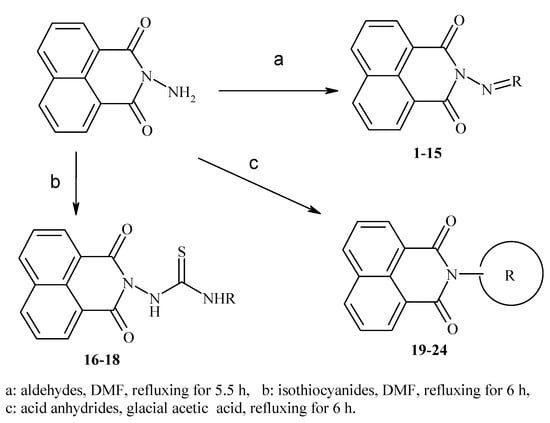
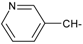
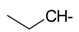
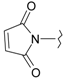
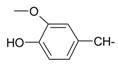
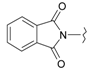
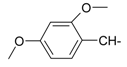
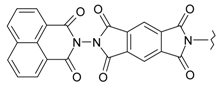
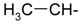Abstract
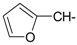
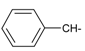
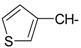
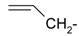
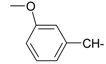
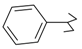
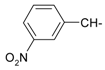
As part of our search for new compounds having antiviral effects, the prepared 2-aminonaphthalimide series was examined for its activity against the herpes simplex viruses HSV-1 and HSV-2. This represents the first study of the antiviral effects of this class of compounds. The new series of 2-amino-1H-benzo[de]isoquinoline-1,3-diones was examined against HSV-1 and HSV-2 using a cytopathic effect inhibition assay. In terms of effective concentration (EC50), furaldehyde, thiophene aldehyde and allyl isothiocyanide derivatives 14‒16 showed potent activity against HSV-1 (EC50 = 19.6, 16.2 and 17.8 μg/mL), compared to acyclovir as a reference drug (EC50 = 1.8 μg/mL). Moreover, 14 and 15 were found to exhibit valuable activity against HSV-2. Many of the tested compounds demonstrated weak to moderate EC50 values relative to their inactive parent compound (2-amino-1H-benzo[de]isoquinoline-1,3-dione), while compounds 7, 9, 13, 14, 15, 16, 21 and 22 were the most active set of antiviral compounds throughout this study. The cytotoxicity (CC50), EC50, and the selectivity index (SI) values were determined. In a molecular docking study, the ligand-receptor interactions of compounds 1–24 and their parent with the HSV-1 thymidine kinase active site were investigated using the Molegro Virtual Docker (MVD) software. Based on the potent anti-HSV properties of the previous naphthalimide condensate products, further exploration of this series of 2-amino-1H-benzo[de]isoquinoline-1,3-diones is warranted.
1. Introduction
HSV-1 and HSV-2, as DNA viruses, belong to the alpha herpes virus subfamily, which also includes the varicella zoster virus (VZV). They are common human pathogens and between 60% and 95% of certain populations are infected with HSV-1, and between 6% and 50% with HSV-2 [1,2]. Herpes simplex viruses are common pathogens that also cause herpes genitalis, herpes labialis, encephalitis and keratitis. The infection caused by the two types is mainly transmitted by close personal contact, and the virus establishes lifelong latent infection in the sensory neurons, with recurrent lesions [3,4]. The frequency of HSV-seropositive males is significantly higher in populations infected with the human immunodeficiency virus (HIV). Moreover, sexually transmitted diseases like genital HSV increase the risk of transmission and acquisition of HIV infection [5]; there is also a synergistic relationship between genital herpes and HIV [6]. It was reported that HSV-suppressive therapy greatly reduced genital and plasma levels in patients co-infected by HIV-1 RNA [7]. Hence, reducing the spread of genital herpes can greatly decrease the risk of acquiring or transmitting HIV infection.
