Functional Characterization of Individual- and Mixed-Burgundian Saccharomyces cerevisiae Isolates for Fermentation of Pinot Noir
Abstract
:1. Introduction
2. Results and Discussion
2.1. Genetic and Phenotypic Characterization of Wine Yeast Strains

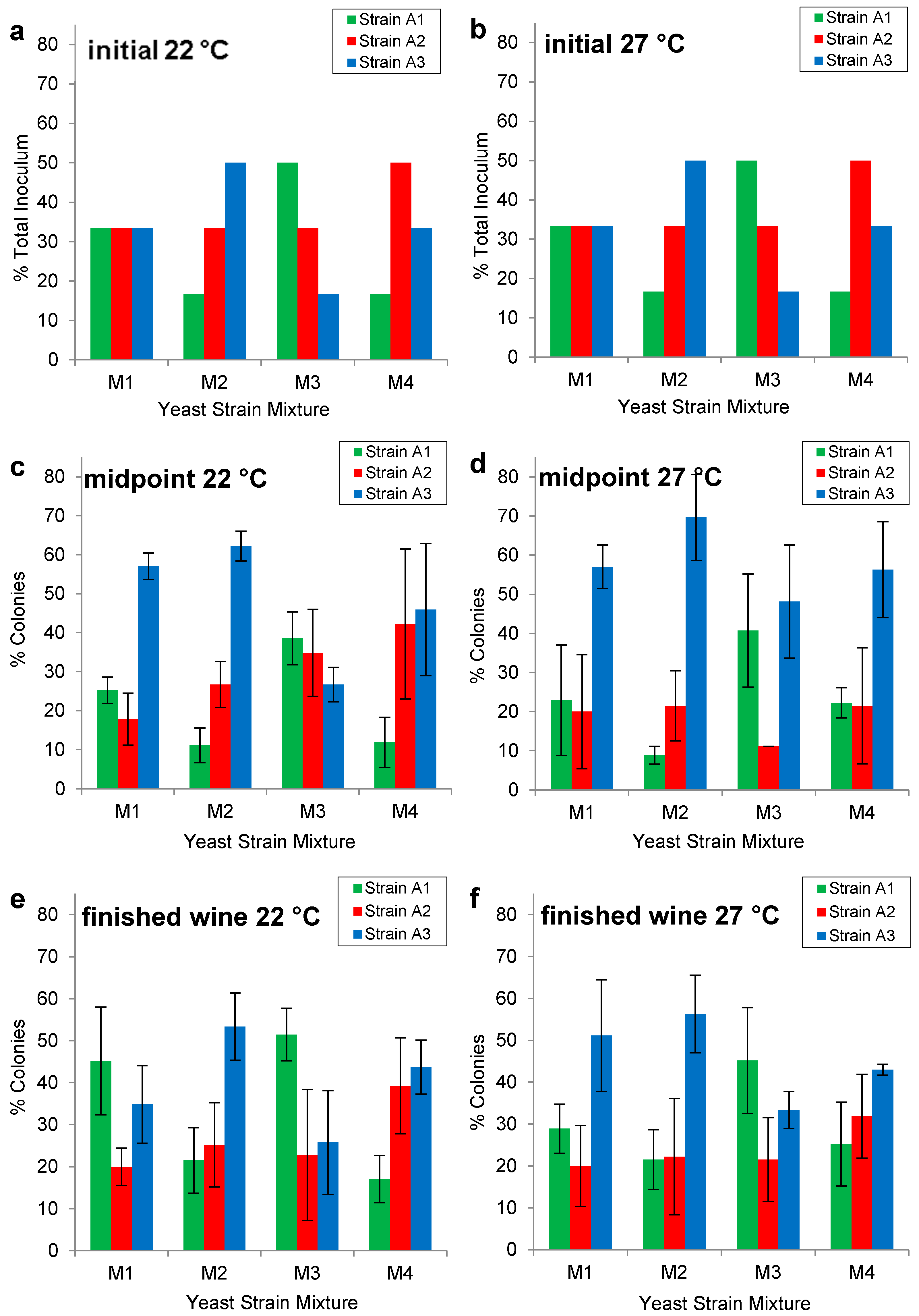
2.2. Enological Characterization of Wine Yeast Strains
| Yeast | Glycerol (g·L−1) | p c | Acetic Acid (g·L−1) | p | Ethanol Tolerance d | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 8.79 d | 10.02 d | *** | 0.124 b | 0.187 cde | *** | 17.68 a | 15.42 a | *** |
| AWRI796 | 9.34 e | 10.64 e | * | 0.132 c | 0.179 bcd | * | 18.30 bc | 17.66 ef | ** | |
| BGY | 8.80 d | 9.65 bcd | ** | 0.337 j | 0.411 h | ** | 18.21 b | 16.32 b | *** | |
| RA17 | 8.43 bc | 9.31 ab | * | 0.224 h | 0.292 g | *** | 18.56 cd | 17.59 e | *** | |
| RC212 | 7.94 a | 9.12 a | ** | 0.246 i | 0.250 f | ns | 18.90 e | 17.58 e | *** | |
| Individual Burgundian | A1 | 8.49 bcd | 9.25 a | ** | 0.119 a | 0.142 a | ** | 18.20 b | 17.56 de | *** |
| A2 | 9.19 e | 9.63 bc | ** | 0.209 g | 0.240 f | * | 18.73 de | 17.35 c | *** | |
| A3 | 8.44 bc | 9.41 ab | *** | 0.135 c | 0.167 b | * | 18.15 b | 17.42 cd | *** | |
| Mixed Burgundian | M1 (1:1:1) | 8.37 b | 9.33 ab | *** | 0.147 d | 0.187 cde | ** | 18.55 cd | 17.75 f | *** |
| M2 (1:2:3) | 8.46 bc | 9.85 cd | ** | 0.160 e | 0.194 de | ns | 18.40 bc | 17.64 ef | *** | |
| M3 (3:2:1) | 8.48 bcd | 9.37 ab | ** | 0.149 d | 0.171 bc | ** | 18.57 cd | 17.72 ef | *** | |
| M4 (1:3:2) | 8.72 cd | 9.65 bcd | ** | 0.177 f | 0.200 e | ** | 18.50 cd | 17.75 f | * | |
| Range | 7.94–9.34 | 9.12–10.64 | 0.119–0.337 | 0.142–0.411 | 17.68–18.73 | 15.42–17.75 | ||||
| Yeast | Final Optical Density e | p | Foam Height f (mm) | p | Sulfur Dioxide f (mg·L−1) | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 1.494 a | 1.667 a | ** | 9.7 a | 7.3 a | ns | 15.0 ab | 12.5 ab | ns |
| AWRI796 | 1.938 d | 1.914 bc | ns | 10.0 a | 18.0 b | * | 9.2 a | 12.1 ab | ns | |
| BGY | 1.658 b | 1.841 b | ns | 34.0 b | 28.0 de | ns | 9.9 a | 6.4 a | ns | |
| RA17 | 1.931 d | 1.992 c | *** | 13.3 a | 20.3 bc | ** | 24.8 c | 50.3 f | ** | |
| RC212 | 1.940 d | 1.989 c | * | 16.3 a | 24.0 cd | ns | 36.5 d | 28.4 cd | ns | |
| Individual Burgundian | A1 | n/a | n/a | 16.0 a | 28.3 de | ns | 26.1 c | 40.9 ef | *** | |
| A2 | 1.819 c | 1.831 b | ns | 15.7 a | 32.3 e | *** | 32.6 d | 30.4 de | ns | |
| A3 | 1.861 cd | 1.979 c | ** | 16.3 a | 20.3 bc | ns | 20.6 bc | 18.2 bc | ns | |
| Mixed Burgundian | M1-M4 | n/a | n/a | n/a | n/a | n/a | n/a | |||
| Range | 1.494–1.938 | 1.667–1.992 | 9.7–34.0 | 7.3–32.3 | 9.2–36.5 | 6.4–50.3 | ||||
2.3. Analysis of Volatile Compounds
| Yeast | 1,3-butanediol | p c | 2,3-butanediol | p | 2-methyl-1-butanol | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 3.150 bcde | 7.216 e | * | 0.929 bcde | 2.001 e | * | 1.693 a | 2.024 a | ns |
| AWRI796 | 2.651 abcde | 5.585 cd | ** | 0.854 abcd | 1.497 cd | * | 2.089 bcd | 2.416 bcd | ns | |
| BGY | 2.773 abcde | 4.336 ab | ** | 0.913 bcde | 1.213 abc | ** | 2.433 ef | 2.481 cd | ns | |
