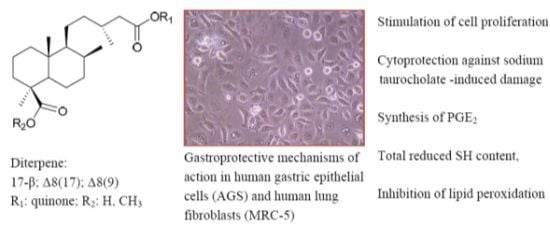
Diterpenylquinone Hybrids: Synthesis and Assessment of Gastroprotective Mechanisms of Action in Human Cells
Abstract
:1. Introduction
2. Results and Discussion
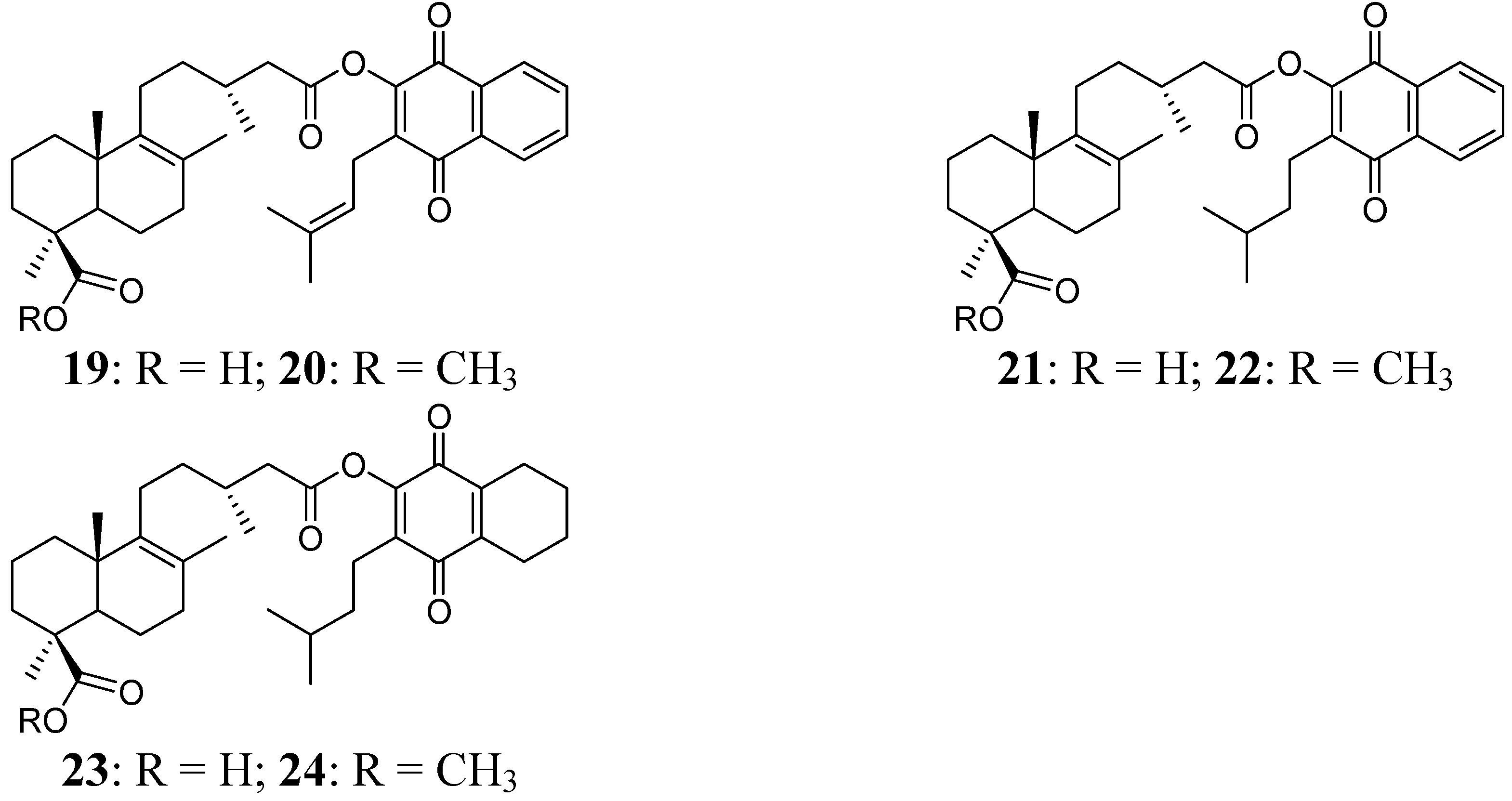
2.1. Hybrid Compounds
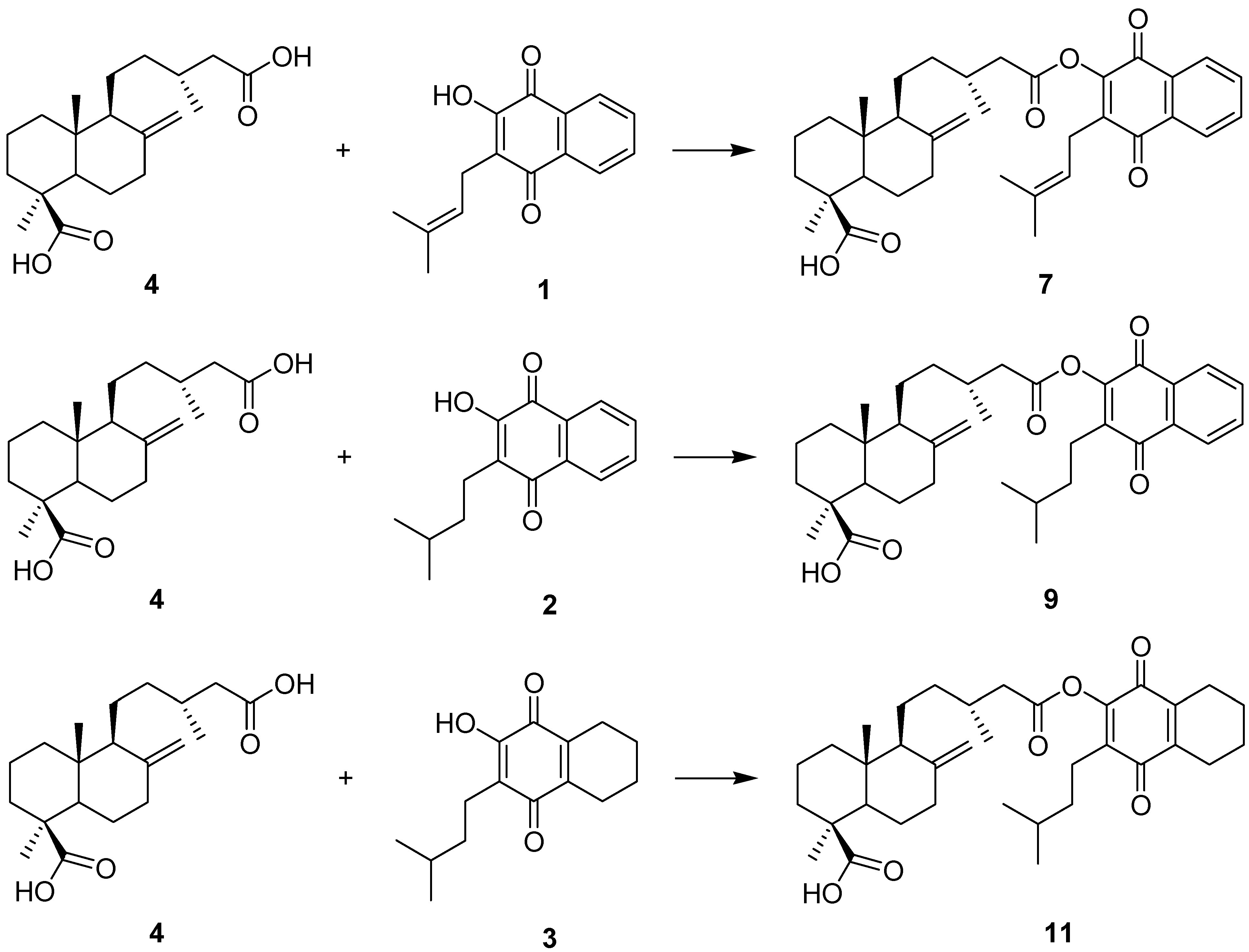
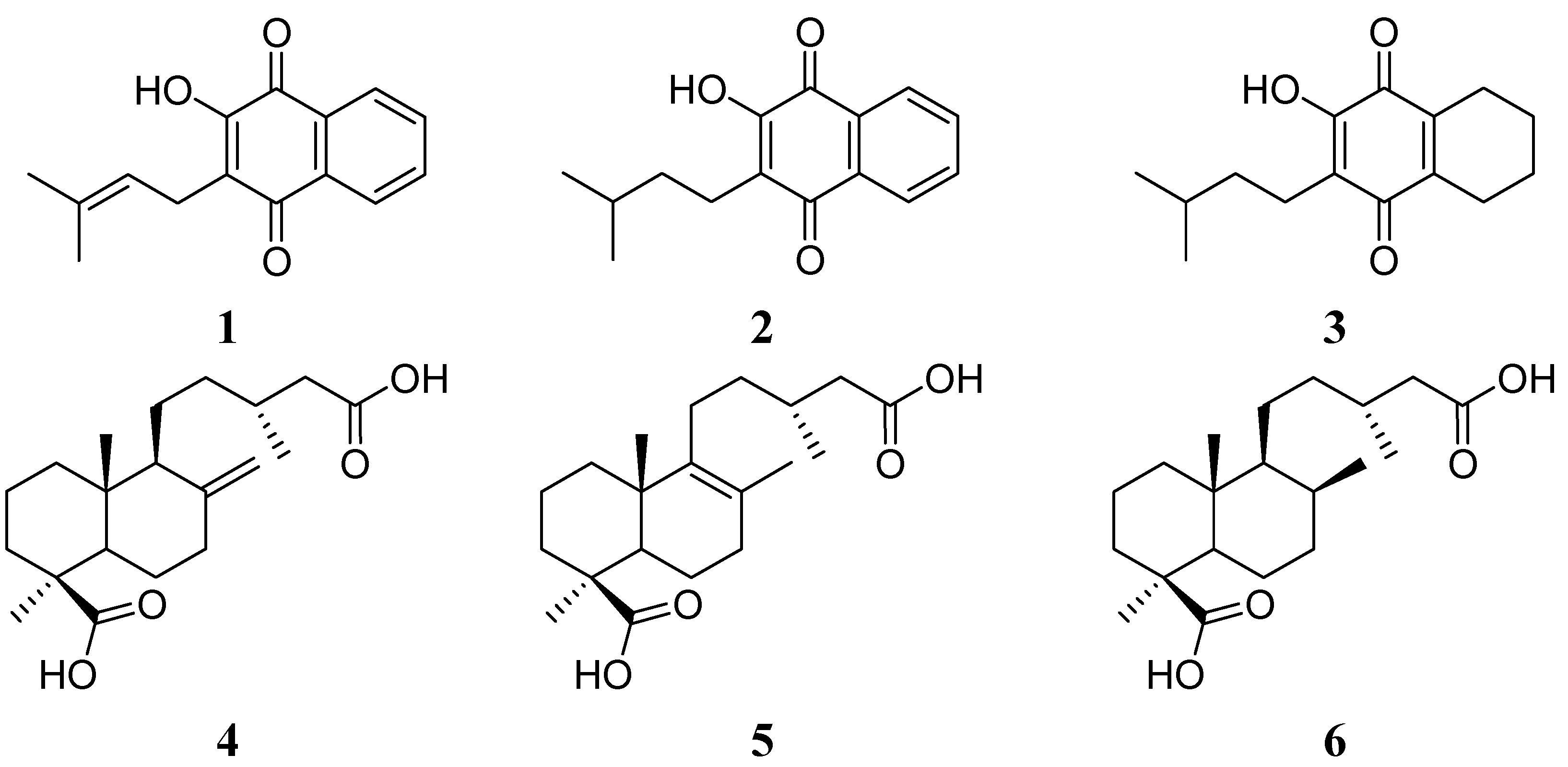
