Abstract
A total of fifty-six chromones, including seven 5,6,7,8-tetrahydro-2-(2-phenylethyl)-chromones (THPECs), five 5,6-epoxy-2-(2-phenylethyl)chromones (EPECs), seven 5,6:7,8-diepoxy-2-(2-phenylethyl)chromones (DEPECs) and thirty-seven 2-(2-phenylethyl)chromones of the flidersia type (FTPECs), were characterized by HPLC/DAD/ESI/MS/MS in three agarwood samples (from Aquilaria crassna) induced by artificial holing with different holing times. The characteristic fragmentation behavior of DEPECs and EPECs, and the methods to distinguish these four types of chromones by MS analysis were described for the first time. In addition, it was found that the relative contents of DEPECs and EPECs were down-regulated, while the relative contents of THPECs and FTPECs were up-regulated for the samples from two, four and five years of the agarwood formation time. However, the relative contents of six most widespread and abundant FTPECs presented roughly upward based on the formation time. These results could be referenced to distinguish different agarwood samples collected from different formation time.
1. Introduction
Agarwood is the dark resinous and aromatic wood from genus Aquilaria trees belonging to the family Thymelaeaceae [1,2,3], and is created as a tree response to various form of injury, including natural injuries, such as lightning strikes, animal grazing, insect attacks or microbial invasion, or artificial injuries, such as cutting, nailing, holing, fire, chemical wounding, and deliberate fungal inoculation [3,4,5]. Agarwood not only plays an important role in Traditional Chinese Medicine, but also has been used for centuries as incense in Buddhist, Hindu and Islamic ceremonies [1,2,3]. Unfortunately, in natural forests, only 7%–10% of the agarwood trees contain resinous material, and wild agarwood is rare due to its exhaustive exploitation. Nowadays, agarwood in trade mostly comes from cultivated Aquilaria trees induced by artificial methods, of which the traditional artificial holing is the most common and popular one. As far as we know, nineteen accepted species have been reported up to date, growing from southeast Asia to the Malay Archipelago [1,2]. However, only the heartwood of A. crassna, A. malaccensis, A. sinensis and A. filarial have been commercially exploited [1]. 2-(2-Phenylethyl)chromone derivatives, of which about one hundred different structures have been reported, are considered to be one of the characteristic and most abundant constituents responsible for the quality of agarwood [1,3,4,6,7,8,9]. These compounds were divided into four types according to the various chromone moiety skeletons, in which the benzyl moiety is the same. In these four types, both 2-(2-phenylethyl)chromones of the flidersia type (FTPECs) and 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones (THPECs) were widely distributed and accumulated most in agarwood, while only four 5,6-epoxy-2-(2-phenylethyl)chromones (EPECs) and three 5,6:7,8-diepoxy-2-(2-phenylethyl) chromones (DEPECs) with one or two epoxy substituents on the chromone moiety respectively, have been reported from 2005 to the present [7,10,11].
Based on the above reports, 2-(2-phenylethyl)chromones were considered as the necessary diagnostic components to evaluate the quality of agarwood. Mei et al. summarized the MS characterization of the FTPECs, which was helpful for the analysis and characterization of FTPECs in agarwood by GC-MS [3]. Xia et al. reported that 29 FTPECs were detected from agarwood by supercritical fluid chromatography in combination with mass spectrometry (SFC-MS) then obtained further detailed structural information by using tandem mass spectrometry (MS/MS) [12]. Li et al. identified and quantified eight characteristic THPECs in Chinese eaglewood, and analyzed the MS fragmentation behavior of THPECs by HPLC/DAD/MS [13]. Lancaster et al. detected diagnostic ions at m/z 319.118 (FTPECs) or 349.129 (FTPECs) and the occurrence of ten or more of the other target chromone ions by real time time-of-flight mass spectrometry (DART-TOFMS) to infer agarwood [5]. Espinoza et al. analyzed the diagnostic chromones ions by using DART-TOFMS followed by discriminant analysis to differentiate wild agarwood from cultivated samples [2]. However, the MS characterization of EPECs and DEPECs have not been reported yet. In this paper, the MS characterization and fragmentation behavior of EPECs and DEPECs was described, and the fragmentation regularity of mass spectra (MS) used to distinguish and identify the four different types of 2-(2-phenylethyl)chromones was concluded for the first time.
Nowadays, artificial holing agarwood from A. crassna tree plays an extremely important role in the market. In this report, we detected and analyzed 2-(2-phenylethyl)chromones in three agarwood samples (from A. crassna) with different holing times by using HPLC/DAD/ESI/MS2, and revealed the variation of relative contents of different types of 2-(2-phenylethyl)chromones in agarwood after two, four and five years of artificial holing. This could be referenced for the variation of 2-(2-phenylethyl)chromones, also identification and quality evaluation of agarwood with different formation time.