| RA17 | 3.359 de | 5.219 bcd | * | 1.000 cde | 1.405 bcd | * | 1.951 ab | 2.392 bcd | ns | |
| RC212 | 2.392 abcd | 3.886 a | *** | 0.773 abc | 1.050 a | ** | 2.053 bc | 2.674 d | ** | |
| Individual Burgundian | A1 | 2.112 a | 4.226 ab | *** | 0.669 a | 1.166 ab | *** | 2.357 def | 2.282 abc | ns |
| A2 | 2.317 abc | 4.663 abc | ** | 0.790 abc | 1.322 abcd | ** | 2.315 cdef | 2.140 ab | ns | |
| A3 | 2.239 ab | 3.818 a | *** | 0.748 ab | 1.057 a | *** | 2.090 bcd | 2.074 a | ns | |
| Mixed Burgundian | M1 (1:1:1) | 3.610 e | 5.893 d | * | 1.110 e | 1.524 d | ns | 2.306 cdef | 2.309 abc | ns |
| M2 (1:2:3) | 3.554 e | 5.801 d | * | 1.087 de | 1.549 d | * | 2.265 cde | 2.484 cd | * | |
| M3 (3:2:1) | 3.234 cde | 5.734 cd | ** | 0.977 bcde | 1.510 cd | ** | 2.330 cdef | 2.278 abc | ns | |
| M4 (1:3:2) | 3.575 e | 5.187 bcd | * | 1.075 de | 1.343 abcd | ns | 2.580 f | 2.299 abc | ns | |
| Range | 2.112–3.651 | 3.818–7.216 | 0.669–1.110 | 1.050–2.001 | 1.693–2.580 | 2.024–2.674 | ||||
| Yeast | 3-methyl-1-butanol | p | Butanol | p | 1-hexanol | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 8.229 a | 9.309 a | ns | 0.185 cd | 0.270 cd | *** | 2.206 ab | 2.520 | ns |
| AWRI796 | 9.840 bcd | 10.815 bcd | ns | 0.169 bc | 0.317 e | *** | 2.413 abc | 2.354 | ns | |
| BGY | 10.949 de | 11.003 cd | ns | 0.169 bc | 0.212 a | * | 2.649 cd | 2.448 | ns | |
| RA17 | 9.486 b | 11.004 cd | ns | 0.157 ab | 0.225 ab | ** | 2.164 a | 2.275 | ns | |
| RC212 | 9.605 bc | 11.843 d | * | 0.263 f | 0.517 f | *** | 2.214 ab | 2.600 | ns | |
| Individual Burgundian | A1 | 11.518 e | 10.888 bcd | ns | 0.147 a | 0.227 ab | *** | 2.499 bc | 2.096 | * |
| A2 | 10.736 cde | 9.745 ab | * | 0.208 e | 0.280 cd | * | 2.497 abc | 2.110 | * | |
| A3 | 9.921 bcd | 9.360 a | ns | 0.189 cde | 0.290 de | *** | 2.351 abc | 2.005 | * | |
| Mixed Burgundian | M1 (1:1:1) | 10.693 bcde | 10.648 bcd | ns | 0.184 cd | 0.271 cd | *** | 2.54 bcd | 2.171 | ns |
| M2 (1:2:3) | 10.492 bcde | 11.197 cd | * | 0.194 de | 0.254 bc | * | 2.487 abc | 2.393 | ns | |
| M3 (3:2:1) | 10.828 cde | 10.541 abc | ns | 0.171 bc | 0.272 cd | *** | 2.533 bcd | 2.151 | ns | |
| M4 (1:3:2) | 11.712 e | 10.209 abc | ns | 0.203 de | 0.261 cd | * | 2.841 d | 2.187 | * | |
| Range | 8.229–11.712 | 9.309–11.843 | 0.169–0.263 | 0.227–0.317 | 2.164–2.841 | 2.005–2.600 | ||||
| Yeast | Isobutanol | p | Phenylethanol | p | Propanol | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 31.380 a | 28.825 a | ns | 0.472 a | 0.827 | ** | 12.845 f | 11.804 e | ns |
| AWRI796 | 50.592 bc | 53.129 b | ns | 0.709 cd | 0.783 | ns | 11.198 de | 12.111 e | ns | |
| BGY | 71.731 d | 72.157 d | ns | 0.680 bcd | 0.829 | ns | 8.654 ab | 7.456 a | ns | |
| RA17 | 53.548 bc | 66.743 cd | ns | 0.565 ab | 0.745 | ns | 8.179 a | 7.837 ab | ns | |
| RC212 | 72.158 d | 110.846 f | ** | 0.570 ab | 0.732 | * | 8.221 a | 9.086 cd | ns | |
| Individual Burgundian | A1 | 62.119 cd | 87.520 e | ** | 0.652 bc | 0.672 | ns | 13.487 f | 14.164 f | ns |
