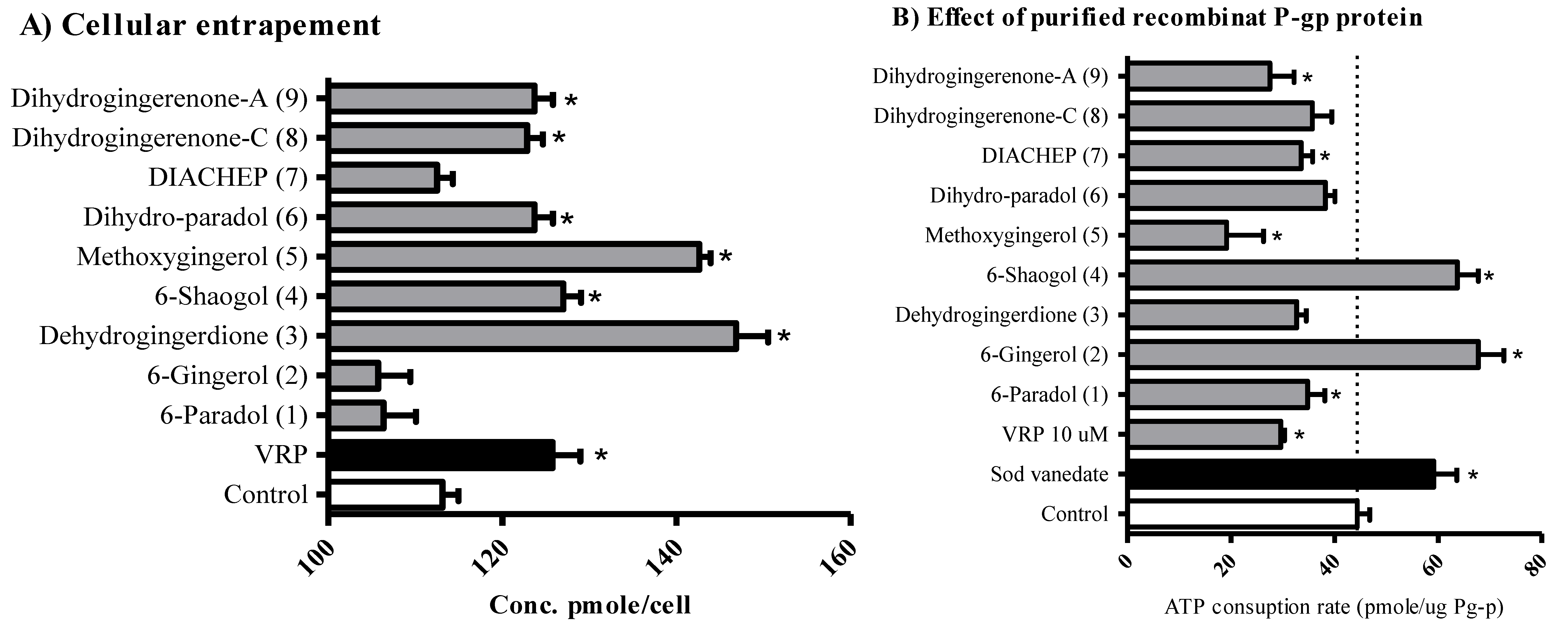
2.4. The Influence of Hydroxyphenylalkanes and Diarylheptanoids on the Cellular Pharmacokinetics
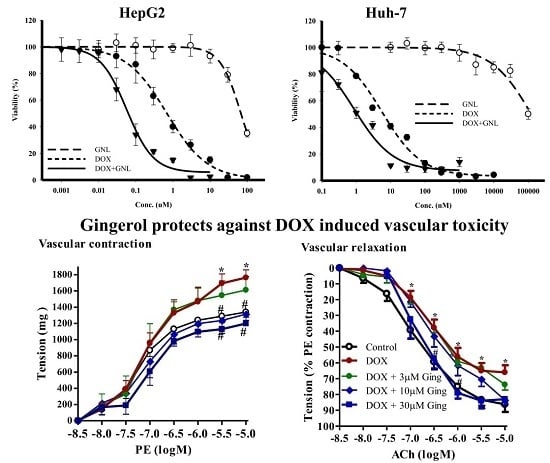
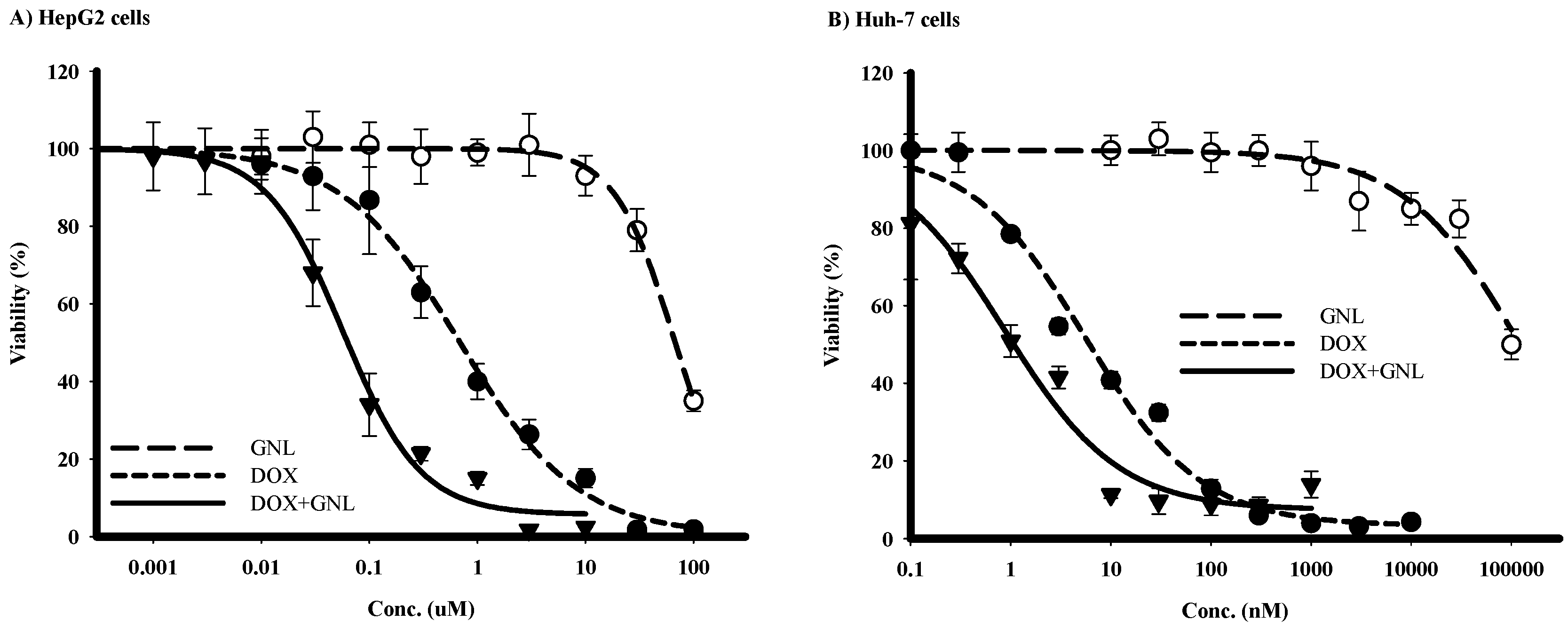
2.5. Chemomodulatory Effect of Gingerol on Doxorubicin within Liver Cancer Cells
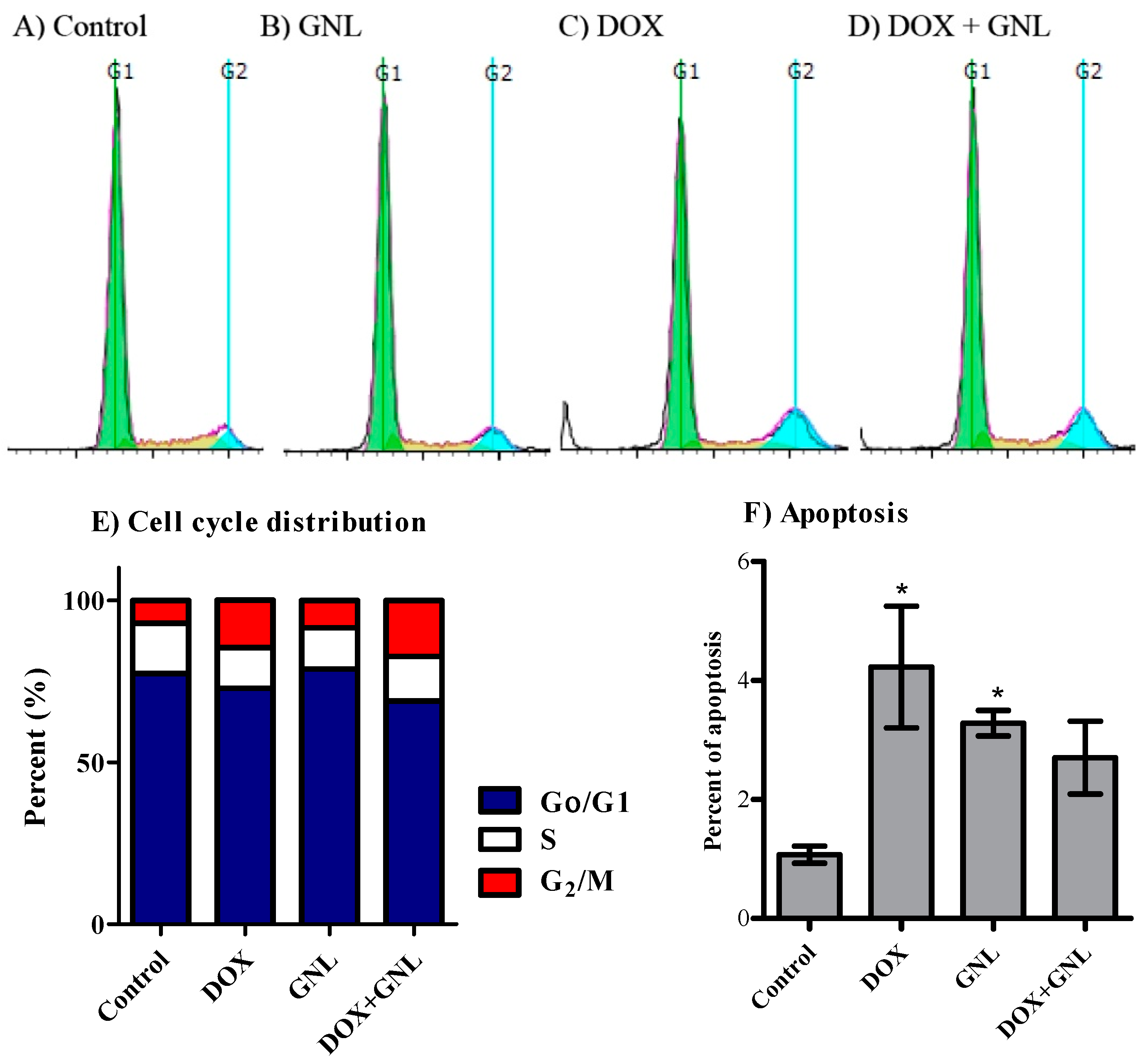
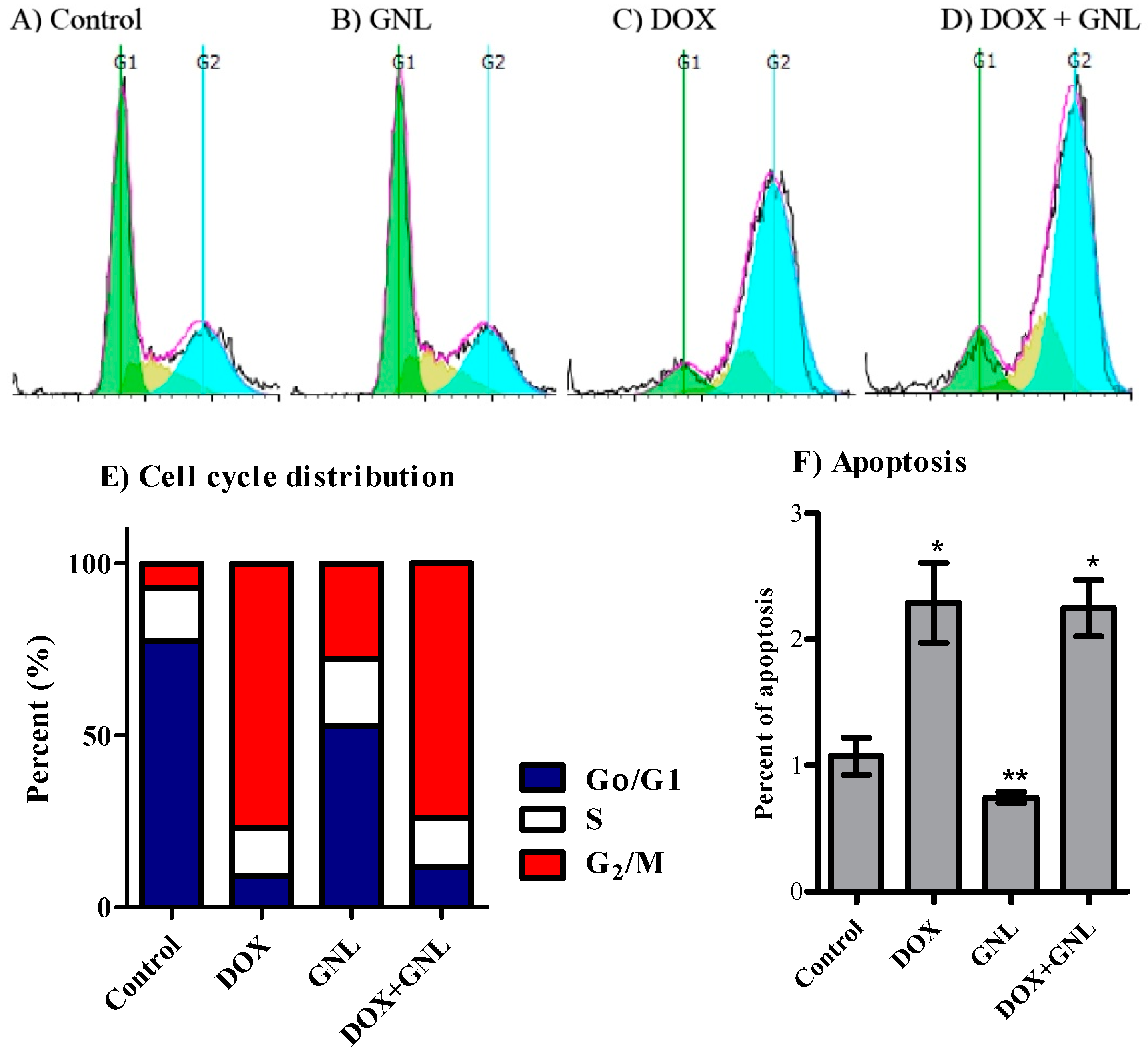
2.6. Cell Cycle Distribution Analysis of Liver Cancer Cells
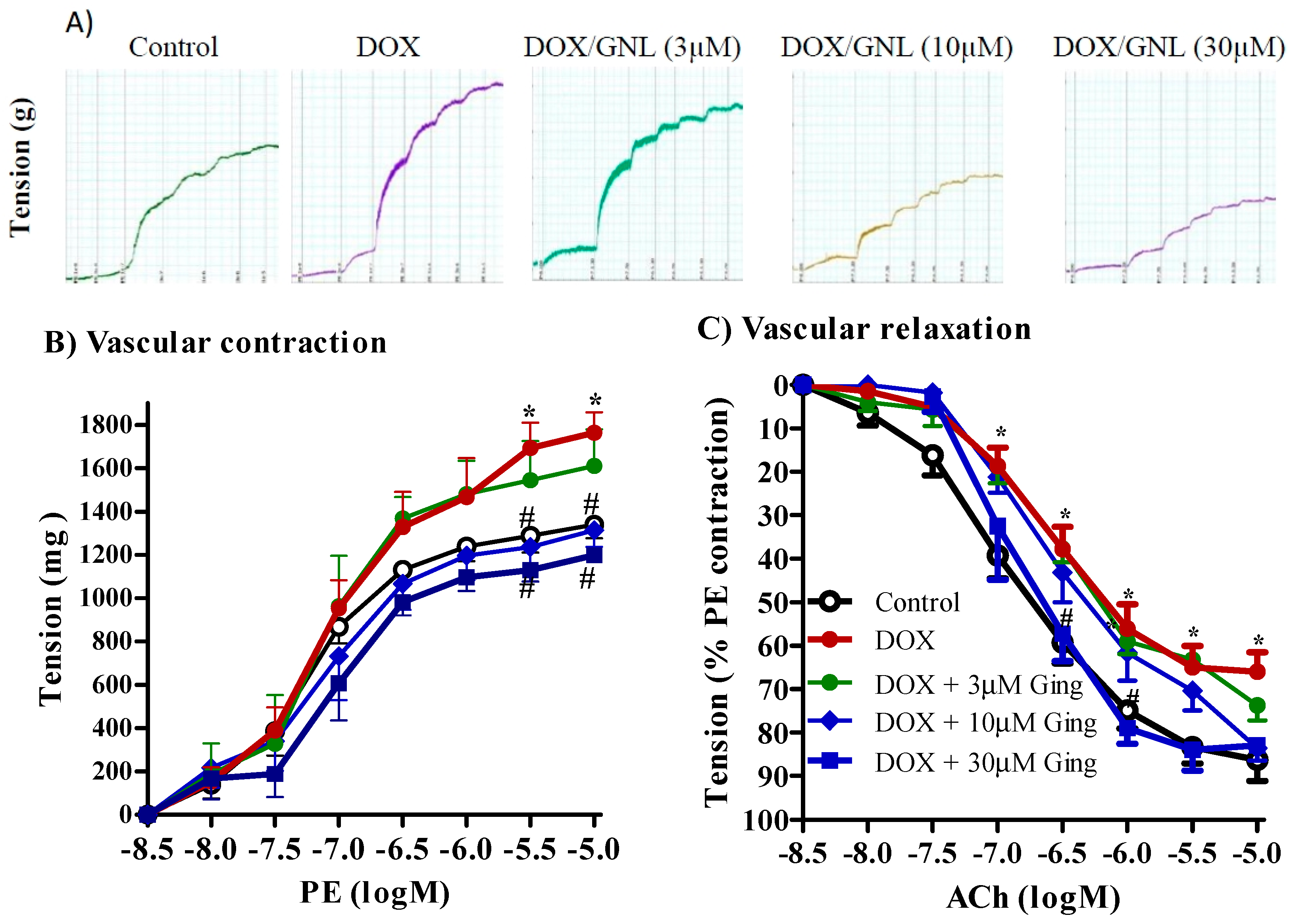
2.7. Gingerol Protects from DOX Induced Vascular Toxicity
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. General Experimental Procedures
4.3. Plant Material
4.4. Extraction and Isolation of Compounds from A. melegueta
4.5. Determining the Antioxidant Activity of Test Compounds Using Cell-Free System (DPPH Assay)
4.6. Cell Culture
4.7. Determining the Antioxidant Activity of Test Compounds within HepG2 Cells
4.8. Cytotoxicity Assessment
4.9. Data Analysis
4.10. The Influence of the Naturally Hydroxyphenylalkanes and Diarylheptanoids on the Cellular Pharmacokinetics of Doxorubicin (DOX)
4.11. Determining Sub-Molecular Interaction Characteristics between P-gp Protein and Naturally Occurring Hydroxyphenylalkanes and Diarylheptanoids
4.12. Chemomodulatory Effect of Gingerol (GNL) to DOX within Liver Cancer Cells
4.13. Analysis of Cell Cycle Distribution
4.14. Animals
4.15. Assessing the Protective Effect of 6-gingerol Against Doxorubicin Induced Vascular Damage
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| CCl4 | Carbon tetrachloride |
| DIACHEP (7) | 3,5-Diacetoxy-1-(3′,4′-dihydroxylphenyl)-7-(3″,4″-dihydroxy-5″-methoxyphenyl) heptane |
| DOX | Doxorubicin |
| GNL | Gingerol |
| GSH | Glutathione (reduced form) |
| GSSG | Glutathione (oxidized form) |
| iu | International Unit |
| P-gp | P-glycoprotein |
| PE | Phenylephrin |
| ROS | Reactive oxygen species |
| VRP | Verapamil |
References
- Duke, J.A. CRC Handbook of Medicinal Spices; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Akendengue, B.; Louis, A.M. Medicinal plants used by the Masango people in Gabon. J. Ethnopharmacol. 1994, 41, 193–200. [Google Scholar] [CrossRef]