2. Results and Discussion
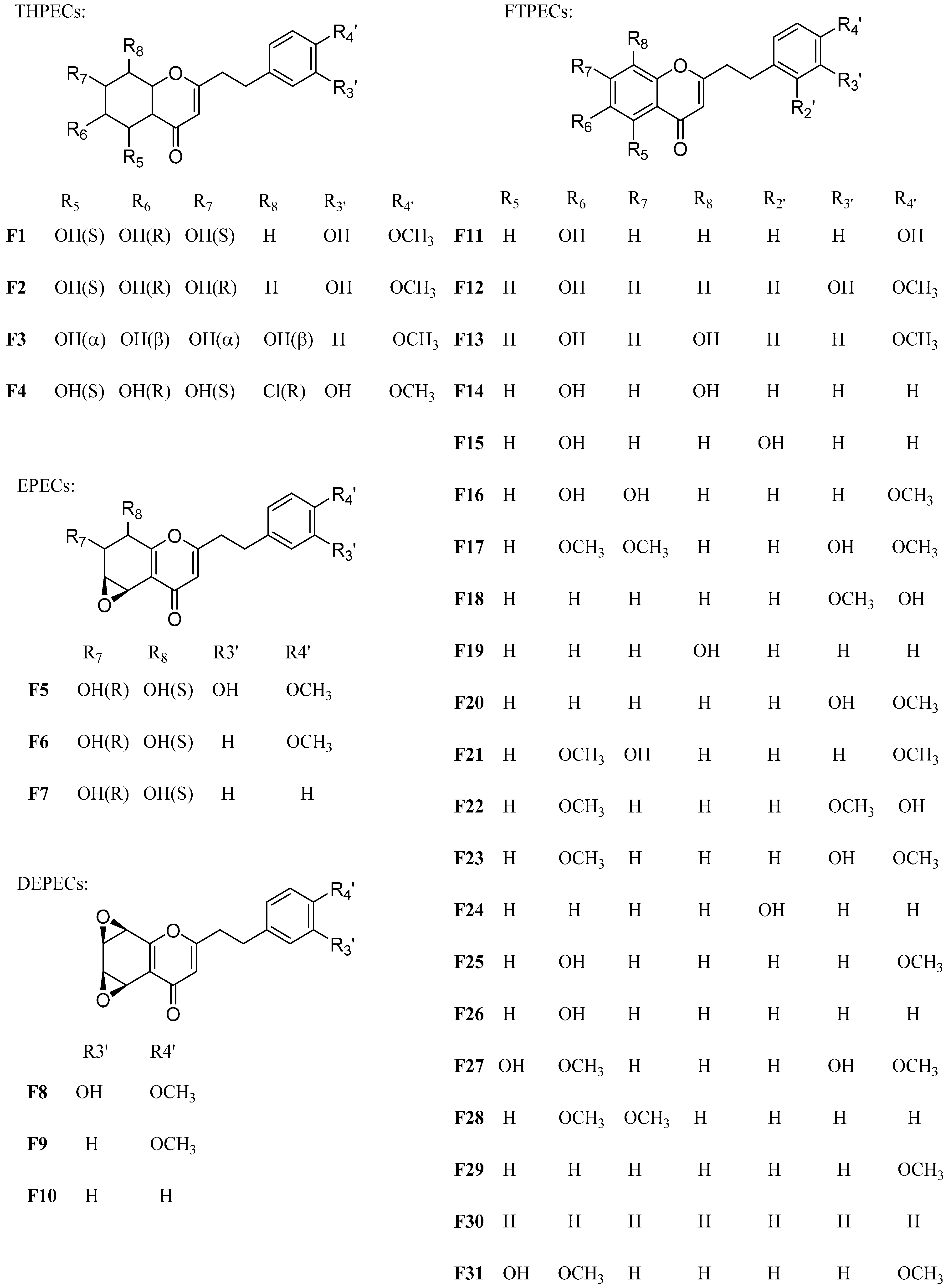
2.1. Characteristic Fragmentation Behavior of Reference Compounds F1–F31
In this study, the different fragmentation behavior of four types of chromones according to their MS spectra was summarized by the reference compounds (Table 1 and Figure 1). The characteristic fragment ions produced by four types of chromones due to their structural difference on the basic skeleton of chromone moiety were used as the basis for identification. For THPECs (compounds F1–F4), the characteristic fragmentations were the loss of a molecule of H2O ([M + H–18]+) and a successive loss of another H2O molecule ([M + H–18–18]+) [13]. For EPECs (compounds F5–F7), the characteristic fragmentations were loss of a molecule of H2O ([M + H–18]+), followed by loss of a molecule of CO ([M + H–18–28]+). For DEPECs F8–F10, the characteristic fragmentation was the loss of a molecule of CO ([M + H–28]+).

Table 1.
Characterization of thirty-one reference compounds by HPLC/ESI-MS/MS.
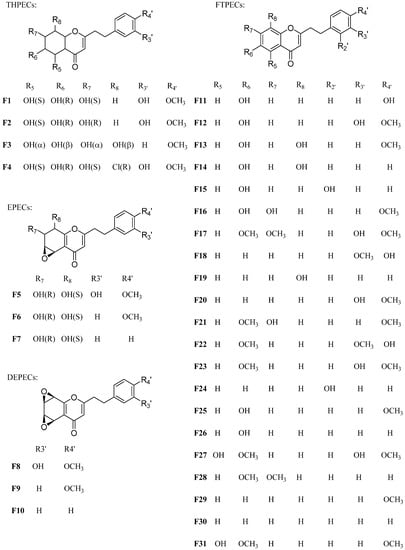
Figure 1.
The structure of reference compounds F1~F31.