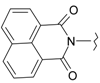
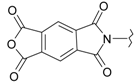
Imides of aromatic dicarboxylic acids like naphthalimides are important in the construction of macromolecules as well as in supramolecular assembly. They are useful fluoroprobes for various studies and also serve as precursors for the protection of the amino group [8]. The 1H-benzo[de]isoquinoline-1,3-diones are also found to play an important role as synthons for the construction of many bioactive compounds such as antitumour and histone deacetylase inhibitors (HDAC) [9,10,11,12,13,14,15,16]. Moreover, they have a high binding affinity towards the 5-HT1A receptor that is expressed in Chinese hamster ovary cells (CHO cells, commonly used in biological, medical research and the most mammalian hosts for industrial production of recombinant protein therapeutics), as determined by fluorescence microscopy [17]. In our recent research, the title compounds were evaluated for their antimicrobial and cytotoxic effects, whereby some of them were found to possess significant activities [18,19]. Despite promising findings in the literature, most such compounds have been poorly studied because of their complicated syntheses [11]. In view of these facts, we aimed to investigate a new prepared series of 2-aminobenzo[de]isoquinoline-1,3-diones as antiviral agents against HSV-1 and HSV-2.
2. Results and Discussion
2.2. Molecular Docking
Thymidine kinase (TK) acts catalytically to phosphorylate thymidine, getting it ready for further phosphorylation and eventual incorporation into DNA [24]. So many researches study the binding capabilities TK and other ligands can serve to develop more effective HSV treatment that would act as inhibitors of this enzyme rather than substrates.
In the present study, the ligand-receptor interactions of compounds 1–24 and the parent compound with HSV-1 thymidine kinase active site were investigated by performing docking studies using the Molegro Virtual Docker (MVD) software. The crystal structure of HSV-1 thymidine kinase in complex with acyclovir (PDB code 1KI5) was obtained from the Protein Data Bank [25] (Figure 1). The accuracy of MVD docking protocol was validated and confirmed by docking the co-crystallized acyclovir inside the active site of TK where the docked acyclovir showed little deviation (Figure 2).
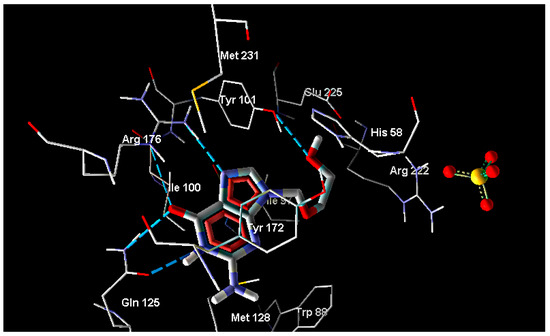
Figure 1.
Interaction of acyclovir with thymidine kinase.
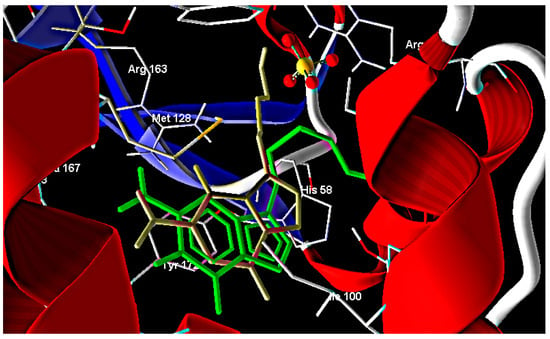
Figure 2.
Comparison between the co-crystallized acyclovir (gray) and docked acyclovir (green).
Snapshots for the most active tested compounds were taken to reveal their molecular interactions (hydrogen, hydrophobic and/or ionic bonds) with the amino acids-HSV-1 thymidine kinase active sites. MolDock scores between these compounds and receptor were calculated (Table 3).

Table 3.