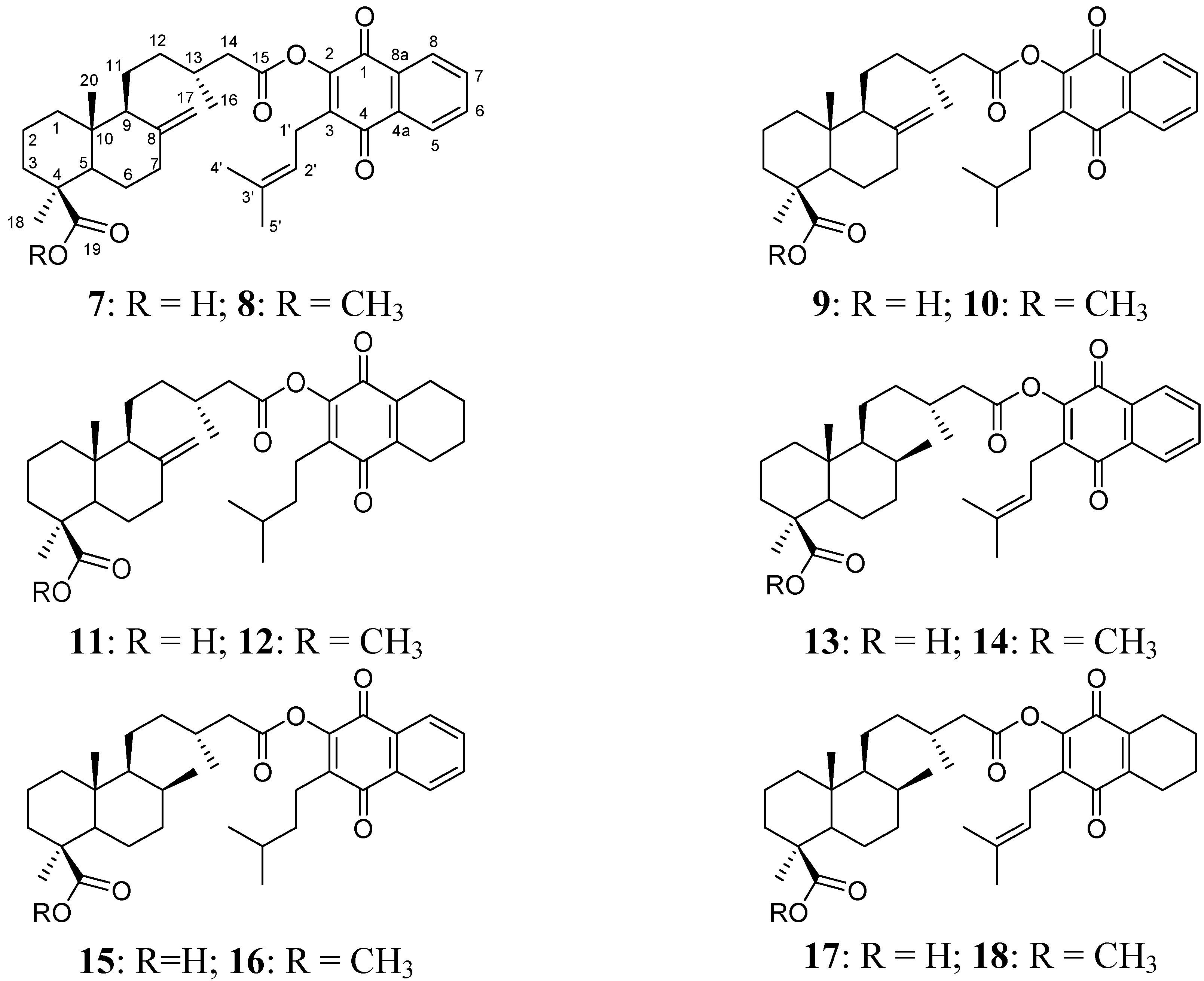
2.2. Cytotoxicity
| Compound | IC50 ± SD (µM) a | |
|---|---|---|
| MRC-5 | AGS | |
| Lapachol (1) | >1000 | 571 ± 28 |
| Dihydroprenyl lapachol (2) | 611 ± 42 | 292 ± 12 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachol (3) | 307 ± 22 | 87 ± 4 |