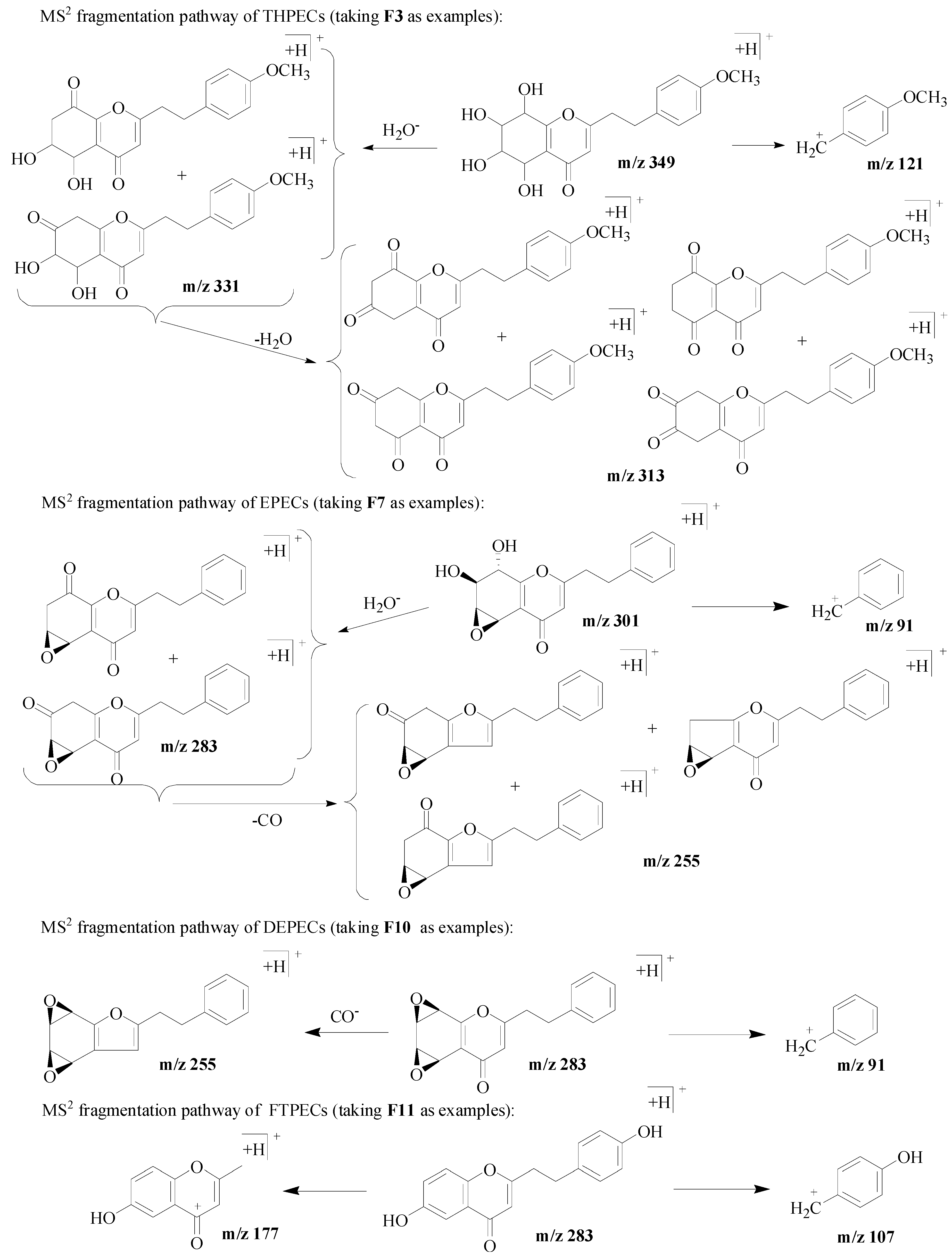
For twenty-one FTPECs in the reference compounds, their characteristic fragmentations were benzyl ions and/or chromone moiety ions. In addition, the benzyl ions observed in the MS spectrum of these four types chromones were the same, and the typical ions included m/z 91 [C7H7]+, 107 [C7H6 + OH]+, 121 [C7H6 + OCH3]+, 137 [C7H5 + OH + OCH3]+, 151 [C7H5 + 2OCH3]+. Figure 2 presents examples of the MS2 fragmentation behavior from each type of chromone.
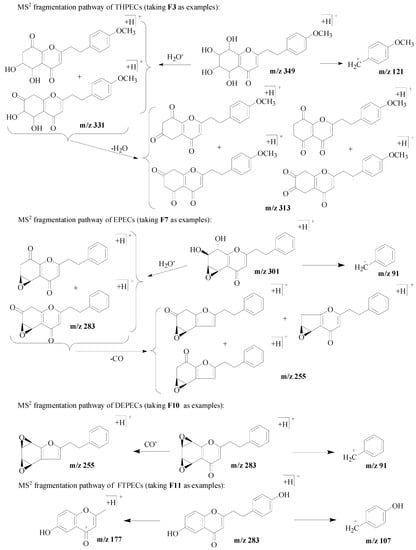
Figure 2.
MS2 fragmentation pathway of four types of chromones.
2.2. The Method Deduced for Identification of Four Types of 2-(2-Phenylethyl)chromone Derivatives
- (1)
- The type of 2-(2-phenylethyl)chromone could be determined by the characteristic fragment ions as discussed above and listed in Figure 3. The present of the ions [M + H–18]+ and [M + H–18–18]+ was proposed to be THPECs. When both ions [M + H–18]+ and[M + H–28]+ were observed in the spectrum, EPECs was deduced. If only [M + H–28]+ was detected, it would be DEPECs. FTPECs only gave the peaks of benzyl ions and/or chromone moiety ions in the MS spectrum. It should be noticed that the [M + H–18]+ ion may appear as a pseudo-characteristic ion during identification, when the CH2-CH2 group between chromone moiety and phenyl moiety was substituted by a hydroxyl group (-OH) [1].
