Soy Isoflavones in Nutritionally Relevant Amounts Have Varied Nutrigenomic Effects on Adipose Tissue
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight, Food Intake and Lipid Profiles
| (A) | ||||
| Body Weight | Food Intake | |||
| g | g/day | |||
| Control diet | 29.1 ± 2.1 | Control diet | 3.0 ± 0.1 | |
| 0.45% Soyselect® | 27.7 ± 3.5 | 0.45% Soyselect® | 2.7 ± 0.1 | |
| (B) | ||||
| Cholesterol | Triglycerides | |||
| mg/dL plasma | mg/dL plasma | |||
| Control diet | 117.5 ± 9.3 | Control diet | 67.6 ± 16.1 | |
| 0.45% Soyselect® | 132.5 ± 2.9 * | 0.45% Soyselect® | 53.7 ± 8.14 * | |
2.2. Leptin Concentrations
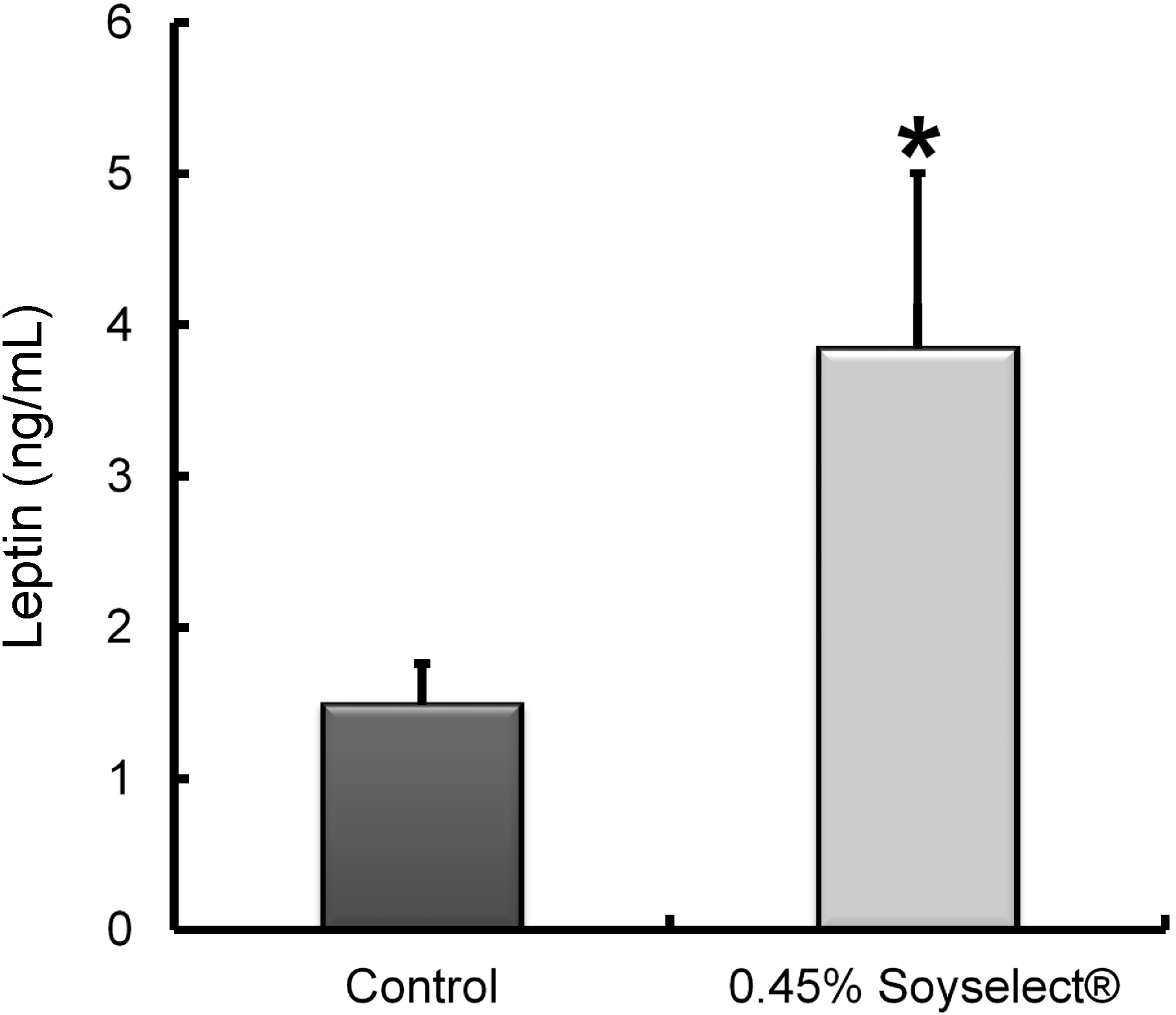
2.3. Peritoneal Fat Genome Expression Profiles
| Genes | NGR | TNGR | NG | TNG | Hyp | Hyp * | Annotations |
|---|---|---|---|---|---|---|---|
| 53 genes | 1999 | 37681 | 53 | 365 | 3.21184 × 10−11 | 4.5287 × 10−9 | GO:0000166: nucleotide binding (MF) |
| 42 genes | 1501 | 37681 | 42 | 365 | 9.08868 × 10−10 | 6.40752 × 10−8 | GO:0016787: hydrolase activity (MF) |
| 38 genes | 1314 | 37681 | 38 | 365 | 2.51857 × 10−9 | 1.18373 × 10−7 | GO:0005524: ATP binding (MF) GO:0000166: nucleotide binding (MF) |
| 39 genes | 1421 | 37681 | 39 | 365 | 6.44848 × 10−9 | 2.27309 × 10−7 | GO:0005524: ATP binding (MF) |
| 61 genes | 2999 | 37681 | 61 | 365 | 2.77158 × 10−8 | 7.81585 × 10−7 | GO:0005515: protein binding (MF) |
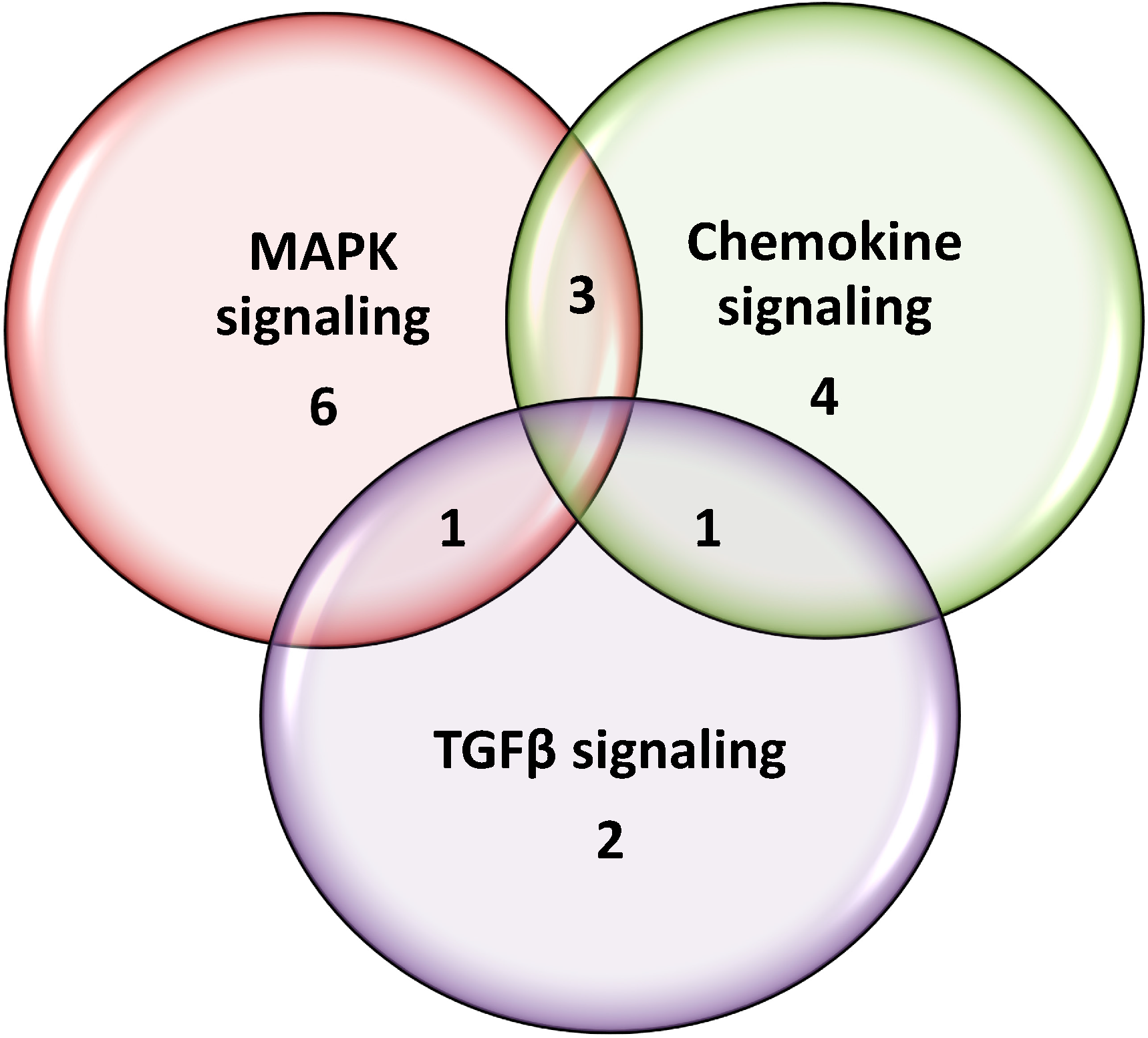
2.4. Gene Expression Validation by qRT-PCR
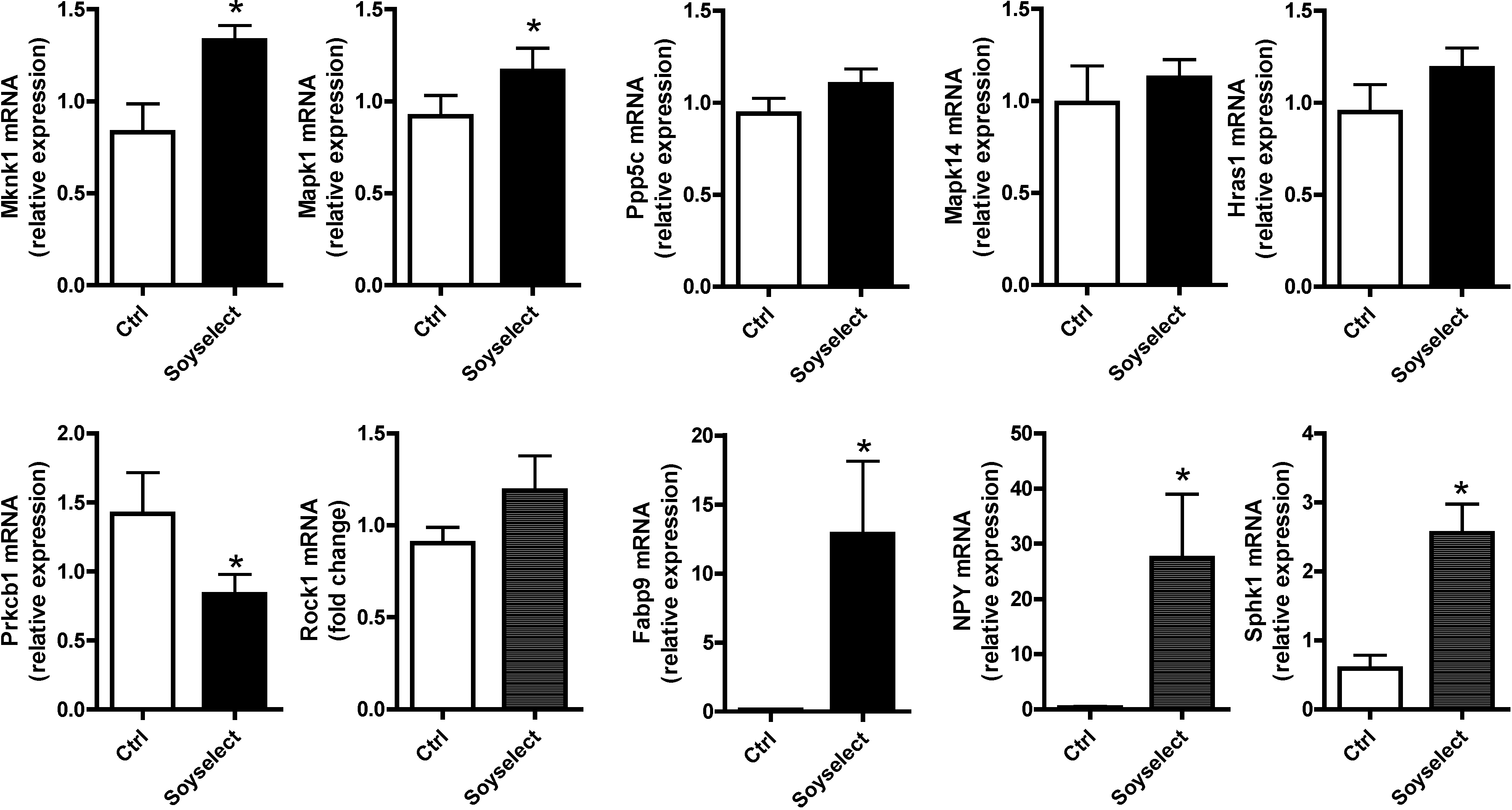
2.5. Discussion
3. Experimental Section
3.1. Materials
3.2. Animals and Diets
| Control | Soyselect® | |||
|---|---|---|---|---|
| g% Kcal% | g% Kcal% | |||
| Protein | 23 | 24 | 23 | 24 |
| Carbohydrate | 60 | 61 | 60 | 61 |
| Fat | 6 | 15 | 6 | 15 |
| Ingredient | g/kg diet | |||
| Casein | 244 | 244 | ||
| l-Cystein | 3 | 3 | ||
| Corn Starch | 318 | 318 | ||