| A2 | 44.142 b | 55.413 b | ns | 0.623 bc | 0.618 | ns | 9.555 bc | 9.217 cd | ns | |
| A3 | 43.416 ab | 51.732 b | * | 0.632 bc | 0.641 | ns | 8.815 ab | 7.767 ab | * | |
| Mixed Burgundian | M1 (1:1:1) | 58.954 c | 56.836 bc | ns | 0.645 bc | 0.682 | ns | 10.984 de | 9.908 d | ns |
| M2 (1:2:3) | 56.976 c | 56.554 bc | ns | 0.636 bc | 0.746 | ns | 9.989 c | 8.589 bc | ns | |
| M3 (3:2:1) | 60.020 cd | 58.112 bc | ns | 0.713 cd | 0.661 | ns | 11.775 e | 11.122 e | ns | |
| M4 (1:3:2) | 53.698 bc | 51.769 b | ns | 0.804 d | 0.673 | ns | 10.258 cd | 8.380 abc | ** | |
| Range | 31.380–72.158 | 28.825–87.520 | 0.472–0.804 | 0.661–0.829 | 8.179–12.845 | 7.456–14.164 | ||||
| Yeast | Ethyl butanoate | p c | Ethyl decanoate | p | Ethyl hexanoate | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 2.689 ab | 1.707 a | *** | 0.031 d | 0.020 ab | ** | 0.060 d | 0.040 ab | *** |
| AWRI796 | 3.232 cde | 2.327 bc | ns | 0.025 cd | 0.024 abc | ns | 0.059 cd | 0.043 bcd | * | |
| BGY | 2.368 a | 2.193 abc | ns | 0.021 bc | 0.017 a | ns | 0.046 ab | 0.036 a | * | |
| RA17 | 2.862 bcd | 2.180 abc | *** | 0.022 bc | 0.024 abc | ns | 0.051 abc | 0.041 abc | ** | |
| RC212 | 3.181 cde | 2.116 abc | * | 0.024 c | 0.023 abc | ns | 0.052 abc | 0.040 abc | ns | |
| Individual Burgundian | A1 | 3.262 de | 3.131 e | ns | 0.025 cd | 0.034 e | * | 0.054 cd | 0.050 d | ns |
| A2 | 2.286 a | 2.107 ab | ns | 0.015 ab | 0.031 cde | *** | 0.046 ab | 0.046 cd | ns | |
| A3 | 3.387 e | 2.893 de | * | 0.023 c | 0.026 bcde | ns | 0.053 bcd | 0.041 abc | ** | |
| Mixed Burgundian | M1 (1:1:1) | 3.275 de | 3.080 e | ns | 0.024 c | 0.030 cde | ns | 0.054 cd | 0.048 d | ns |
| M2 (1:2:3) | 3.316 e | 2.626 cde | ns | 0.024 c | 0.024 abc | ns | 0.053 bcd | 0.042 abc | ns | |
| M3 (3:2:1) | 3.252 de | 3.117 e | ns | 0.025 cd | 0.033 de | ns | 0.054 cd | 0.050 d | ns | |
| M4 (1:3:2) | 2.826 bc | 2.494 bcd | ns | 0.013 a | 0.025 bcd | ns | 0.045 a | 0.041 abc | ns | |
| Range | 2.368–3.316 | 1.707–3.131 | 0.013–0.031 | 0.017–0.034 | 0.045–0.060 | 0.036–0.050 | ||||
| Yeast | Ethyl lactate | p | Ethyl laurate | p | Ethyl octanoate | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 0.548 a | 0.682 b | * | 0.006 def | 0.004 a | ** | 0.068 d | 0.035 a | *** |
| AWRI796 | 0.847 ab | 0.882 cd | ns | 0.007 ef | 0.008b cd | ns | 0.053 bc | 0.040 ab | ns | |
| BGY | 1.773 c | 0.671 b | ns | 0.004 bcd | 0.004 a | ns | 0.046 abc | 0.033 a | ns | |
| RA17 | 0.631 ab | 0.726 b | ns | 0.004 abc | 0.005 ab | ns | 0.047 abc | 0.040 ab | ns | |
| RC212 | 0.753 ab | 0.939 d | ** | 0.005 cd | 0.006 ab | ns | 0.046 abc | 0.035 a | ns | |
| Individual Burgundian | A1 | 0.973 b | 0.936 d | ns | 0.007 f | 0.009 cd | ** | 0.057 cd | 0.052 cd | ns |
| A2 | 0.749 ab | 0.671 b | ns | 0.002 ab | 0.007 bc | ** | 0.043 ab | 0.052 cd | *** | |
| A3 | 0.724 ab | 0.719 b | ns | 0.004 abc | 0.006 ab | * | 0.055 bc | 0.040 ab | * | |
| Mixed Burgundian | M1 (1:1:1) | 0.817 ab | not quantifiable | *** | 0.005 cd | 0.009 d | * | 0.057 cd | 0.048 bcd | ns |