- Umukoro, S.; Ashorobi, B.R. Further pharmacological studies on aqueous seed extract of Aframomum melegueta in rats. J. Ethnopharmacol. 2008, 115, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Kamtchouing, P.; Mbongue, G.; Dimo, T.; Watcho, P.; Jatsa, H.; Sokeng, S. Effects of Aframomum melegueta and Piper guineense on sexual behaviour of male rats. Behav. Pharmacol. 2002, 13, 243–247. [Google Scholar] [CrossRef] [PubMed]
- El-Halawany, A.M.; El Dine, R.S.; El Sayed, N.S.; Hattori, M. Protective effect of Aframomum melegueta phenolics against CCl4-induced rat hepatocytes damage; role of apoptosis and pro-inflammatory cytokines inhibition. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- El-Halawany, A.M.; Hattori, M. Anti-oestrogenic diarylheptanoids from Aframomum melegueta with in silico oestrogen receptor alpha binding conformation similar to enterodiol and enterolactone. Food Chem. 2012, 134, 219–226. [Google Scholar] [CrossRef]
- Seo, H.B.; Kwon, T.D.; Song, Y.J. The effect of ginger extract ingestion and swimming exercise on insulin resistance and skeletal muscle antioxidant capacity and apoptosis in hyperglycemic rats fed a high-fructose diet. J. Exerc. Nutr. Biochem. 2011, 15, 41–48. [Google Scholar] [CrossRef]
- Surh, Y.-J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999, 428, 305–327. [Google Scholar] [CrossRef]
- El-Bakly, W.M.; Louka, M.L.; El-Halawany, A.M.; Schaalan, M.F. 6-Gingerol ameliorated doxorubicin-induced cardiotoxicity: Role of nuclear factor kappa B and protein glycation. Cancer Chemother. Pharmacol. 2012, 70, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorganic Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Saba, A.B.; Azeez, O.I. Molecular targets of 6-gingerol: Its potential roles in cancer chemoprevention. Biofactors 2010, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Manoharan, S.; Arokia Vijayaanand, M.; Sugunadevi, G. Chemopreventive and antioxidant efficacy of (6)-paradol in 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis. Pharmacol. Rep. 2010, 62, 1178–1185. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Kim, J.; Lee, K.H.; Park, K.K.; Surh, Y.-J.; Lee, J.M.; Lee, S.-S.; Yoon, J.H.; Joo, S.Y.; Cha, I.H. Induction of apoptosis and caspase-3 activation by chemopreventive 6-paradol and structurally related compounds in KB cells. Cancer Lett. 2002, 177, 41–47. [Google Scholar] [CrossRef]