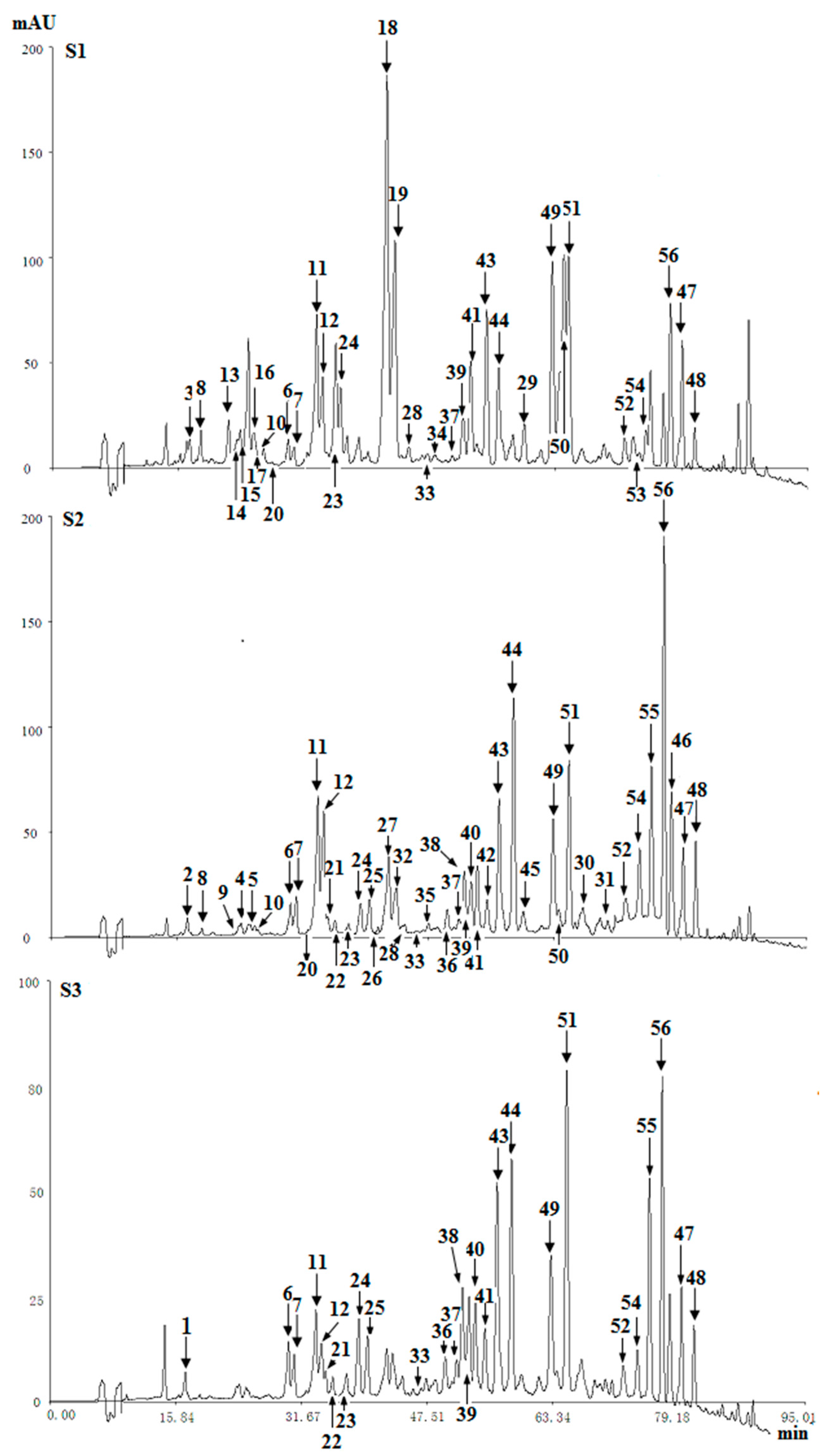
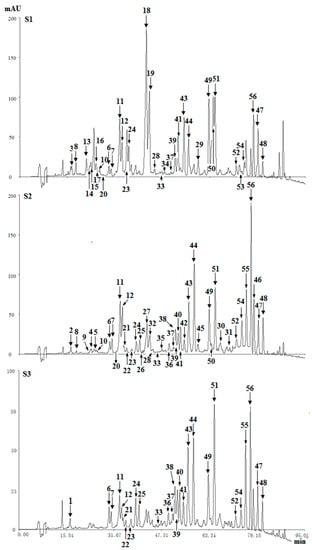 Figure 3. Chromatograms of three agarwood samples (S1, S2, S3) at 254 nm.
Figure 3. Chromatograms of three agarwood samples (S1, S2, S3) at 254 nm. - (2)
- The four types of 2-(2-phenylethyl)chromone derivatives in agarwood have different basic skeletons (the epoxy group was assigned as a part of the basic skeletons), but substituted with similar substituent groups (mainly hydroxyl, methoxyl group and/ or chlorine atom). The molecular weights of the basic skeletons of THPECs, EPECs, DEPECs and FTPECs were 254, 268, 282 and 250, respectively. Thus, the number of different substituents of the structure could be deduced by Formula (1), where “MW” meant the molecular weight, “a” meant the number of methoxy groups, “b” represented the number of hydroxyl groups, and “c” meant the number of chlorine atom.
- (3)
- The four types of chromones presented the same characteristic benzyl ion, while it could not be observed in some compounds. The benzyl moiety without substituent group was 91 [C7H7]+, therefore, the number of hydroxyl and/or methoxyl groups substituted on the benzyl moiety could be deduced according to Formula (2), where “MWbm” means the molecular weight of benzyl ion, “abm” means the number of methoxy groups on the benzyl moiety, “bbm” represents the number of hydroxyl groups on the benzyl moiety. For example, according to Formula (2), the hydroxyl- or methoxyl-substituted benzyl moiety provided characteristic ions at m/z 107 [C7H6 + OH]+, 121 [C7H6 + OCH3]+, 137 [C7H5 + OH + OCH3]+, 151 [C7H5 + 2OCH3]+:MWbm − 91 = 30abm + 16bbm
- (4)
- Except for FTPECs, the fragment ion of the chromone moiety was rarely observed in the MS2 spectra of THPECs, EPECs and DEPECs, nevertheless the number of substituent groups of the chromone moiety could still be calculated by subtracting the number of substituents on the benzyl moiety deduced by Formula (2) from the number of substituents on the whole structure deduced by Formula (1).For FTPECs with hydroxyl-substituted benzyl moieties, according to [3,6], if one of characteristic fragment ions at m/z 161 [C10H8O2 + H], 177 [C10H7O2 + OH + H], 191 [C10H7O2 + OCH3 + H], 193 [C10H6O2 + 2OH + H], 207 [C10H6O2 + OCH3 + OH + H], 221 [C10H6O2 + 2OCH3 + H] from different substituted chromone moieties was observed, a 4′/2′-OH substituent on the benzyl moiety was deduced. Otherwise, if none of them was observed, a hydroxyl group substituted at the 3′ position of the benzyl moiety was deduced, whether an ion of chromone moiety at m/z 160, 176, 190, 192, 206, 220 appeared or not. The above mentioned MS characterization has been confirmed by the FTPEC reference compounds in Table 1. The observed fragment ions of the chromone moieties of F11, F15, F18, F22 and F24 with 4′/2′-OH on the benzyl moiety were m/z 177, 177, 161, 191 and 161, respectively, which were not observed for the F12, F17, F20, F23 and F27 with 3′-OH on the benzyl moiety, while only a fragment ion at m/z 220 of F17 was observed. These chromone moiety ions could be used to suggest the position of hydroxyl groups on the benzyl moieties.