| M2 (1:2:3) | 0.780 ab | 0.799 bc | ns | 0.004 cd | 0.007 bcd | ns | 0.056 cd | 0.040 ab | ns | |
| M3 (3:2:1) | 0.857 ab | 0.766 bc | ns | 0.005 cde | 0.009 d | * | 0.057 cd | 0.055 d | ns | |
| M4 (1:3:2) | 0.902 ab | 0.698 b | ns | 0.002 a | 0.006 b | * | 0.036 a | 0.042 abc | ns | |
| Range | 0.548–1.773 | 0.671–0.939 | 0.002–0.007 | 0.004–0.009 | 0.036–0.068 | 0.035–0.055 | ||||
| Yeast | Ethyl palmitate | p | ||||||||
| Strain or Isolate | 22 °C | 27 °C | ||||||||
| Industrial | AMH | 0.045 a | 0.054 a | ns | ||||||
| AWRI796 | 0.118 ef | 0.133 d | ns | |||||||
| BGY | 0.072 abc | 0.083 b | ns | |||||||
| RA17 | 0.068 abc | 0.101 bc | * | |||||||
| RC212 | 0.103 def | 0.143 d | ns | |||||||
| Individual Burgundian | A1 | 0.123 f | 0.145 d | ns | ||||||
| A2 | 0.056 ab | 0.101 bc | * | |||||||
| A3 | 0.090 cde | 0.101 bc | ns | |||||||
| Mixed Burgundian | M1 (1:1:1) | 0.105 ef | 0.139 d | ns | ||||||
| M2 (1:2:3) | 0.108 ef | 0.120 cd | ns | |||||||
| M3 (3:2:1) | 0.114 ef | 0.127 cd | ns | |||||||
| M4 (1:3:2) | 0.077 bcd | 0.090 b | ns | |||||||
| Range | 0.045–0.123 | 0.054–0.133 | ||||||||
| Yeast | Ethyl acetate | pc | Hexyl acetate | p | Isoamyl acetate | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 10.039 bc | 8.968 ab | ns | 0.029 | 0.017 a | ns | 0.203 abcd | 0.148 a | ns |
| AWRI796 | 9.889 bc | 10.191 bcd | ** | 0.022 | 0.020 ab | ns | 0.188 abc | 0.213 bcd | ns | |
| BGY | 10.083 bc | 9.636 abc | ns | 0.016 | 0.018 a | ns | 0.171 a | 0.184 abc | ns | |
| RA17 | 9.944 bc | 10.111 bcd | * | 0.027 | 0.027 abcd | ns | 0.228 bcd | 0.255 def | ns | |
| RC212 | 8.824 a | 8.615 a | ns | 0.022 | 0.016 a | ns | 0.190 abc | 0.178 ab | ns | |
| Individual Burgundian | A1 | 10.467 c | 11.271 de | ns | 0.024 | 0.036 d | ns | 0.235 cd | 0.320 g | * |
| A2 | 9.335 ab | 10.342 bcd | * | 0.015 | 0.033 cd | *** | 0.167 a | 0.267 defg | *** | |
| A3 | 10.077 bc | 9.342 ab | ns | 0.025 | 0.025 abcd | ns | 0.211 abcd | 0.222 bcd | ns | |
| Mixed Burgundian | M1 (1:1:1) | 10.613 c | 11.471 de | ns | 0.027 | 0.030 bcd | ns | 0.234 cd | 0.291 efg | ns |
| M2 (1:2:3) | 10.850 c | 10.994 cde | * | 0.027 | 0.020 ab | ns | 0.237 d | 0.225 bcd | ns | |
| M3 (3:2:1) | 10.599 c | 11.807 e | ns | 0.027 | 0.032 cd | ns | 0.241 d | 0.305 fg | ns | |
| M4 (1:3:2) | 10.360 c | 9.751 abc | ns | 0.016 | 0.024 abc | ns | 0.182 ab | 0.241 cde | ns | |
| Range | 8.824–10.850 | 8.615–11.807 | 0.016–0.029 | 0.016–0.033 | 0.167–0.241 | 0.148–0.320 | ||||
| Yeast | Isobutyl acetate | p | Methyl acetate | p | Acetaldehyde | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 0.00091 a | 0.00071 a | ** | 0.889 a | 0.784 a | ns | 1.026 cd | 0.967 bcd | ns |
| AWRI796 | 0.00105 ab | 0.00137 b | ns | 1.245 cd | 1.041 bc | ns | 0.504 a | 0.614 a | * | |
| BGY | 0.00148 de | 0.00187 cd | ns | 1.105 bc | 1.077 bc | ns | 0.670 a | 0.690 ab | ns | |
| RA17 | 0.00125 bcd | 0.00194 cde | ns | 0.996 ab | 0.932 ab | ns | 1.339 e | 1.357 ef | ns | |
| RC212 | 0.00158 e | 0.00202 cde | ** | 1.304 d | 1.126 cd | ns | 0.871 bc | 0.803 ab | ns | |