- Lee, K.K.; Bahler, B.D.; Hofmann, G.A.; Mattern, M.R.; Johnson, R.K.; Kingston, D.G. Isolation and structure elucidation of new pkcα inhibitors from Pinus flexilis. J. Nat. Prod. 1998, 61, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Li, G.; Kim, S.H.; Lee, C.-S.; Woo, M.-H.; Lee, S.-H.; Jhang, Y.-D.; Son, J.-K. Cytotoxic diarylheptanoids from the roots of Juglans mandshurica. J. Nat. Prod. 2002, 65, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Novaković, M.; Pešić, M.; Trifunović, S.; Vučković, I.; Todorović, N.; Podolski-Renić, A.; Dinić, J.; Stojković, S.; Tešević, V.; Vajs, V. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry 2014, 97, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from s. Peucetius var. Caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, J.; Guardia, J.; Bacardi, R.; Monne, J. Doxorubicin for liver cancer. Lancet 1978, 1, 1367. [Google Scholar] [CrossRef]
- Maeng, J.H.; Lee, D.H.; Jung, K.H.; Bae, Y.H.; Park, I.S.; Jeong, S.; Jeon, Y.S.; Shim, C.K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Licata, S.; Saponiero, A.; Mordente, A.; Minotti, G. Doxorubicin metabolism and toxicity in human myocardium: Role of cytoplasmic deglycosidation and carbonyl reduction. Chem. Res. Toxicol. 2000, 13, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Lopez, M.C.; Sanchez-Mas, J.; Pascual-Figal, D.A.; Abenza, S.; Perez-Martinez, M.T.; Valdes, M.; Lax, A. Involvement of ferritin heavy chain in the preventive effect of metformin against doxorubicin-induced cardiotoxicity. Free Radic. Biol. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.A.; El-Shenawy, S.M.; Al-Shabka, A.M. Aqueous fenugreek seed extract ameliorates adriamycin-induced cytotoxicity and testicular alterations in albino rats. Reprod. Sci. 2012, 19, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kapuscinski, J.; Ardelt, B.; Piosik, J.; Zdunek, M.; Darzynkiewicz, Z. The modulation of the DNA-damaging effect of polycyclic aromatic agents by xanthines. Part I. Reduction of cytostatic effects of quinacrine mustard by caffeine. Biochem. Pharmacol. 2002, 63, 625–634. [Google Scholar] [CrossRef]
- Greenlee, H.; Shaw, J.; Lau, Y.K.; Naini, A.; Maurer, M. Lack of effect of coenzyme Q10 on doxorubicin cytotoxicity in breast cancer cell cultures. Integr. Cancer Ther. 2012, 11, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Al-Abassi, F.A.; Asaad, G.F.; Abdel-Naim, A.B. Didox potentiates the cytotoxic profile of doxorubicin and protects from its cardiotoxicity. Eur. J. Pharmacol. 2013, 718, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Mahmoud, A.M.; El-Sherbiny, G.A.; El-Moselhy, M.A.; Nofal, S.M.; El-Latif, H.A.; El-Eraky, W.I.; El-Shemy, H.A. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Prolif. 2011, 44, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, X.D.; Liu, Q.; Wang, F.P.; Bao, X.Q.; Zhang, D. Reversal of P-glycoprotein-mediated multidrug resistance by the novel tetrandrine derivative W6. J. Asian Nat. Prod. Res. 2015, 17, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Al-Abd, A.M.; El-Dine, R.S.; El-Halawany, A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J. Adv. Res. 2015, 6, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Timmermann, B.N.; Gang, D.R. Characterization and identification of diarylheptanoids in ginger (Zingiber officinale rosc.) using high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, S.; Duan, W.; Wang, W. An enantioselective synthesis of (+)-(S)-[n]-gingerols via the l-proline-catalyzed aldol reaction. Bioorg. Med. Chem. Lett. 2009, 19, 3909–3911. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; Lajide, L.; Mizutani, J. Termite antifeedant activity in Aframomum melegueta. Phytochemistry 1995, 40, 1097–1099. [Google Scholar] [CrossRef]