| Maltodextrin 10 | 45 | 45 | ||
| Dextrose | 250 | 250 | ||
| Cellulose | 75 | 75 | ||
| Inulin | 25 | 25 | ||
| Sunflower Oil | 29.5 | 29.5 | ||
| Olive Oil | 18.6 | 18.6 | ||
| Lard | 18.5 | 18.5 | ||
| Mineral Mix S10026 | 10 | 10 | ||
| Dicalcium Phosphate | 13 | 13 | ||
| Calcium Carbonate | 5.5 | 16.5 | ||
| Potassium Citrate | 5.5 | 16.5 | ||
| Vitamin Mix V10001 | 10 | 10 | ||
| Retinyl Acetate, 500,000 IU/g | 0.048 | 0.048 | ||
| Choline Bitartrate | 2 | 2 | ||
| Genistein/Daidzein mix | 0 | 4.5 | ||
| Cholesterol | 0.146 | 0.146 | ||
| Total | 1083.84 | 1088.34 | ||
3.3. Determination of Circulating Leptin Concentrations
3.4. Determination of Plasma Lipid Concentrations
3.5. RNA Isolation and Analysis
3.6. Microarray Hybridization
3.7. Quantitative Real-Time PCR (qRT-PCR)
3.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clifton, P.M. Protein and coronary heart disease: The role of different protein sources. Curr. Atheroscler. Rep. 2011, 13, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy protein, isoflavones, and cardiovascular health: A summary of a statement for professionals from the american heart association nutrition committee. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Koh, W.P.; van Dam, R.M.; Yuan, J.M.; Pan, A. Dietary soy intake is not associated with risk of cardiovascular disease mortality in Singapore Chinese adults. J. Nutr. 2014, 144, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F. Nutritional support in the pharmacological treatment of metabolic syndrome. Eur. J. Pharmacol. 2011, 668, S43–S49. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Niu, K.; Zeng, Y.; Liu, P.; Yi, L.; Zhang, T.; Zhang, Q.Y.; Zhu, J.D.; Mi, M.T. Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst. Rev. 2013, 6, CD009518. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Penalvo, J.L.; Gil, J.I.; Medina, S.; Horcajada, M.N.; Lafay, S.; Silberberg, M.; Llorach, R.; Zafrilla, P.; Garcia-Mora, P.; et al. Soy isoflavones and cardiovascular disease epidemiological, clinical and -omics perspectives. Curr. Pharm. Biotechnol. 2012, 13, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS One 2013, 8, e81968. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, M.; Binetruy, B.; Caron, L.; Le Marchand-Brustel, Y.; Bost, F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 2006, 88, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.; Taylor, A.A.; Smith, E.O.; Barnes, S.; Hachey, D.L. Effect of soy isoflavone supplementation on nitric oxide metabolism and blood pressure in menopausal women. Am. J. Clin. Nutr. 2012, 95, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S. Methodological challenges conducting epidemiological research on nutraceuticals in health and disease. PharmaNutrition 2014, 2, 120–125. [Google Scholar] [CrossRef]
- Food and Drug Administration. Food labeling: Health claims; soy protein and coronary heart disease. Fed. Regist. 1999, 64, 57700–57733. [Google Scholar]
- Sirtori, C.R.; Eberini, I.; Arnoldi, A. Hypocholesterolaemic effects of soya proteins: Results of recent studies are predictable from the anderson meta-analysis data. Br. J. Nutr. 2007, 97, 816–822. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piccolella, M.; Crippa, V.; Messi, E.; Tetel, M.J.; Poletti, A. Modulators of estrogen receptor inhibit proliferation and migration of prostate cancer cells. Pharmacol. Res. 2014, 79, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Li, F.; Yu, X.; White, R.E. GPER: A novel target for non-genomic estrogen action in the cardiovascular system. Pharmacol. Res. 2013, 71, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Valera, M.C.; Payrastre, B.; Lenfant, F.; Gourdy, P. Structure-function relationship of