MolDock scores of the acyclovir, parent and tested compounds 1–24.
| Ligand | MolDock Score | Ligand | MolDock Score | Ligand | MolDock Score |
|---|---|---|---|---|---|
| Parent | −101.205 | 9 | −112.001 | 18 | −134.765 |
| 18 | −97.8437 | 10 | −108.487 | 19 | −112.904 |
| 2 | −102.893 | 11 | −104.531 | 20 | −118.027 |
| 3 | −102.959 | 12 | −115.317 | 21 | −93.0316 |
| 4 | −95.8232 | 13 | −101.817 | 22 | −91.99 |
| 5 | −113.363 | 14 | −116.126 | 23 | −73.5124 |
| 6 | −127.137 | 15 | −112.552 | 24 | −133.133 |
| 7 | −109.968 | 16 | −129.001 | acyclovir | −111.737 |
| 8 | −121.392 | 17 | −132.879 |
The results showed that all tested compounds have affinity for HSV-1 thymidine kinase comparable to that of the reference compound. The docking interaction of the most active compound 15 with the the active site of TK is represented in Figure 3. It is observed that the 2-amino group, carbonyl groups and the nitrogen atom of the benzoisoquinoline-1,3-dione moiety form five H-bond interactions with Glu63 and Tyr172, with bond lengths of 2.53, 3.27, 3.01, 3.38 and 3.31 Å, respectively. In addition, the hydrophobic interactions with Tyr172 alongside the phenyl ring with Tyr132 and Trp88 appear to constrain the molecule in close proximity with the amino acids forming the aforementioned hydrogen bonding [24,25,26].
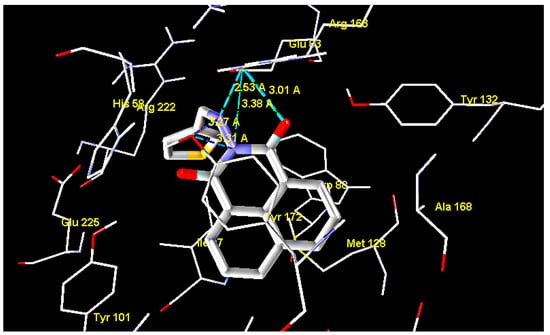
Figure 3.
Interaction of compound 15 with thymidine kinase.
3. Experimental Section
3.1. Mammalian Cell Line
Vero cells (derived from the kidney of a normal African green monkey) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The viral strains used were GHSV-UL46 for HSV-1 and the G strain for HSV-2. The Vero cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated foetal bovine serum (FBS), 1% L-glutamine, HEPES buffer and 50 µg/mL gentamycin. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and were subcultured two times a week [19,27].
3.3. Cytotoxicity Evaluation Using Viability Assay
The Vero cell lines in the cytotoxicity assay were seeded in 96-well plates at a cell concentration of 1 × 104 cells per well in 100 µL of the growth medium [29]. Fresh medium containing different concentrations of the tested sample was added after 24 h of seeding. Serial two-fold dilutions of the tested compound were added to confluent cell monolayers dispensed into 96-well, flat-bottomed microtiter plates (Falcon, Jersey, NJ, USA) using a multichannel pipette. The microtiter plates were incubated at 37 °C in a humidified incubator with 5% CO2 for a period of 48 h. Three wells were used for each concentration of the tested sample. Control cells were incubated without test samples and with or without DMSO. The small percentage of DMSO present in the wells (maximal 0.1%) was not found to affect the experiment [19,27].
After the end of the incubation period, the viable cell yield was determined by a colorimetric method [32,33,34,35,36]. In brief, the process of treatment the media with crystal violet solution and measurement of absorbance at 590 nm using a microplate reader was mentioned above. The absorbance was proportional to the number of surviving cells in the culture plate. All the results were corrected for background absorbance detected in wells without added stain. Treated samples were compared with the cell control in the absence of the tested compounds. All experiments were carried out in triplicate. The cell cytotoxicity effect of each tested compound was calculated [30,37].