- Tackie, A.; Dwuma-Badu, D.; Ayim, J.; Dabra, T.; Knapp, J.; Slatkin, D.; Schiff, P. Hydroxyphenylalkanones from Aframomum melegueta. Phytochemistry 1975, 14, 853–854. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially processed dry ginger (Zingiber officinale): Composition and effects on LPS-stimulated PGE2 production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef] [PubMed]
- Dev, S. Impact of natural products in modern drug development. Indian J. Exp. Biol. 2010, 48, 191–198. [Google Scholar] [PubMed]
- Aboul-Enein, A.M.; Al-Abd, A.M.; Shalaby, E.; Abul-Ela, F.; Nasr-Allah, A.A.; Mahmoud, A.M.; El-Shemy, H.A. Eichhornia crassipes (mart) solms: From water parasite to potential medicinal remedy. Plant Signal. Behav. 2011, 6, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Mohamed, G.A. Naturally occurring thiophenes: Isolation, purification, structural elucidation, and evaluation of bioactivities. Phytochem. Rev. 2015, 15, 197–220. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Elnagar, A.Y.; Khanfar, M.A.; Sallam, A.A.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Design of semisynthetic analogues and 3D-QSAR study of eunicellin-based diterpenoids as prostate cancer migration and invasion inhibitors. Eur. J. Med. Chem. 2011, 46, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure-activity relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Selassie, C.D.; Kapur, S.; Verma, R.P.; Rosario, M. Cellular apoptosis and cytotoxicity of phenolic compounds: A quantitative structure-activity relationship study. J. Med. Chem. 2005, 48, 7234–7242. [Google Scholar] [CrossRef] [PubMed]
- Ghareib, S.A.; El-Bassossy, H.M.; Elberry, A.A.; Azhar, A.; Watson, M.L.; Banjar, Z.M. 6-Gingerol alleviates exaggerated vasoconstriction in diabetic rat aorta through direct vasodilation and nitric oxide generation. Drug Des. Dev. Ther. 2015, 9, 6019–6026. [Google Scholar]
- Chen, Y.L.; Zhuang, X.D.; Xu, Z.W.; Lu, L.H.; Guo, H.L.; Wu, W.K.; Liao, X.X. Higenamine combined with 6-gingerol suppresses doxorubicin-triggered oxidative stress and apoptosis in cardiomyocytes via upregulation of PI3K/Akt pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 970490. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of mdr and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Nakumura, T.; Sakaeda, T.; Ohmoto, N.; Moriya, Y.; Komoto, C.; Shirakawa, T.; Gotoh, A.; Matsuo, M.; Okmura, K. Gene expression profiles of ABC transporters and cytochrome P450 3A in CaCo-2 and human colorectal cancer cell lines. Pharm. Res. 2003, 20, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Leonard, G.D.; Fojo, T.; Bates, S.E. The role of ABC transporters in clinical practice. Oncologist 2003, 8, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, Z.J.; Chang, J.Y.; Zhang, Z.F.; Chu, R.; Li, Y.L.; Dai, K.H.; Mo, G.Q.; Chang, Q.Y. Sinomenine sensitizes multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by downregulation of mdr-1 expression. PLoS ONE 2014, 9, e98560. [Google Scholar] [CrossRef] [PubMed]
- Van der Bliek, A.M.; Baas, F.; Ten Houte de Lange, T.; Kooiman, P.M.; Van der Velde-Koerts, T.; Borst, P. The human Mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987, 6, 3325–3331. [Google Scholar] [PubMed]
- Behling, E.B.; Sendao, M.C.; Francescato, H.D.; Antunes, L.M.; Costa, R.S.; Bianchi Mde, L. Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol. Rep. 2006, 58, 526–532. [Google Scholar] [PubMed]
- Topal, T.; Oztas, Y.; Korkmaz, A.; Sadir, S.; Oter, S.; Coskun, O.; Bilgic, H. Melatonin ameliorates bladder damage induced by cyclophosphamide in rats. J. Pineal Res. 2005, 38, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Wang, L.; Lei, Y.; Hu, T.; Zhang, F.L.; Cho, C.H.; To, K.K. Reversal of P-gp and BCRP-mediated MDR by tariquidar derivatives. Eur. J. Med. Chem. 2015, 101, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wisniewska, A.; Halas-Wisniewska, M.; Tadrowski, T.; Gagat, M.; Grzanka, D.; Grzanka, A. Paclitaxel and the dietary flavonoid fisetin: A synergistic combination that induces mitotic catastrophe and autophagic cell death in A549 non-small cell lung cancer cells. Cancer Cell Int. 2016, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Songgang, L.; Jiyu, L.; Gongwei; Yingbin, L.; Yiyu, Q.; Zhiwei, Q. Somatostatin enhances the chemosensitivity of GBC-SD cell line to doxorubicin through arresting the cell cycle to S phase rather than through the P53/Bax-depended apoptosis way in vitro. Hepato Gastroenterol. 2009, 56, 1253–1260. [Google Scholar]