- (5)
- Following the above procedure, a compound could be characterized as one of the four types of 2-(2-phenylethyl)chromones, as well as calculated the numbers of substituent groups of the chromone moiety and benzyl moiety. Furthermore, the position of substituent groups could be deduced sometimes. Besides, if more than one structure were suggested for a single peak, the comparison of retention time and MS spectra with reference compounds was performed. The compound was identified when the data were the same to the reference compound, otherwise, it might be a new compound.
2.3. Identification of 2-(2-Phenylethyl)chromones According to MS Characterization
In the HPLC chromatograms of the ether extract of three agarwood samples (S1, S2, S3) detected at UV 254 nm, a total of fifty-six compounds (four types of chromones) were detected (Figure 3). By comparing their retention time and MS spectra with reference compounds, twenty-six of them were identified, and thirty compounds were tentative identified. The identified or characterized results of THPECs, EPECs and DEPECs were listed in Table 2, and the retention time of THPECs, EPECs and DEPECs were in the range of 12~30 min, 18~34 min, 21~43 min, respectively. The results of FTPECs were showed in Table 3, and the retention time of FTPECs were in the range of 34~80 min, except for compound 20 (26.1 min).

Table 2.
The MS characterization and identified or characterized results of DEPECs, EPECs and THPECs from S1 to S3.

Table 3.
The MS characterization and identified or characterized results of FTPECs from S1 to S3 by HPLC/DAD/ESI/MS2.
2.3.1. Structural Analysis of THPECs
Taking compounds 1 and 3 for example, the characteristic fragment ions at m/z 331([M + H–18]+) and 313 ([M + H–18–18]+), indicated both were THPECs. The molecular weight (MW) of these two compounds were determined as 348 based on the protonated precursor ion at m/z 349. According to formula (1) (MW − (30a + 16b +34c) = 254), a, b and c were calculated as 1, 4 and 0, respectively, which meant compounds 1 and 3 had four hydroxyl and one methoxyl substituent groups on the whole compound. Furthermore, the benzyl ion at m/z 121 was found in compound 1, which meant one methoxy substitution occurred on its benzyl moiety according to Formula (2), thus, the other four hydroxyl substitutions occurred on its chromone moiety. Neither benzyl ion nor chromone moiety ions were observed in compound 3, thus the positions of substituent groups were uncertain. By searching the literature, we found six THPECs with the MW = 348 were reported, including four of them possessing one methoxy substitution occurring on the benzyl moiety, and four hydroxyl substitutions occurring on their chromone moiety, which matched the above deduced structural characterization of compound 1, so compound 1 was tentative identified as one of the four reported compounds. The positions of the substituent groups of compound 3 were uncertain, so it was tentative identified as one of the six reported compounds while different from compound 1. By comparing the retention time and MS spectra with the reference compounds, compound 1 was identified as F3, and further proved that the above identification method was reasonable.
According to above method of identification, compounds 1–7 were deduced as THPECs. Except for compound 5 (m/z 317), the other six compounds, with [M + H]+ ions of m/z 319, 303, 367, 337, have been reported as THPECs. Compound 5 was identified as a new compound.
2.3.2. Structural Analysis of EPECs
Taking compounds 10 and 11 for example, according to the characteristic fragment ions of m/z 313 ([M + H–18]+) and 285 ([M + H–18–28]+), they were identified as EPECs. The protonated precursor ions at m/z 331 meant their molecular weight (MW) was 330. According to the Formula (1), a, b and c were calculated as 1, 2 and 0, respectively, which meant they had two hydroxyl and one methoxyl substitutions on the whole compound. The benzyl ion at m/z 121 indicated one methoxy substitution occurred on its benzyl moiety according to Formula (2), therefore, the two hydroxyl groups substitutions occurred on the chromone moiety. Subsequently, by literature searching, only one EPEC with MW = 330 was reported, which meant that one of them would be a new compound. By comparing the retention time and MS spectra with reference compound, compound 11 was identified as F6, which proved that the above identification method was feasible, and meanwhile compound 10 should be a new compound.
By the same method, compounds 8–12 were deduced as EPECs. Except for compound 9 (which [M + H]+ ion is m/z 285), all the other compounds, which [M + H]+ ions were at m/z 347 or 301, have been reported. By comparing retention time and MS spectra with reference compounds, compounds 8 and 12 were identified as reference compounds F5 and F7, respectively. Compounds 9 and 10 were proposed to be new compounds as listed in Table 2, because only four EPECs have been reported until now [7,14].
2.3.3. Structural Analysis of DEPECs
According to the above method of identification, compounds 13–19 were assigned as DEPECs. By comparing the retention time and MS spectra with the reference compounds, compounds 16, 18, and 19 were identified as the reference compounds F8~F10, respectively. The other DEPECs were proposed to be new compounds, as only a total of three DEPECs (F8~F10) have been reported until now [10]. The results are shown in Table 2.
2.3.4. Structural Analysis of FTPECs
Taking compounds 22~24, 27 and 30 for example, only benzyl ions (m/z 137 and 121) and chromone moiety ion (m/z 177) were detected, leading to identification of these compounds as FTPECs. All of them showed protonated precursor ions at m/z 313, which meant their molecular weight (MW) was 312. According to Formula (1), the a, b and c values were calculated as 1, 2 and 0, respectively, which meant they have two hydroxyl and one methoxyl substituent group on the whole compound. Then, according to Formula (2), the benzyl ion at m/z 137 was found in compounds 22 and 23, which meant one methoxyl and one hydroxyl substitution occurred on their benzyl moiety, while the benzyl ion at m/z 121 was detected in compounds 24, 27 and 30, which meant one methoxy substitution occurred on their benzyl moiety. Consequently, one hydroxyl substitution occurred on the chromone moiety of compounds 22 and 23, and two hydroxyl substitutions occurred on the chromone moiety of compounds 24, 27 and 30. In particular a chromone moiety ion (m/z 177) was detected in compound 22, which indicated the hydroxyl group should be substituted at the 4′/2′ position of the benzyl moiety. Five FTPECs with MW = 312 have been traced from the literature, so the five FTPECs were tentatively identified as reported compounds. By comparing the retention time and MS spectra with reference compounds, compounds 22, 24 and 27 were identified as F12, F13 and F16, respectively, and the results proved that the above identification method was reasonable.