3.4. Molecular Docking
Molecular docking studies were carried out on a laptop PC, Intel® Core ™ i7-3630 QM CPU @ 2.40 GHz, RAM 8 GB operating under the Windows 7 Professional OS. It consists of several steps; first, the 3D crystal structures of HSV-1 thymidine kinase in complex with acyclovir (PDB code 1KI5) [25] was downloaded from the Brookhaven Protein Data Bank PDB and loaded into Molegro Virtual Docker (MVD 2013.6.0.0 [win32] program fully functional free trial version with time limiting license [38]. All types of atoms, charges and bond hybridization were carefully checked. The MolDock Score [GRID] and MolDock Optimizer routines as implemented in Molegro Virtual Docker (MVD version 2013.6.0.0). The non-bonded oxygen atoms of water molecules, present in the crystal structure, were removed. ChemBio3D Ultra 10 was used to draw the 3D structures of different ligands that were further pre-optimized using free version of Marvinsketch 4.1.13 from Chemaxon Ltd. with MM force field and saved in Tripos mol2 file format [39]. MolDock score functions were used with a 0.3 Å grid resolution. Prior to the calculations of the examined compounds, the MVD software was benchmarked by docking the acyclovir.
3.5. Data Analysis
Statistics were done using a one-way ANOVA test [40], and the percentage cell viability was calculated using Microsoft Excel®, as follows:
where Abs equals the absorbance at 590 nm. The CC50 was estimated from graphic plots and the STATA statistical analysis package was used for the drawing of the dose response curve, in order to calculate CC50. Concerning antiviral evaluation, all experiments and data analysis were performed in RCMB, Al-Azhar University, Cairo, Egypt.
% Cell viability = [(Mean Abscontrol− Mean Abstest metabolite)/Mean Abscontrol] × 100
4. Conclusions
This study has revealed that compounds 14, 15 and 16 are active agents against both herpes simplex viruses. These compounds could be useful as templates for furthering development and design of more potent antiviral agents.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No RG-1435-068.
Author Contributions
Rashad Al-Salahi has made a substantial contribution to the experimental design, significant contributions to the acquisition of data, manuscript preparation (writing and revision) and approved the final form of the manuscript. Ibrahim Alswaidan has participated in the reading and revision processes and approved the final form of the manuscript. Essam Ezzeldin has written and analyzed the statistical data and participated in the revision process and approved the final form of the manuscript. Hazem A. Ghabbour has performed the molecular docking work. Mahmoud Elaasser has performed substantial biological investigation tests. Mohamed Marzouk has made a substantial contribution to the experimental design, significant contributions to the acquisition of data and manuscript preparation (writing and revision) and approved the final form of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, J.S.; Robinson, J.N. Age-specific prevalence of infection with herpes simplex virus types 1 and 2: A global review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef] [PubMed]
- Akanitapichata, P.; Wangmaneerata, A.; Wilairatb, P.; Bastowc, K.F. Anti-herpes virus activity of Dunbari abella Prain. J. Ethnopharmacol. 2006, 105, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Cowan, F.M.; French, R.S.; Mayaud, P.; Gopal, R.; Robinson, N.J.; de Oliveira, S.A.; Faillace, T.; Uusküla, A.; Nygård-Kibur, M.; Ramalingam, S.; et al. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex. Transm. Infect. 2003, 79, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Safrin, S.; Crumpacker, C.; Chatis, P.; Davis, R.; Hafner, R.; Rush, J.; Kessler, H.A.; Landry, B.; Mills, J. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant muco cutaneous herpes simplex in the acquired immunodeficiency syndrome: The AIDS clinical trials group. N. Eng. J. Med. 1991, 325, 551–555. [Google Scholar] [CrossRef]