- Ehrhardt, H.; Pannert, L.; Pfeiffer, S.; Wachter, F.; Amtmann, E.; Jeremias, I. Enhanced anti-tumour effects of vinca alkaloids given separately from cytostatic therapies. Br. J. Pharmacol. 2013, 168, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Li, Z.M.; Huang, Y.; Huang, Y.; Tian, Y.; He, X.X.; Xiao, J.; Lin, T.Y. Schedule-dependent inhibition of T-cell lymphoma cells by cotreatment with the mTOR inhibitor everolimus and anticancer drugs. Investig. New Drugs 2012, 30, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Melissinos, K.G.; Delidou, A.Z.; Varsou, A.G.; Begietti, S.S.; Drivas, G.J. Serum and erythrocyte glutathione reductase activity in chronic renal failure. Nephron 1981, 28, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R.; Oh, S.H.; Hoekstra, W.G.; Ganther, H.E. Glutathione peroxidase: Inhibition by cyanide and release of selenium. Biochem. Biophys. Res. Commun. 1977, 74, 64–71. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al-Abd, A.M.; Lightfoot, D.A.; El-Shemy, H.A. Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely P53-dependent. J. Enzyme Inhib. Med. Chem. 2012, 27, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; El-Moselhy, M.A.; Mahmoud, M.F. Pentoxifylline alleviates vascular impairment in insulin resistance via TNF-alpha inhibition. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 384, 277–285. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; El-Fawal, R.; Fahmy, A. Arginase inhibition alleviates hypertension associated with diabetes: Effect on endothelial dependent relaxation and no production. Vasc. Pharmacol. 2012, 57, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all compounds are available from the authors.
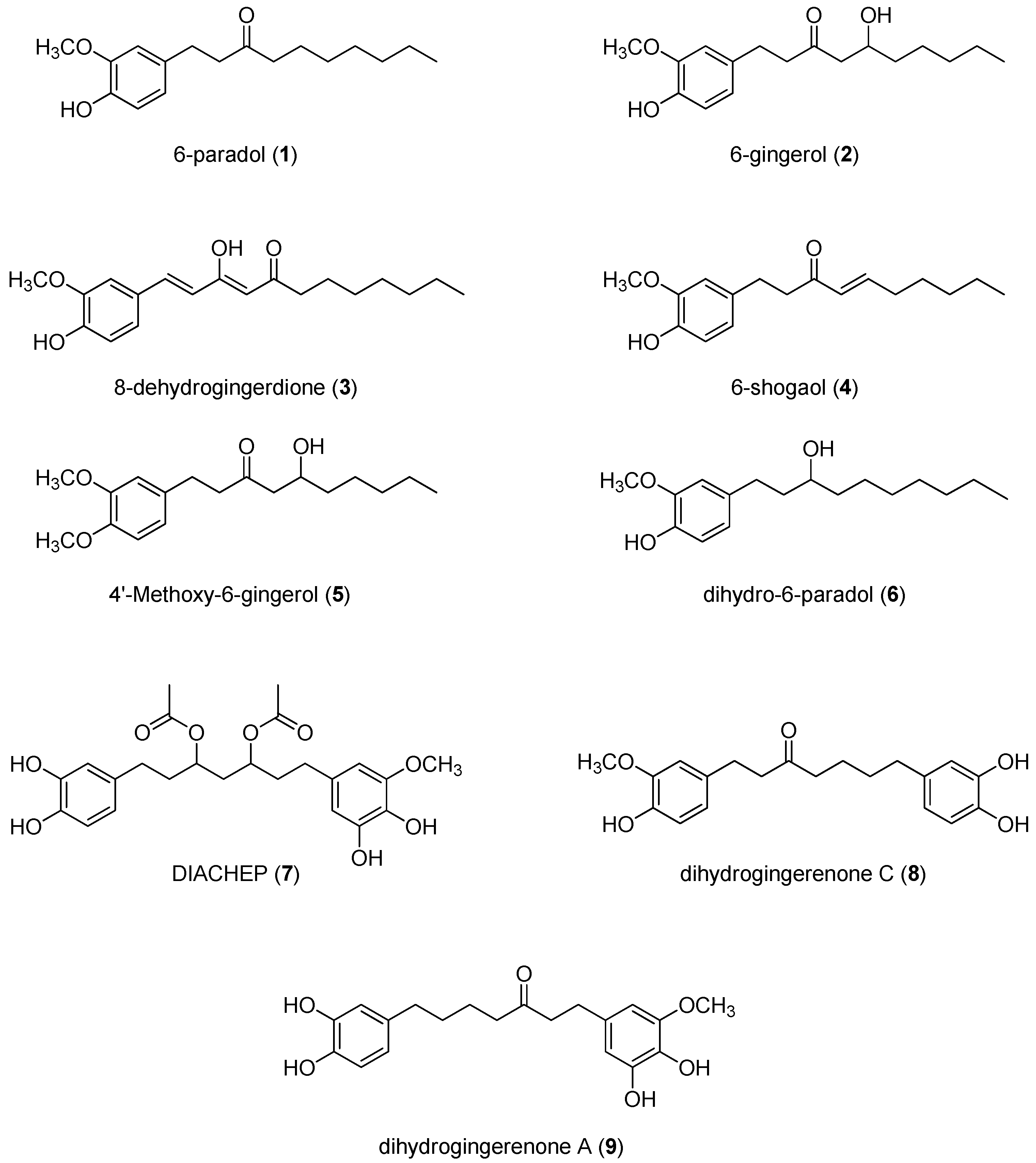
| Compound | HCT-116 | HepG2 | MCF-7 | HeLa | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | R-Value (%) | IC50 (µM) | R-Value (%) | IC50 (µM) | R-Value (%) | IC50 (µM) | R-Value (%) | |
| 6-Paradol (1) | 10.4 | 15.1 | >100 | N/A | 20.4 | 42.9 | 57.7 | 37.9 |