estrogen receptors in cardiovascular pathophysiological models. Thromb. Res. 2012, 130, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Valeri, A.; Fiorenzani, P.; Rossi, R.; Aloisi, A.M.; Valoti, M.; Pessina, F. The soy phytoestrogens genistein and daidzein as neuroprotective agents against anoxia-glucopenia and reperfusion damage in rat urinary bladder. Pharmacol. Res. 2012, 66, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Jang, S.; Agostini, B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol. Ther. 2014, 144, 202–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013, 93, 1317–1542. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Weber, C. Chemokines in atherosclerosis: Proceedings resumed. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Christia, P.; Frangogiannis, N.G. Targeting inflammatory pathways in myocardial infarction. Eur. J. Clin. Investig. 2013, 43, 986–995. [Google Scholar] [CrossRef]
- van der Velpen, V.; Geelen, A.; Hollman, P.C.; Schouten, E.G.; van't Veer, P.; Afman, L.A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: A double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 1269–1277. [Google Scholar]
- Mark, A.L. Selective leptin resistance revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R566–R581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.L.; He, L.P.; Ling, W.H.; Liu, Z.M.; Chen, Y.M. Soy food consumption, cardiometabolic alterations and carotid intima-media thickness in Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Woo, J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: a 6-month randomized controlled trial among postmenopausal women. J. Hypertens. 2013, 31, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; van Eck, M. Mouse Models of Disturbed HDL Metabolism. Handb. Exp. Pharmacol. 2015, 224, 301–336. [Google Scholar] [PubMed]