According to the method of identification, compounds 20–56 were deduced as FTPECs (Table 3), among which nineteen compounds were identified according to the reference compounds.
Finally, fifty-six 2-(2-phenylethyl)chromone derivatives were identified or characterized from the three samples according to the fragmentation behavior and the comparison of retention time and MS spectra with reference compounds (Table 1). A total of 37 (seven DEPECs, four EPECs, three THPECs and 23 FTPECs), 43 (five EPECs, five THPECs and 33 FTPECs), and 29 (one DEPEC, two EPECs, three THPECs and 23 FTPECs) 2-(2-phenylethyl)chromones were identified or characterized in S1, S2 and S3, respectively. The respective relative content of 2-(2-phenylethyl)chromone derivatives was 66.42%, 81.39% and 79.20% in S1, S2 and S3 (Table 4).

Table 4.
The statistical results of 2-(2-phenylethyl)chromone derivatives.
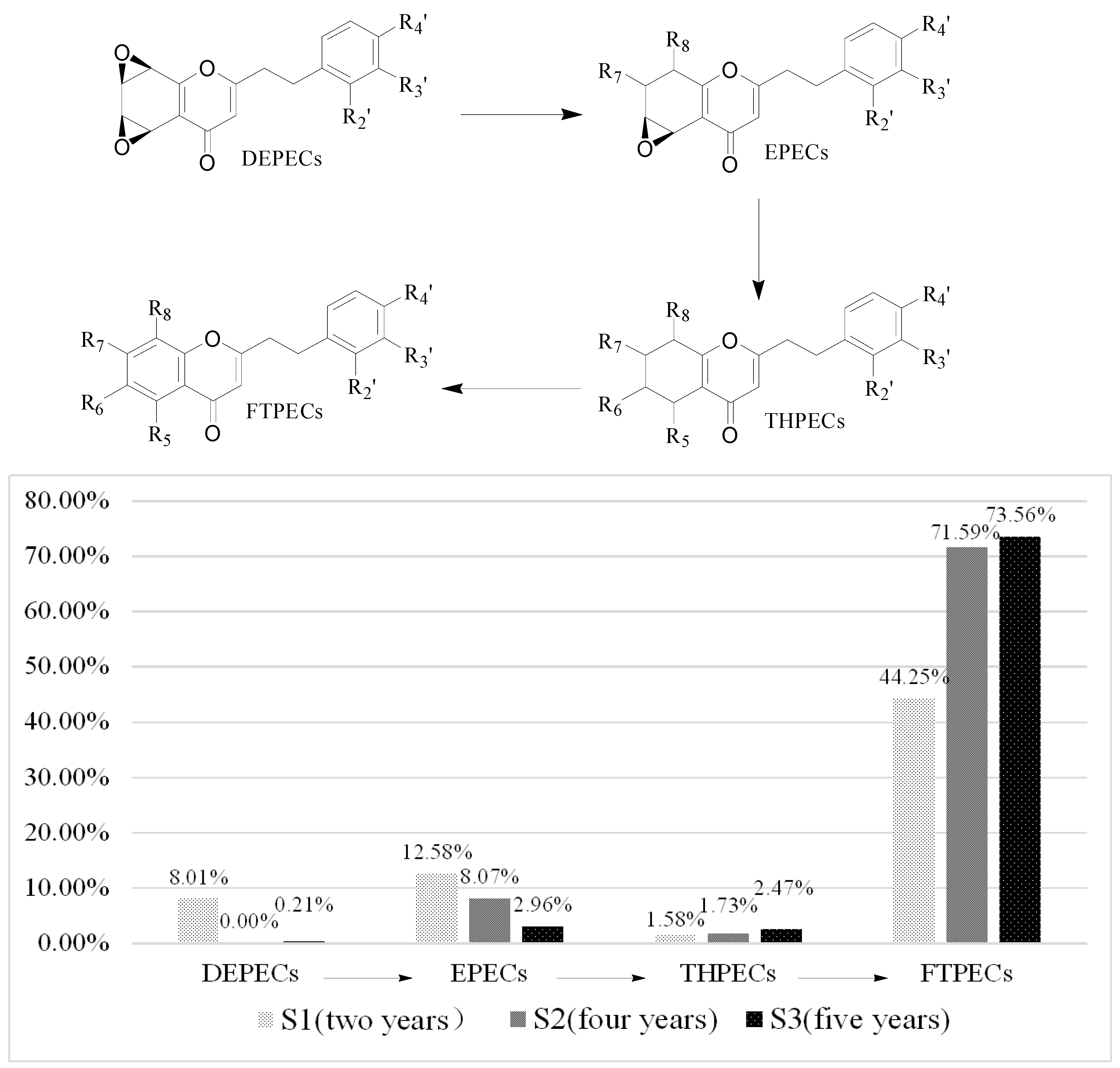
Based on the above data, it was found that the number and relative content of DEPECs (7/8.01%) from S1 was the highest among the three samples, while they were merely traces in S2 and S3 (Table 4, Figure 4). The relative content of EPECs showed a downtrend from S1 to S3 (12.58%, 8.07% and 2.96%, respectively), while the relative content of THPECs and FTPECs showed an uptrend from S1 to S3 (1.58%/1.73%/2.47%, 44.25%/71.59%/73.56%, respectively). It was noticeable that both the total number and relative content of DEPECs, and EPECs decreased obviously (20.59%, 8.07% and 3.17%, respectively) from S1 to S3, and this was consistent with that observed in the HPLC chromatograms (Figure 3), in which the height of peaks for the DEPECs, EPECs (before 43 min) showed a remarkable decreasing trend. This finding was in agreement with the proposed biosynthetic pathways of the four types of chromones (Figure 4) [11], which showed transformations among them during different agarwood formation time. DEPECs have been recognized as precursors, and only accumulated at the early stage of agarwood formation. Then, the next group was EPECs, where the low occurrence rate also suggested they were early intermediates during the agarwood formation. The following group was the highly oxidized THPECs, and the last presented group were FTPECs, both of which were widely distributed in agarwood.