| Individual Burgundian | A1 | 0.00155 e | 0.00286 f | ns | 1.311 d | 1.175 cde | ns | 0.839 b | 0.947 bcd | ns |
| A2 | 0.00096 a | 0.00165 bc | ** | 0.946 a | 1.017 bc | ns | 0.979 bcd | 1.156 cde | ns | |
| A3 | 0.00111 abc | 0.00159 bc | ns | 1.196 cd | 1.006 bc | * | 0.901 bcd | 0.737 ab | ns | |
| Mixed Burgundian | M1 (1:1:1) | 0.00133 cde | 0.00215 de | ** | 1.183 cd | 1.252 de | ns | 0.923 bcd | 1.377 ef | *** |
| M2 (1:2:3) | 0.00133 cde | 0.00176 bcd | * | 1.198 cd | 1.335 e | ns | 1.009 cd | 1.538 f | ns | |
| M3 (3:2:1) | 0.00140 de | 0.00235 e | ** | 1.192 cd | 1.333 e | ns | 1.049 d | 1.245 def | ns | |
| M4 (1:3:2) | 0.00104 ab | 0.00166 bc | ns | 1.172 cd | 1.065 bc | ns | 0.937 bcd | 0.939 bc | ns | |
| Range | 0.0009–0.00155 | 0.00071–0.00235 | 0.889–1.311 | 0.784–1.335 | 0.504–1.339 | 0.737–1.538 | ||||
| Yeast | Benzaldehyde | p | Acetic acid | p | 1,1-diethoxyacetal | p | ||||
| 22 °C | 27 °C | 22 °C | 27 °C | 22 °C | 27 °C | |||||
| Industrial | AMH | 0.029 ab | 0.056 abcd | * | 0.387 a | 0.427 ab | ns | 2.833 b | 3.357 d | ns |
| AWRI796 | 0.056 fg | 0.061 bcdef | ns | 0.394 a | 0.375 a | ** | 2.049 a | 2.364 b | ns | |
| BGY | 0.045 de | 0.056 abcd | * | 0.662 b | 0.658 cd | ns | 1.885 a | 2.808 c | ns | |
| RA17 | 0.063 g | 0.068 f | ns | 0.450 a | 0.864 d | ns | 4.677 e | 4.827 f | ns | |
| RC212 | 0.050 ef | 0.063 def | ** | 0.480 a | 0.693 cd | ns | 3.210 bcd | 2.750 c | ns | |
| Individual Burgundian | A1 | 0.034 bc | 0.055 abc | *** | 0.332 a | 0.586 bc | ns | 3.303 bcd | 3.569 d | ns |
| A2 | 0.026 a | 0.053 ab | ** | 0.439 a | 0.347 a | ns | 3.362 bcd | 3.336 d | ns | |
| A3 | 0.025 a | 0.052 a | *** | 0.356 a | 0.847 d | ns | 3.169 bc | 2.373 b | ns | |
| Mixed Burgundian | M1 (1:1:1) | 0.039 cd | 0.064 ef | *** | 0.445 a | 0.726 cd | ns | 3.294 bcd | not quantifiable | ns |
| M2 (1:2:3) | 0.037 cd | 0.062 cde | ** | 0.415 a | 0.533 abc | ** | 3.780 cd | not quantifiable | ns | |
| M3 (3:2:1) | 0.043 de | 0.057 abc | * | 0.342 a | 0.586 bc | ** | 3.909 d | 4.163 e | ns | |
| M4 (1:3:2) | 0.050 ef | 0.059 abc | ns | 0.383 a | 0.629 bc | * | 3.601 cd | 3.498 d | ns | |
| Range | 0.029–0.063 | 0.052–0.068 | 0.332–0.662 | 0.347–0.864 | 1.885–4.677 | 2.364–4.827 | ||||
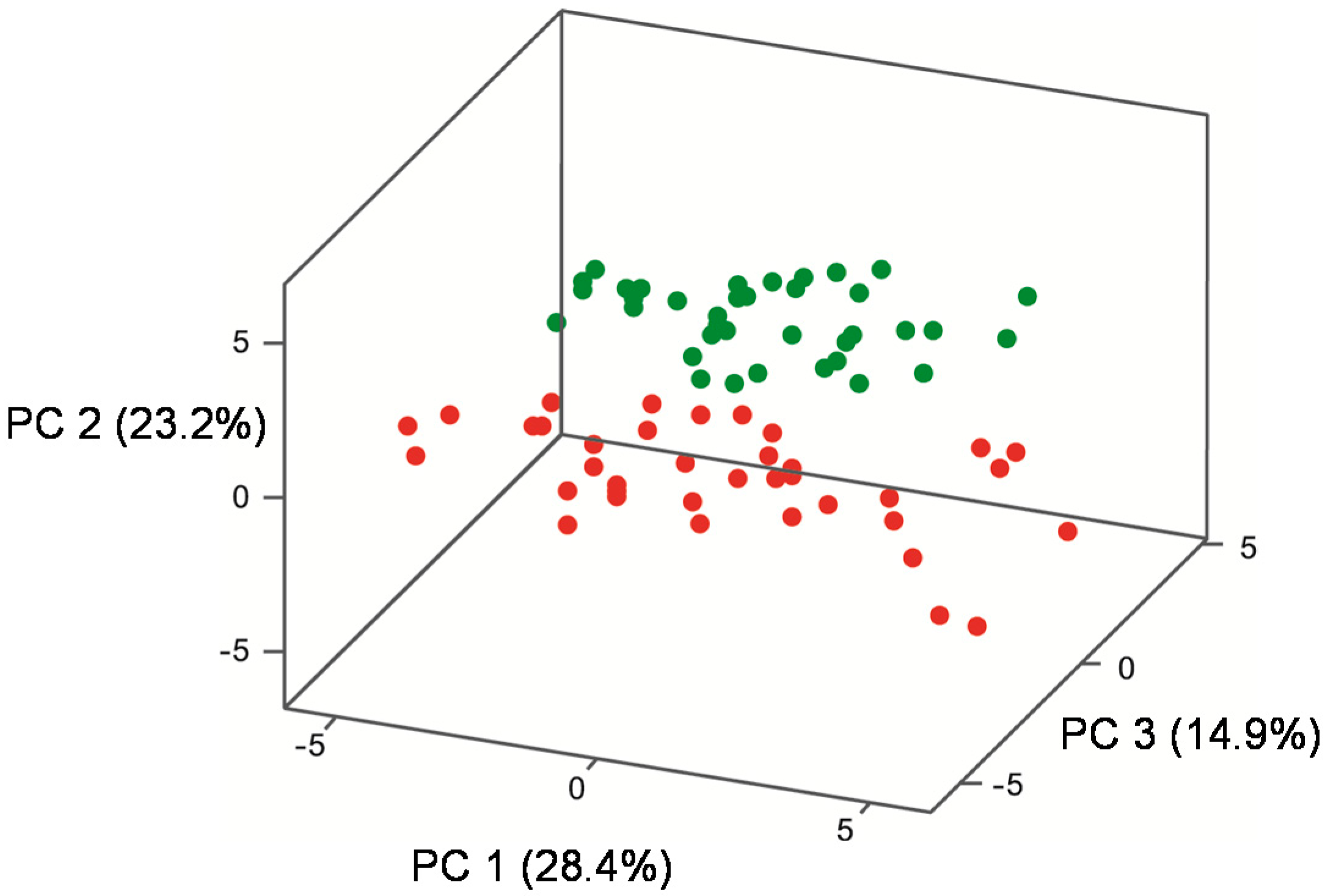
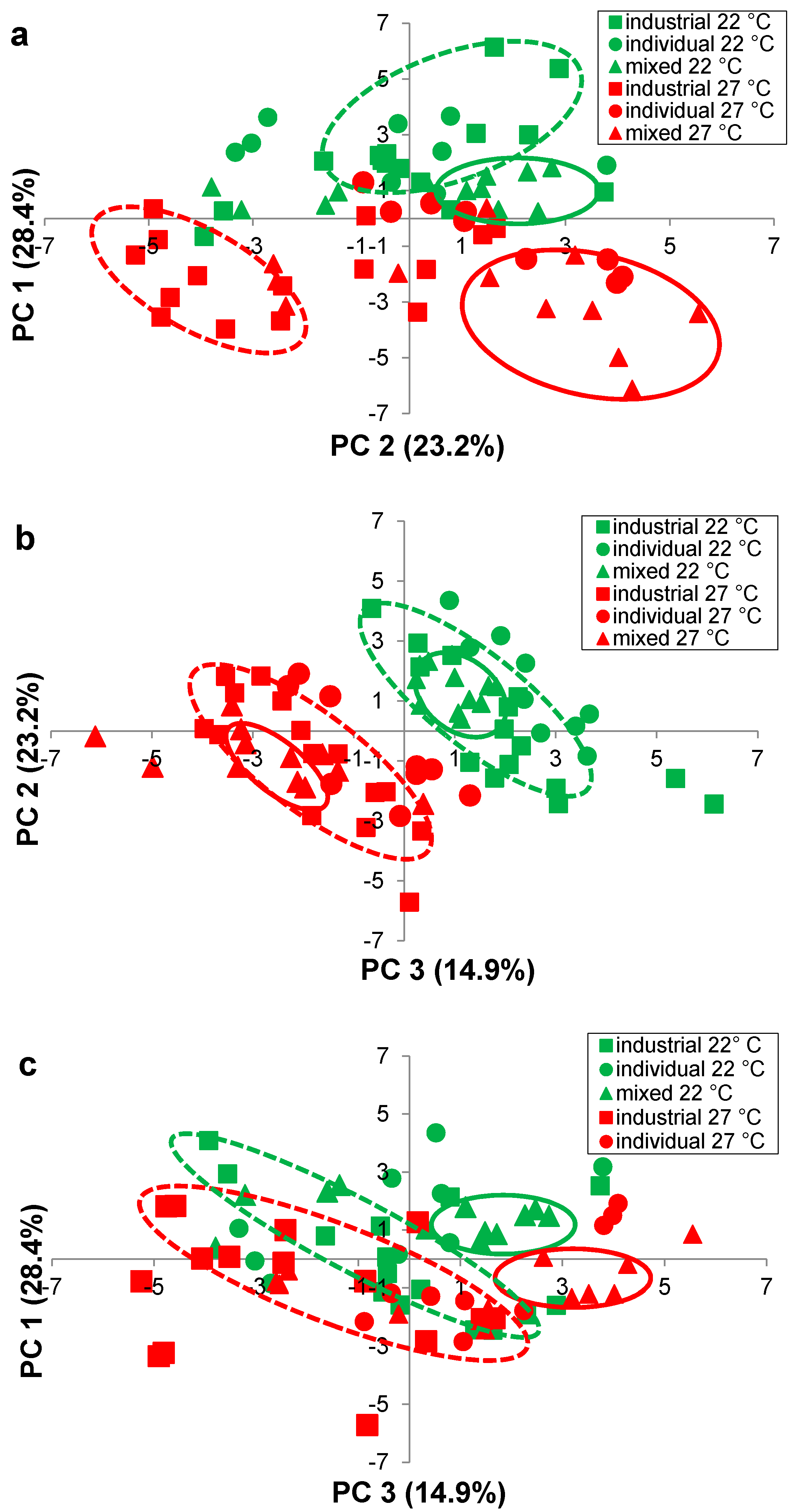
| Quadrant in Figure 4a | Volatile Compound | Volatile Class | Loading a PC 1 (x) 28.4% | Loading a PC 2 (y) 23.2% | Loading a PC 3 (z) 14.9% | Temperature that volatile predominates |
|---|---|---|---|---|---|---|
| 1 | Propanol | Higher alcohol | 1.10 | 0.20 | 1.02 | Inconclusive |
| 1 | Ethyl octanoate | Ethyl ester | 2.11 | 1.25 | 0.72 | 22 °C |
| 1 | Ethyl hexanoate | Ethyl ester | 1.76 | 1.44 | 1.29 | 22 °C |
| 1 | Ethyl butanoate | Ethyl ester | 1.86 | 0.62 | 1.74 | 22 °C |
| 1 | 1,1-Diethyoxyacetal | Acetal | 0.17 | 0.82 | 0.10 | 22 °C |
| 3 | 2,3-Butanediol | Higher alcohol | −0.03 | −1.98 | −1.93 | 27 °C |
| 3 | Isobutanol | Higher alcohol | −0.24 | −1.35 | 1.83 | 27 °C |
| 3 | Butanol | Higher alcohol | −0.42 | −1.70 | −0.49 | 27 °C |