- White, M.K.; Gorrill, T.S.; Khalili, K. Reciprocal transactivation between HIV-1 and other human viruses. Virology 2006, 352, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes 2004, 11, 70–76. [Google Scholar] [PubMed]
- Barooah, N.; Tamuly, C.; Baruah, J.B. Synthesis, characterisation of few N-substituted 1,8-naphthalimide derivatives and their copper(II) complexes. J. Chem. Sci. 2005, 117, 117–122. [Google Scholar]
- Xu, Y.; Qu, B.; Qian, X.; Li, Y. Five-member thio-heterocyclic fused naphthalimides with aminoalkyl side chains: Intercalation and photocleavage to DNA. Bioorg. Med. Chem. Lett. 2005, 15, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Hazra, S.; Dutta, S.; Muthiah, S.; Mondhe, D.M.; Sharma, P.R.; Singh, S.K.; Saxena, A.K.; Qazi, G.N.; Sanyal, U. Antitumor efficacy and apoptotic activity of substituted chloroalkyl 1H-benzo[de]isoquinoline-1,3-diones: A new class of potential antineoplastic agents. Investg. New Drugs 2011, 29, 434–442. [Google Scholar] [CrossRef]
- Brana, M.F.; Castellano, J.M.; Moran, M.; Pérez de Vega, M.J.; Romerdahl, C.R.; Qian, X.D.; Bousquet, P.; Emling, F.; Schlick, E.; Keilhauer, G. Bis-naphthalimides: A new class of antitumor agents. Anti-Cancer Drug Des. 1993, 8, 257–268. [Google Scholar]
- Aibin, W.; Jide, L.L.; Shaoxiong, Q.; Ping, M. Derivatives of 5-nitro-1H-benzo[de]isoquinoline-1,3(2H)-dione: Design, synthesis, and biological activity. Monatsh. Chem. 2010, 141, 95–99. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dutta, S.; Shanmugavel, M.; Mondhe, D.M.; Sharma, P.R.; Singh, S.K.; Saxena, A.K.; Sanyal, U. 6-Nitro-2-(3 hydroxypropyl)-1H-benz[de]isoquinoline-1,3-dione, a potent antitumor agent, induces cell cycle arrest and apoptosis. J. Exp. Clin. Cancer Res. 2010, 29, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Qazi, G.N.; Saxena, A.K.; Muthiah, S.; Mondhe, D.M.; Sharma, P.R.; Singh, S.K.; Sanyal, U.; Mukherjee, A.; Hazra, S.; Dutta, S. Preparation of benzisoquinolinedione derivatives for us as antitumor agents. WO 2008084496 A1, 17 July 2008. [Google Scholar]
- Cholody, W.M.; Kosakowska-Cholody, T.; Michejda, C.J. Preparation of 1,8-naphthalimido-linked imidazo[4,5,1-de]acridones as bis-intercalating antitumor agents. WO 2001066545 A2, 13 September 2001. [Google Scholar]
- Vaisburg, A.; Bernstein, N.; Frechette, S.; Allan, M.; Abou-Khalil, E.; Leit, S.; Moradei, O.; Bouchain, G.; Wang, J.; Woo, S.H.; et al. (2-Aminophenyl)-amides of omega-substituted alkanoic acids as new histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Leopoldo, M.; Masotti, A.C.; Inglese, C.; Berardi, F.; Perrone, R.; Ganguly, S.; Jafurulla, M.; Chattopadhyay, A. Synthesis and characterization of environment-sensitive fluorescent ligands for human 5-HT1A receptors with 1-arylpiperazine structure. J. Med. Chem. 2009, 52, 7892–7896. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Marzouk, M. Some 2-amino-benzo[de]isoquinolin-1,3-diones as antimicrobial agents. Asian J. Chem. 2014, 26, 8163–8165. [Google Scholar]
- Al-Salahi, R.; Alswaidan, I.; Marzouk, M. Cytotoxicity evaluation for a new set of 2-aminobenzo[de]iso-quinolin-1,3-diones. Int. J. Mol. Sci. 2014, 15, 22483–22491. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Marzouk, M. Synthesis of novel 2-aminobenzo[de]isoquinolin-1,3-dione derivatives. Asian J. Chem. 2014, 26, 2166–2172. [Google Scholar]
- Garett, R.; Romanos, M.T.V.; Borges, R.M.; Santos, M.G.; Rocha, L.; da Silva, A.J.R. Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Braz. J. Pharmacogn. 2012, 22, 306–313. [Google Scholar] [CrossRef]