- Selvaraj, V.; Asano, A.; Page, J.L.; Nelson, J.L.; Kothapalli, K.S.; Foster, J.A.; Brenna, J.T.; Weiss, R.S.; Travis, A.J. Mice lacking FABP9/PERF15 develop sperm head abnormalities but are fertile. Dev. Biol. 2010, 348, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Fujishita, C.; Komatsu, T.; Kim, S.E.; Chiba, T.; Mori, R.; Shimokawa, I. NPY antagonism reduces adiposity and attenuates age-related imbalance of adipose tissue metabolism. FASEB J. 2014, 28, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mottillo, E.P.; Zhao, J.; Gartung, A.; VanHecke, G.C.; Lee, J.F.; Maddipati, K.R.; Xu, H.; Ahn, Y.H.; Proia, R.L.; et al. Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. J. Biol. Chem. 2014, 289, 32178–32185. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.; Young, R.L.; Steinberg, F.M.; Murray, M.J.; Lewis, R.D.; Cramer, M.A.; Barnes, S.; Ellis, K.J.; Shypailo, R.J.; Fraley, J.K.; et al. Effect of soy isoflavone supplementation on menopausal quality of life. Menopause 2013, 20, 443–447. [Google Scholar] [PubMed]
- Reverri, E.J.; LaSalle, C.D.; Franke, A.A.; Steinberg, F.M. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol. Nutr. Food Res. 2014. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Murray, M.J.; Lewis, R.D.; Cramer, M.A.; Amato, P.; Young, R.L.; Barnes, S.; Konzelmann, K.L.; Fischer, J.G.; Ellis, K.J.; et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am. J. Clin. Nutr. 2011, 93, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Mantuano, E.; Fabrizi, M.; Ferlini, C.; Mozzetti, S.; De Stefano, I.; Scambia, G. Effects of a phytoestrogen-containing soy extract on the growth-inhibitory activity of ICI 182 780 in an experimental model of estrogen-dependent breast cancer. Endocr. Relat. Cancer 2007, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Zannoni, G.F.; De Stefano, I.; Mosca, M.; Ferlini, C.; Mantuano, E.; Scambia, G. Soy phytochemicals decrease nonsmall cell lung cancer growth in female athymic mice. J. Nutr. 2008, 138, 1360–1364. [Google Scholar] [PubMed]