| 3 | 3-Methyl-1-butanol | Higher alcohol | −0.88 | −1.69 | 2.38 | 27 °C |
| 3 | 2-Methyl-1-butanol | Higher alcohol | −1.19 | −1.88 | 1.96 | 27 °C |
| 3 | Phenylethanol | Higher alcohol | −1.23 | −1.86 | 0.70 | 27 °C |
| 3 | 1-Hexanol | Higher alcohol | −1.62 | −0.23 | 2.07 | Inconclusive |
| 3 | Ethyl lactate | Ethyl ester | −0.80 | −0.03 | 2.09 | Inconclusive |
| 3 | Benzaldehyde | Aldehyde | −0.03 | −2.22 | −1.09 | 27 °C |
| 3 | Acetic acid | Acid | −0.14 | −1.88 | −1.08 | 27 °C |
| 4 | 1,3-Butanediol | Higher alcohol | 0.10 | −2.10 | −1.97 | 27 °C |
| 4 | Ethyl laurate | Ethyl ester | 1.93 | −1.46 | 0.00 | 27 °C |
| 4 | Ethyl decanoate | Ethyl ester | 2.35 | −0.43 | −0.41 | 27 °C |
| 4 | Ethyl palmitate | Ethyl ester | 1.00 | −1.87 | 1.48 | 27 °C |
| 4 | Methyl acetate | Acetate ester | 1.01 | −0.96 | 2.45 | 27 °C |
| 4 | Ethyl acetate | Acetate ester | 1.95 | −0.96 | 0.73 | 27 °C |
| 4 | Hexyl acetate | Acetate ester | 2.45 | −0.02 | −0.51 | Inconclusive |
| 4 | Isobutyl acetate | Acetate ester | 1.36 | −1.89 | 0.42 | 27 °C |
| 4 | Isoamyl acetate | Acetate ester | 2.31 | −0.95 | −0.07 | 27 °C |
| 4 | Acetaldehyde | Aldehyde | 1.10 | −0.90 | −1.12 | 27 °C |
3. Experimental Section
3.1. Yeast and Bacterial Strains Employed
3.2. Media and Culture Conditions
3.3. Genetic Fingerprinting and Monitoring of Mixed Strains During Fermentation
3.4. Killer Factor Phenotyping
3.5. Model Fermentations—Fermentation Characteristics
3.6. Growth Phenotype Assay
3.7. Foam Production Assay
3.8. Quantification of Compounds Using HPLC
3.9. Identification and Quantification of Volatile Compounds Using GC-MS
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Husnik, J.; Delaquis, P.A.; Cliff, M.A.; van Vuuren, H.J.J. Functional analysis of the malolactic wine yeast ML01. Am. J. Enol. Vitic. 2007, 58, 42–52. [Google Scholar]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Influence of sequential yeast mixtures on wine fermentation. Int. J. Food Microbiol. 2005, 98, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef] [PubMed]
- King, E.S.; Swiegers, J.H.; Travis, B.; Francis, I.L.; Bastian, S.E.P.; Pretorius, I.S. Coinoculated fermentations using Saccharomyces yeasts affect the volatile composition and sensory properties of Vitis vinifera L. cv. Sauvignon blanc wines. J. Agric. Food Chem. 2008, 56, 10829–10837. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Cliff, M.A.; van Vuuren, H.J.J. Impact of mixed S. cerevisiae strains on the production of volatiles and estimated sensory profiles of Chardonnay wines. Food Res. Internat. 2012, 48, 725–735. [Google Scholar] [CrossRef]
- Carrau, F.; Gaggero, C.; Aguilar, P.S. Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol. 2015, 33, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zambonelli, C. Microbiologia e Biotecnologia dei Vini; Edagricole: Bologna, Italy, 1998. [Google Scholar]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Moio, L.; Etievant, P.X. Ethyl anthranilate, ethyl cinnamate, 2,3-dihydrocinnamate, and methyl anthranilate: Four important odorants identified in Pinot noir wines of Burgundy. Am. J. Enol. Vitic. 1995, 46, 392–398. [Google Scholar]