| 6-Gingerol (2) | 1.5 | 60.6 | 71.9 | N/A | >100 | 85.6 | 15.5 | 43.8 |
| Dehydrogingerdione (3) | 12.6 | N/A | >100 | N/A | 63.7 | N/A | 55.8 | 1.1 |
| 6-Shogoal (4) | 3.1 | N/A | 18.7 | N/A | 7.5 | 1.1 | 10.2 | 1.1 |
| 4′-Methoxy-6-gingerol (5) | 11.4 | 4.6 | 19.4 | N/A | 12.0 | N/A | 9.2 | 0.5 |
| Dihydro-6-paradol (6) | 12.0 | N/A | 13.5 | 50.2 | 63.5 | 42.9 | 14.7 | 0.9 |
| DIACHEP (7) | 11.2 | 4.1 | >100 | N/A | 61.5 | N/A | 20.2 | 0.94 |
| Dihydrogingerenone-C (8) | 10.5 | 10.7 | N/A | 75.5 | 20.4 | 65.5 | 16.6 | 15.1 |
| Dihydrogingerenone-A (9) | 12.2 | 7.8 | N/A | N/A | 59.4 | 0.7 | 17.5 | 5.5 |
| Treatment | HepG2 | Huh-7 | ||
|---|---|---|---|---|
| IC50 | R-Value (%) | IC50 | R-Value (%) | |
| DOX | 680 ± 60 nM | 2.7 ± 0.8 | 4.6 ± 0.9 nM | 3.0 ± 0.9 |
| GNL | 71.9 ± 2.8 µM | N/A | 103.1 ± 3.0 µM | 4.3 ± 1.7 |
| DOX + GNL | 67.4 ± 9.1 nM | 3.3 ± 0.7 | 1.2 ± 0.13 nM | 4.7 ± 1.1 |
| CI-index/CI-value | Synergism/0.19 | Synergism/0.29 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Abbasi, F.A.; Alghamdi, E.A.; Baghdadi, M.A.; Alamoudi, A.J.; El-Halawany, A.M.; El-Bassossy, H.M.; Aseeri, A.H.; Al-Abd, A.M. Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity. Molecules 2016, 21, 886. https://doi.org/10.3390/molecules21070886
Al-Abbasi FA, Alghamdi EA, Baghdadi MA, Alamoudi AJ, El-Halawany AM, El-Bassossy HM, Aseeri AH, Al-Abd AM. Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity. Molecules. 2016; 21(7):886. https://doi.org/10.3390/molecules21070886
Chicago/Turabian StyleAl-Abbasi, Fahad A., Eman A. Alghamdi, Mohammed A. Baghdadi, Abdulmohsin J. Alamoudi, Ali M. El-Halawany, Hany M. El-Bassossy, Ali H. Aseeri, and Ahmed M. Al-Abd. 2016. "Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity" Molecules 21, no. 7: 886. https://doi.org/10.3390/molecules21070886
APA StyleAl-Abbasi, F. A., Alghamdi, E. A., Baghdadi, M. A., Alamoudi, A. J., El-Halawany, A. M., El-Bassossy, H. M., Aseeri, A. H., & Al-Abd, A. M. (2016). Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity. Molecules, 21(7), 886. https://doi.org/10.3390/molecules21070886