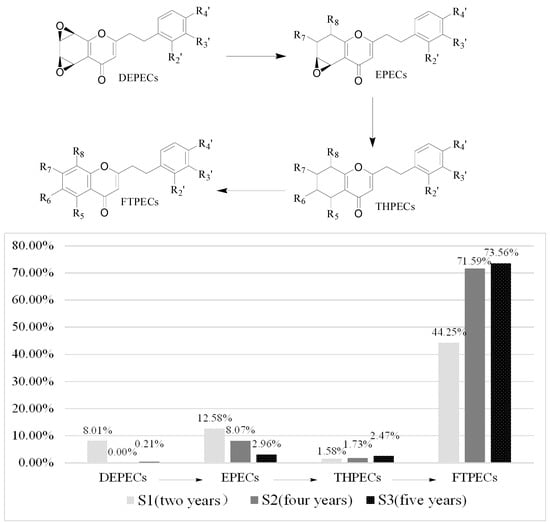
Figure 4.
Biosynthetic progress of chromones and the varieties of the four types of chromones in S1 to S3.
In total, twenty-one 2-(2-phenylethyl)chromones (two EPECs, two THPECs and 17 FTPECs) were detected in all three samples, while ten (six DEPECs, one THPEC and three FTPECs), twelve (one EPEC, three THPECs and eight FTPECs), and one (THPEC) 2-(2-phenylethyl)chromone were only found in S1, S2, and S3, respectively. Furthermore, we found that the relative content of six FTPECs, 6,8-dihydroxy-2-[2-(4-methoxy)phenylethyl]chromone, 6-methoxy-7-hydroxy-2-[2-(4-methoxy)-phenylethyl]chromone, 6-hydroxy-2-(2-phenylethyl)chromone, 6,7-dimethoxy-2-(2-phenylethyl) chromone, 2-[2-(4-methoxy)phenylethyl]chromone, and 2-(2-phenylethyl)chromone, which are reported as the main constituents of agarwood, showed a uptrend from S1 to S3. This finding suggested that the relative content of the six 2-(2-phenylethyl)chromones was the significant factor to evaluate the formation time of agarwood.
3. Experimental Section
3.1. Chemicals and Materials
HPLC-grade acetonitrile (ACN) and methanol were supplied by Tedia (Fairfield, CR, USA). Chromatographic grade absolute formic acid was purchased from Roe Scientific Inc. (Shanxi, HPLC, China). Water was purified using a Milli-Q Plus185 system from Millipore (Milford, MA, USA). The times of artificial holing into the trunk of A. crassna tree were Aug of 2011 (S1), 2009 (S2) and 2008 (S3), respectively, so the agarwood formation times of S1–S3 were 2 years, 4 years and 5 years, respectively (Figure 5). The samples were collected by Dr. Haofu Dai from Guangnan Province of Vietnam at 14 August 2013. Voucher specimens were deposited at Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences.

Figure 5.
Pictures of the three batches of agarwood.
Thirty-one references compounds were isolated and purified from agarwood in our previous work [11,14,15,16,17]. Their structures were identified by spectroscopic methods (MS and NMR), and they are listed in Table 1 and Figure 1. The purity of each compound was determined to be higher than 97% by high-performance liquid chromatography (HPLC).
3.2. Preparation of Sample Solutions and Reference Compound Solutions
In this study, the crushed agarwood (100g, dry weight) was soaked in Et2O (3 × 200 mL) and extracted by ultrasonication (3 × 15 min). The Et2O extracts were filtered and evaporated to get brownish yellow oil. The yields of S1, S2 and S3 were 0.56%, 0.46%, and 2.08%, respectively. Then each extract oil sample was diluted to 1 mg/mL with methanol in a volumetric flask. The solution was filtered through 0.45 µm membranes and stored at 4 °C until use. Accurately weighed samples of thirty-one reference compounds were dissolved in methanol (1.0 mg/mL), respectively, and filtered through 0.45 µm membranes to get the standard solutions. All standard solutions were stored at 4 °C until use.
3.3. HPLC Chromatographic Condition
A Dionex 3000 series HPLC instrument composed of a diode array detector, a vacuum degasser, a quaternary pump and an auto-sampler was used (Bruker Daltonics Inc., Bremen, HPLC/DAD/VD/QP/AS, Germany). The chromatographic separation was carried out on a Dionex-Acclaim 120 C18column (250 mm × 4.6 mm, 5 µm). The mobile phase consisted of acetonitrile (A) and water-acetic acid (99.5:0.5, v/v) (B). The gradient elution program was as follows: 25%–55% A in 0–60 min, 55%–80% A in 60–80 min, 80%–100% A in 80–90 min, and 100% A in 90–95 min. The flow rate was 0.4 mL/min, the column temperature was maintained at 26 °C, and the injection volume was 20 µL. The detection wavelength was set at 254 nm for all the tested compounds.
3.4. MS Spectrometry
MS experiments were performed using an electrospray ionization tandem mass spectrometry system (Amazon SL, Bruker Daltonics Inc., Bremen, Germany) mainly in positive-ion mode. Helium gas was used as the collision gas and high-purity nitrogen gas as the nebulizer and drying gas at flow rates of 0.4 L/min and 6.0 L/min, respectively. The ESI source conditions were as follows: capillary voltage, −4000 V (position); end plate voltage, −500 V (position); drying gas temperature of 250 °C; and nebulizer pressure 15 psi. Scan spectra from m/z 70 to 2200. All the mass data was processed using Bruker Compass Data Analysis 4.0 software (Bruker Daltonics: Germany, 2009).