- Aubry, V.; Etievant, P.X.; Ginies, C.; Henry, R. Quantitative determination of potent flavor compounds in Burgundy Pinot noir wines using a stable isotope dilution assay. J. Agric. Food Chem. 1997, 45, 2120–2123. [Google Scholar] [CrossRef]
- Etievant, P.; Issanchou, S.; Bayonove, C.L. The flavor of Muscat wine: The sensory contribution of some volatile compounds. J. Sci. Food Agric. 1983, 34, 497–504. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M.C. Quantification of Selected Aroma-Active Compounds in Pinot noir Wines from Different Grape Maturities. J. Agric. Food Chem. 2006, 22, 8567–8573. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The role and use of non-Saccharomyces yeasts in wine production. S. Afr. J. Enol. Vitic. 2006, 27, 15–39. [Google Scholar]
- King, E.S.; Kievit, R.L.; Curtin, C.; Swiegers, J.H.; Pretorius, I.S.; Bastian, S.E.P.; Francis, I.L. The effect of multiple yeasts co-inoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chem. 2010, 122, 618–626. [Google Scholar] [CrossRef]
- Howell, K.S.; Cozzolino, D.; Bartowsky, E.J.; Fleet, G.H.; Henschke, P.A. Metabolic profiling as a tool for revealing Saccharomyces interactions during wine fermentation. FEMS Yeast Res. 2006, 6, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Schuller, D.; Valero, E.; Dequin, S.; Casal, M. Survey of molecular methods for the typing of wine yeast strains. FEMS Microbiol. Lett. 2004, 231, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, S.; Pretorius, I.S. Selection and improvement of wine yeasts. Ann. Microbiol. 2000, 50, 15–31. [Google Scholar]
- Van Vuuren, H.J.J.; Jacobs, C.J. Killer yeasts in the wine industry: A review. Am. J. Enol. Vitic. 1992, 43, 119–128. [Google Scholar]
- Saberi, S.; Cliff, M.A.; van Vuuren, H.J.J. Comparison of genetic and enological characteristics of new and existing S. cerevisiae strains for Chardonnay wine fermentations. Food Biotechnol. 2014, 28, 195–215. [Google Scholar] [CrossRef]
- Remize, F.; Roustan, J.L.; Sablayrolles, J.M.; Barre, P.; Dequin, S. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microbiol. 1999, 65, 143–149. [Google Scholar] [PubMed]
- Eglinton, J.; Henschke, P.A. The occurrence of volatile acidity in Australian wines. Aust. Grapegr. Winemaker. 1999, 426a, 7–12. [Google Scholar]
- Arriagada-Carrazanaa, J.P.; Sáez-Navarretea, C.; Bordeu, E. Membrane filtration effects on aromatic and phenolic quality of Cabernet Sauvignon wines. J. Food Eng. 2005, 68, 363–368. [Google Scholar] [CrossRef]
- Romano, P. Metabolic characteristics of wine strains during spontaneous and inoculated fermentation. Food Technol. Biotechnol. 1997, 35, 255–260. [Google Scholar]
- Dubois, P. Les arȏmes des vins et leurs défauts. Revue Francaise d'œnologie 1994, 145, 27–40. [Google Scholar]
- Rankine, B.C. Formation of higher alcohols by wine yeasts, and relationship to taste threshold. J. Sci. Food Agric. 1967, 18, 583–589. [Google Scholar] [CrossRef]