- Bombardelli, E.; Gabetta, B. Soya Extract, Process for its Preparation and Pharmaceutical Composition. U.S. Patent 6280777, 19 August 2003. [Google Scholar]
- Bombardelli, E.; Gabetta, B. Isoflavones and Saponins in Defined Ratios, Menopause, Cancer. U.S. Patent 6607757, 19 September 2006. [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Carmona-Saez, P.; Chagoyen, M.; Tirado, F.; Carazo, J.M.; Pascual-Montano, A. GENECODIS: A web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Davalos, A.; Visioli, F. Chronic hydroxytyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, E.; Dávalos, A.; Crespo, M.C.; Tomé-Carneiro, J.; Gómez-Coronado, D.; Visioli, F. Soy Isoflavones in Nutritionally Relevant Amounts Have Varied Nutrigenomic Effects on Adipose Tissue. Molecules 2015, 20, 2310-2322. https://doi.org/10.3390/molecules20022310
Giordano E, Dávalos A, Crespo MC, Tomé-Carneiro J, Gómez-Coronado D, Visioli F. Soy Isoflavones in Nutritionally Relevant Amounts Have Varied Nutrigenomic Effects on Adipose Tissue. Molecules. 2015; 20(2):2310-2322. https://doi.org/10.3390/molecules20022310
Chicago/Turabian StyleGiordano, Elena, Alberto Dávalos, Maria Carmen Crespo, Joao Tomé-Carneiro, Diego Gómez-Coronado, and Francesco Visioli. 2015. "Soy Isoflavones in Nutritionally Relevant Amounts Have Varied Nutrigenomic Effects on Adipose Tissue" Molecules 20, no. 2: 2310-2322. https://doi.org/10.3390/molecules20022310
APA StyleGiordano, E., Dávalos, A., Crespo, M. C., Tomé-Carneiro, J., Gómez-Coronado, D., & Visioli, F. (2015). Soy Isoflavones in Nutritionally Relevant Amounts Have Varied Nutrigenomic Effects on Adipose Tissue. Molecules, 20(2), 2310-2322. https://doi.org/10.3390/molecules20022310