- Bag, P.; Ojha, D.; Mukherjee, H.; Halder, U.C.; Mondal, S.; Chandra, N.S.; Nandi, S.; Sharon, A.; Sarkar, M.C.; Chakrabarti, S.; et al. An indole alkaloid from a tribal folklore inhibits immediate early event in HSV-2 infected cells with therapeutic efficacy in vaginally infected mice. PLoS One 2013, 8, e77937. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Vogel, J.L.; Narayanan, A.; Peng, H.; Kristie, T.M. Inhibition of the histone demethylase LSD1 blocks α-herpesvirus lytic replication and reactivation from latency. Nat. Med. 2009, 15, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Champness, J.N.; Bennett, M.S.; Wien, F.; Viss, R.; Summers, W.C.; Herdewijn, P.; Ostrowski, E.; Jarvest, R.L.; Sanderson, M.R. Exploring the active site of herpes simplex virus type-1 thymidine kinase by X-ray crystallography of complexes with Aciclovir and other ligands. Proteins Struct. Funct. Genet. 1998, 32, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.S.; Wien, F.; Champness, J.N.; Batuwangala, T.; Rutherford, T.; Summers, W.C.; Sun, H.; Wright, G.; Sanderson, M.R. Structure to 1.9 Å resolution of a complex with herpes simplex virus type-1 thymidine kinase of a novel, non-substrate inhibitor: X-ray crystallographic comparison with binding of acyclovir. FEBS Lett. 1999, 443, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Luyten, I.; de Winter, H.; Busson, R.; Lescrinier, T.; Creuven, I.; Durant, F.; Baizarini, J.; de Clercq, E.; Herdwijn, P. Synthesis of 2'-deoxy-5-(isothiazol-5-yl)uridine and its interaction with the HSV-1 thymidine kinase. Helv. Chim. Acta 1996, 79, 1462–1474. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, M.; Alswaidan, I.; Al-Omar, M. Antiviral activity of 2-phenoxy-4H-[1,2,4]triazolo[1,5-a]quinazoline derivatives. Life Sci. J. 2013, 10, 2164–2169. [Google Scholar]
- Hu, J.M.; Hsiung, G.D. Evaluation of new antiviral agents I: In vitro prospective. Antivir. Res. 1989, 11, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, P.; Raghu, C.; Ashok, G.; Dhanaraj, S.A.; Suresh, B. Antiviral activity of medicinal plants of Nilgiris. Indian J. Med. Res. 2004, 120, 24–29. [Google Scholar] [PubMed]
- Dargan, D.J. Investigation of the anti-HSV activity of candidate antiviral agents. In Methods in Molecular Medicine, Herpes Simplex Virus Protocols; Brown, S.M., MacLean, A.R., Eds.; Humana Press Inc.: Totowa, NJ, USA, 1998; Volume 10, pp. 387–405. [Google Scholar]
- Zandi, K.; Zadeh, M.A.; Sartavi, K.; Rastian, Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Biotechnol. 2007, 6, 1770–1773. [Google Scholar]
- Vega-Avila1, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assesssurvival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar] [PubMed]
- Bajbouj, K.; Schulze-Luehrmann, J.; Diermeier, S.; Amin, A.; Schneider-Stock, R. The anticancer effect of saffron in two p53 isogenic colorectal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Ušaj, M.; Trontelj, K.; Hudej, R.; Kandušer, M.; Miklavčič, D. Cell size dynamics and viability of cells exposed to hypotonic treatment and electroporation for electrofusion optimization. Radiol. Oncol. 2009, 43, 108–119. [Google Scholar] [CrossRef]
- Bernhardt, G.; Reile, H.; Birnboeck, H.; Spruss, T.; Schoenenberger, H. Standardized kinetic microassay to quantify differential chemosensitivity on the basis of proliferative activity. J. Cancer Res. Clin. Oncol. 1992, 118, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ait Mbarek, L.; ait Mouse, H.; Elabbadi, N.; Bensalah, M.; Gamouh, A.; Aboufatima, R.; Benharref, A.; Chait, A.; Kamal, M.; Dalal, A.; et al. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Braz. J. Med. Biol. Res. 2007, 40, 839–847. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, S.M. ChemBioOffice Ultra 2010 suite. J. Am. Chem. Soc. 2010, 132, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Serna, L.; Cravioto, J. Simply Statistic for Health Investigation, 1st ed.; Trillas: Mexico, Mixico, 1999. [Google Scholar]
- Sample Availability: Samples of the compounds (1–24) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).