- Giudici, P.; Zambonelli, C.; Kunkee, R.E. Increased production of n-propanol in wine by yeast strains having an impaired ability to form hydrogen sulfide. Am. J. Enol. Vitic. 1993, 44, 17–21. [Google Scholar]
- Clarke, R.J.; Bakker, J. Wine Flavor Chemistry; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Ferreira, V.; Fernández, P.; Peña, C.; Escudero, A.; Cacho, J.F. Investigation on the role played by fermentation esters in the aroma of young Spanish wines by multivariate analysis. J. Sci. Food Agric. 1995, 67, 381–392. [Google Scholar] [CrossRef]
- Cheraiti, N.; Guezenec, S.; Salmon, J.M. Redox interactions between Saccharomyces cerevisiae and Saccharomyces uvarum in mixed culture under enological conditions. Appl. Environ. Microbiol. 2005, 71, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Linsemeyer, H.; Muno, H.; Rapp, A. Use of oligo-strain yeast cultures to increase complexity of wine aroma. Vitic. Enol. Sci. 1996, 51, 175–179. [Google Scholar]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Short Protocols in Molecular Biology; Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Van Vuuren, H.J.J.; Wingfield, B.D. Killer yeasts—The cause of stuck fermentations in a wine cellar. S. Afr. J. Enol. Vitic. 1986, 7, 113–118. [Google Scholar]
- Regodón, J.A.; Peréz, F.; Valdés, M.E.; de Miguel, C.; Ramírez, M. A simple and effective procedure for selection of wine yeast strains. Food Microbiol. 1997, 14, 247–254. [Google Scholar] [CrossRef]
- Adams, C.; van Vuuren, H.J.J. The timing of diammonium phosphate addition to fermenting grape must affects the production of ethyl carbamate in wine. Am. J. Enol. Vitic. 2010, 61, 125–129. [Google Scholar]
- Danzer, K.; Garcia, D.D.; Thiel, G.; Reichenbacher, M. Classification of wine samples according to origin and grape varieties on the basis of inorganic and organic trace analyses. Am. Lab. 1999, 31, 26–34. [Google Scholar]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrell, E.; Cliff, M.A.; Van Vuuren, H.J.J. Functional Characterization of Individual- and Mixed-Burgundian Saccharomyces cerevisiae Isolates for Fermentation of Pinot Noir. Molecules 2015, 20, 5112-5136. https://doi.org/10.3390/molecules20035112
Terrell E, Cliff MA, Van Vuuren HJJ. Functional Characterization of Individual- and Mixed-Burgundian Saccharomyces cerevisiae Isolates for Fermentation of Pinot Noir. Molecules. 2015; 20(3):5112-5136. https://doi.org/10.3390/molecules20035112
Chicago/Turabian StyleTerrell, Emily, Margaret A. Cliff, and Hennie J. J. Van Vuuren. 2015. "Functional Characterization of Individual- and Mixed-Burgundian Saccharomyces cerevisiae Isolates for Fermentation of Pinot Noir" Molecules 20, no. 3: 5112-5136. https://doi.org/10.3390/molecules20035112
APA StyleTerrell, E., Cliff, M. A., & Van Vuuren, H. J. J. (2015). Functional Characterization of Individual- and Mixed-Burgundian Saccharomyces cerevisiae Isolates for Fermentation of Pinot Noir. Molecules, 20(3), 5112-5136. https://doi.org/10.3390/molecules20035112