4. Conclusions
HPLC/ESI-MS/MS is a powerful tool to identify or characterize 2-(2-phenylethyl)chromone derivatives in agarwood. Thirty-one reference compounds including four types of chromones were analyzed by ESI-MS/MS, thus the characteristic fragmentation behavior of DEPECs and EPECs and the methods to distinguish the four types of chromones by their MS characteristic fragmentations were described for the first time, on the basis of the MS spectra of the thirty-one reference compounds. A total of fifty-six 2-(2-phenylethyl)chromone derivatives comprising seven DEPECs, five EPECs, seven THPECs and thirty-seven FTPECs were identified or characterized by their fragmentation pathways and characteristic fragment ions. It was found, with the increase of artificial holing time of agarwood (two years to five years), the total relative content of DEPECs and EPECs showed a downtrend, while the relative content of THPECs and FTPECs showed an increasing trend, which was consistent with the biosynthetic pathway of the formation of agarwood. The relative content of six FTPECs went upward from S1 to S3. This finding could be useful to distinguish agarwood samples with different formation times, and further studies on more agarwood samples with different formation times are needed to validate these conjectures.
Acknowledgments
Supported by Special Fund for Agro-scientific Research in the Public Interest (201303117), Special Fund for The Young Talents’s Science and Technology Project of Hainan Association for Science and Technology (HASI201628), Special Fund for Key Research Project of Hainan Province in 2016 (ZDYF2016210), and Major Technology Project of Hainan Province (ZDKJ2016004).
Author Contributions
The list authors contributed to this work as follows: J.L. Yang performed the HPLC/MS/MS and prepared the manuscript. W.H. Dong, F.D. Kong and G. Liao contributed to the revision of this manuscript. G. Liao and W. Li provided the reference compounds, and J. Wang dealt with the picture. The whole research was performed based on the planning of H.F. Dai and W.L. Mei. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Fragr. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Espinoza, E.O.; Lancaster, C.A.; Kreitals, N.M.; Hata, M.; Cody, R.B.; Blanchette, R.A. Distinguishing wild from cultivated agarwood (Aquilaria spp.) using direct analysis in real time and time of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.L.; Yang, D.L.; Wang, H.; Yang, J.L.; Zeng, Y.B.; Guo, Z.K.; Dong, W.H.; Li, W.; Dai, H.F. Characterization and Determination of 2-(2-Phenylethyl)chromones in Agarwood by GC-MS. Molecules. 2013, 18, 12324–12345. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Wei, J.H.J.S.; Zheng, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical Constituents of Agarwood Originating from the Endemic Genus Aquilaria Plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, C.; Espinoza, E. Evaluating agarwood products for 2-(2-phenylethyl)chromones using direct analysis in real time time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Xia, B.; Jiang, Y.; Mei, W.L.; Kuckc, D. Fragmentation of protonated 2-(2-phenylethyl)chromones from agarwood: The diagnostic role of ion/neutral complexes as reactive intermediates. Eur. J. Mass Spectrom. 2015, 21, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Bo, W.; Sung, W.K.; Gwi, S.H.; Jeong, H.P. Eight New 2-(2-Phenylethyl)chromone (¼2-(2-Phenylethyl)-4H-1-benzopyran-4-one) Derivatives from Aquilaria malaccensis Agarwood. Helv. Chim. Acta 2012, 95, 1657–1665. [Google Scholar] [CrossRef]
- Dai, H.F.; Liu, J.; Zeng, Y.B.; Han, Z.; Wang, H.; Mei, W.L. A New 2-(2-Phenylethyl)Chromone from Chinese Eaglewood. Molecules 2009, 14, 5165–5168. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, Z.R.; Chai, X.Y.; Zeng, K.W.; Jia, Y.X.; Bi, D.; Ma, Z.Z.; Tu, P.F. Nine 2-(2-Phenylethyl)chromone Derivatives from the Resinous Wood of Aquilaria sinensis and Their Inhibition of LPS-Induced NO Production in RAW 264.7 Cells. Eur. J. Org. Chem. 2012, 2012, 5389–5397. [Google Scholar] [CrossRef]
- Yagura, T.; Shibayama, N.; Ito, M.; Kiuchib, F.; Honda, G. Three novel diepoxy tetrahydrochromones from agarwood artificially produced by intentional wounding. Tetrahedron Lett. 2005, 46, 4395–4398. [Google Scholar] [CrossRef]
- Wei, L.; Cai, H.C.; Wen, H.D.; Zhi, K.G.; Hao, W.; Wen, L.M.; Hao, F.D. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar]
- Xia, B.; Li, J.R.; Yang, D.L.; Mei, W.L.; Ding, L.S.; Zhou, Y. A rapid and highly specific method to evaluate the presence of 2-(2-phenylethyl)chromones in agarwood by supercritical fluid chromatography-mass spectrometry. Eur. J. Mass Spectrom. 2014, 20, 395–402. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Jiang, Y.; Zhang, Q.; Zhang, L.; Tu, P.F. Identification and quantification of 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones in Chinese eaglewood by HPLC with diode array detection and MS. J. Sep. Sci. 2013, 36, 3733–3740. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, J.; Zhao, Y.X.; De, Y.Y.; Mei, W.L.; Dai, H.F. A new cytotoxic 2-(2-phenylethyl)chromone from Chinese eaglewood. Chin. Chem. Lett. 2008, 9, 934–936. [Google Scholar] [CrossRef]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in Chinese Agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.F.; Liu, J.; Han, Z.; Zeng, Y.B.; Wang, H. Two new 2-(2-phenylethyl)chromones from Chinese eaglewood. J. Asian Nat. Prod. Res. 2010, 12, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Dong, W.H.; Mei, W.L.; Dai, H.F. A new 2-(2-phenylethyl)chromone derivative in Chinese agarwood ‘Qi-Nan’ from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2014, 16, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).