| Junicedric acid (4) | 439 ± 26 | 383 ± 23 |
| Δ8(9) Junicedric acid (5) | 834 ± 51 | 666 ± 35 |
| 17β-Dihydrojunicedric acid (6) | 163 ± 5 | 283 ± 17 |
| Lapachoyl junicedrate (7) | 221 ± 13 | 143 ± 6 |
| Lapachoyl junicedrate methyl ester (8) | >1000 | >1000 |
| Dihydroprenyl lapachol junicedrate (9) | 449 ± 27 | 49 ± 10 |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | >1000 | 659 ± 42 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate(11) | 42 ± 2 | 63 ± 4 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (12) | 667 ± 40 | >1000 |
| Lapachoyl 17β-dihydrojunicedrate (13) | 355 ± 21 | 351 ± 29 |
| Lapachoyl 17β-dihydrojunicedrate methyl ester (14) | 727 ± 48 | 916 ± 54 |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | >1000 | >1000 |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16) | 596 ± 44 | 643 ± 39 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17) | 44 ± 4 | 60 ± 5 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 369 ± 29 | 884 ± 62 |
| Lapachoyl Δ8(9) junicedrate (19) | 129 ± 9 | 148 ± 9 |
| Lapachoyl Δ8(9) junicedrate methyl ester (20) | 483 ± 31 | 321 ± 24 |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | 601 ± 26 | 278 ± 14 |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 848 ± 51 | 340 ± 20 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23) | 20 ± 2 | 29 ± 2 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (24) | >1000 | >1000 |
| Lansoprazole b | 316 ± 11 | 168 ± 8 |
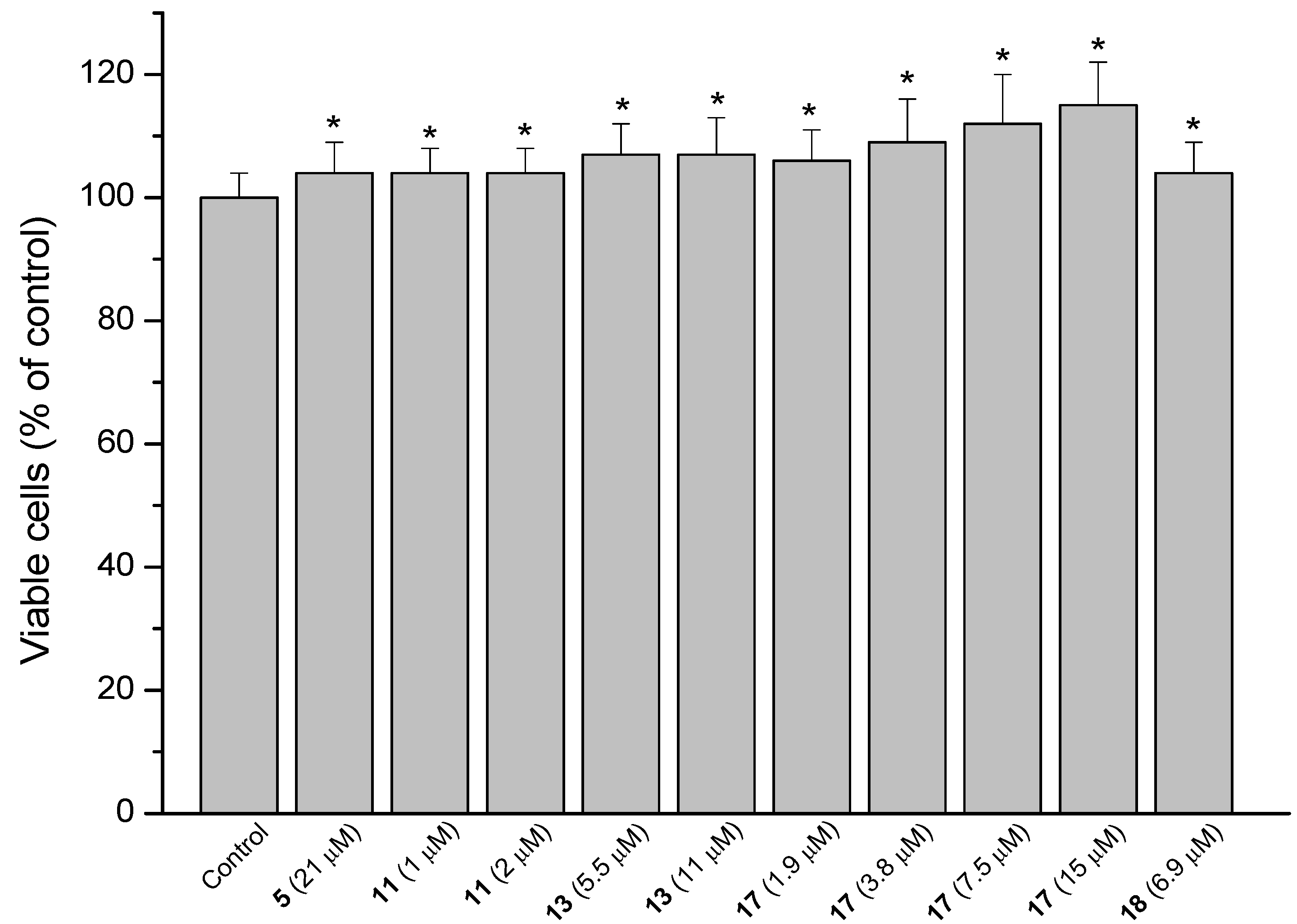
2.3. MRC-5 Fibroblast Proliferation
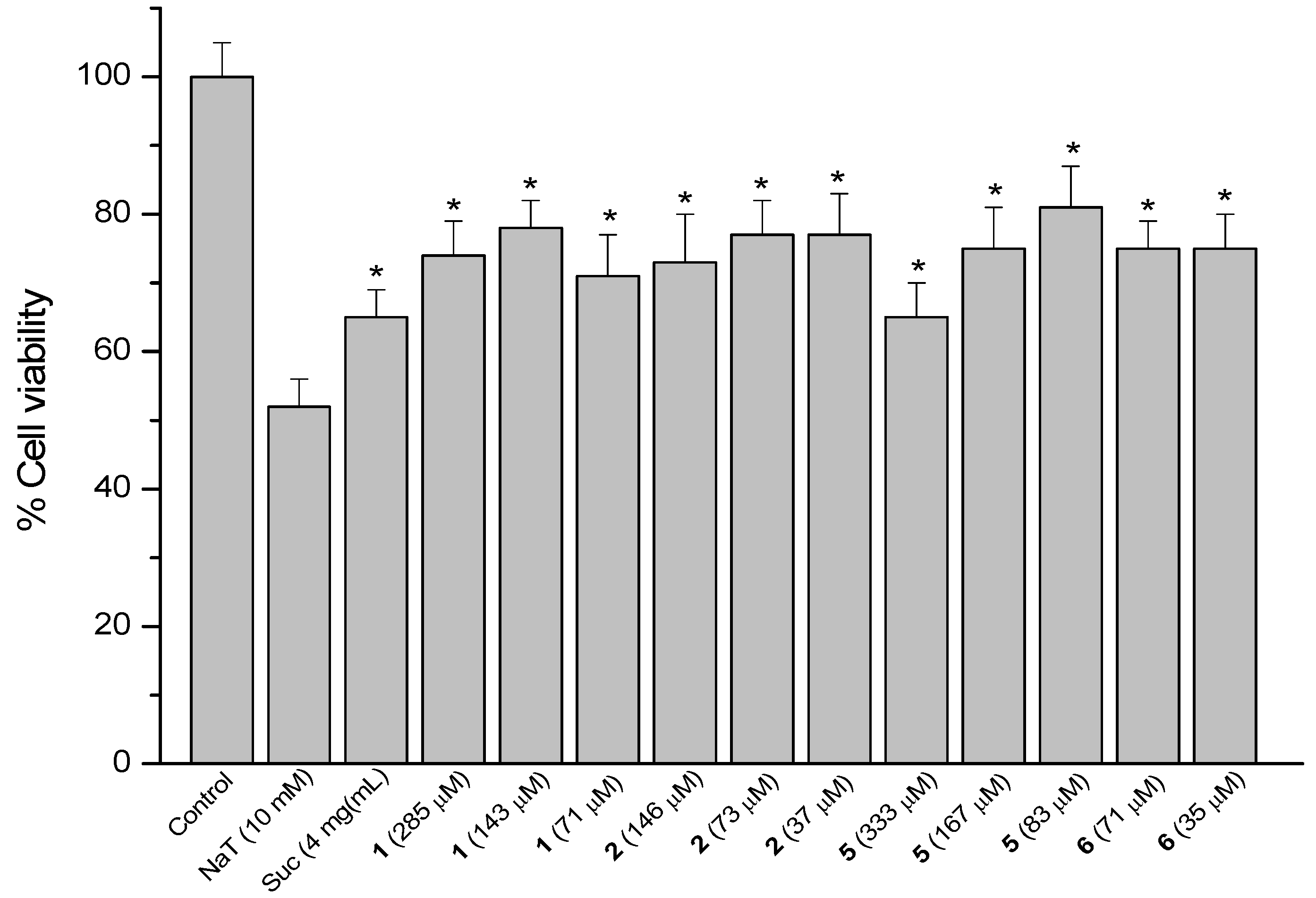
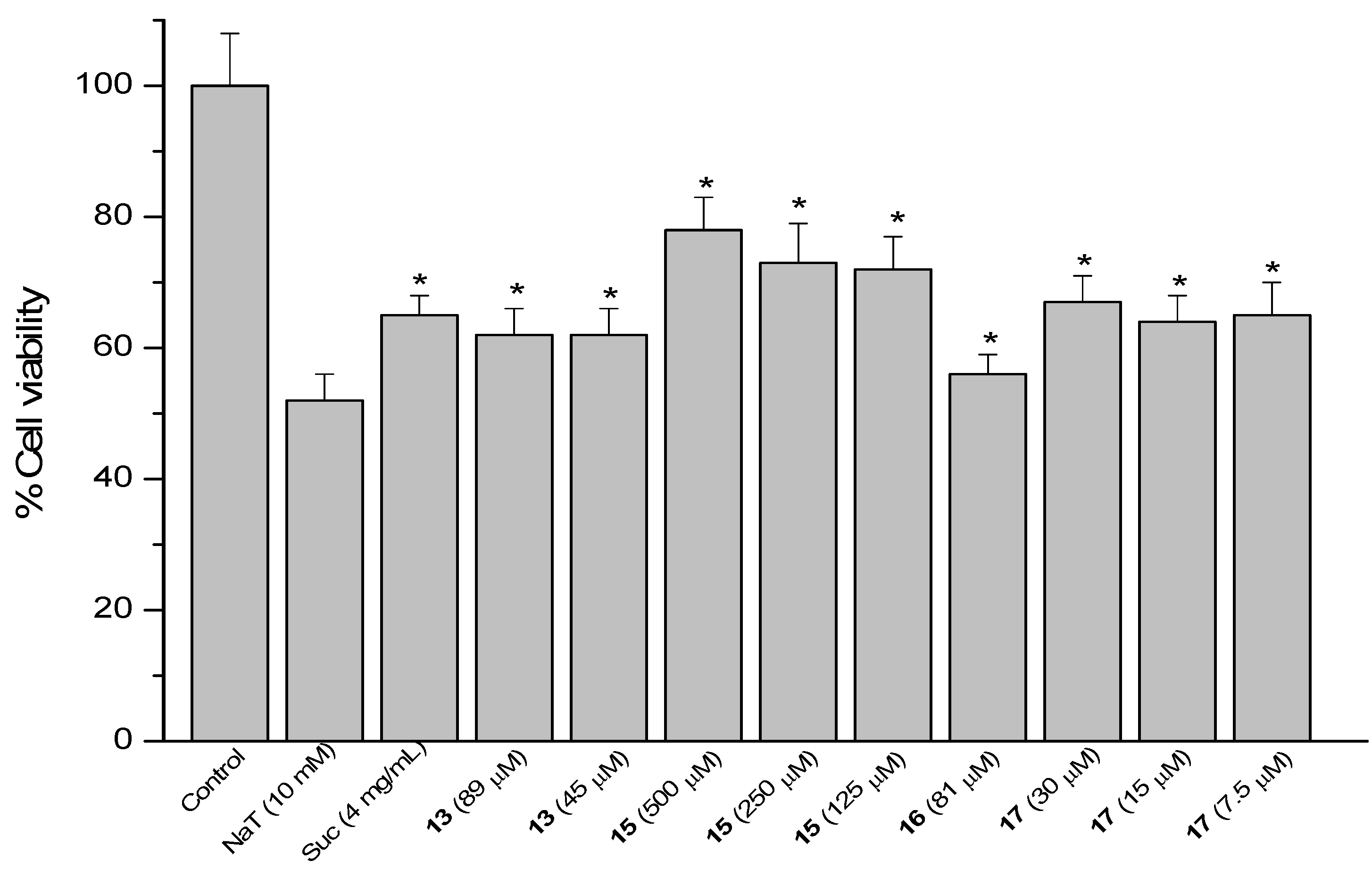
2.4. Sodium Taurocholate (NaT)-Induced Damage
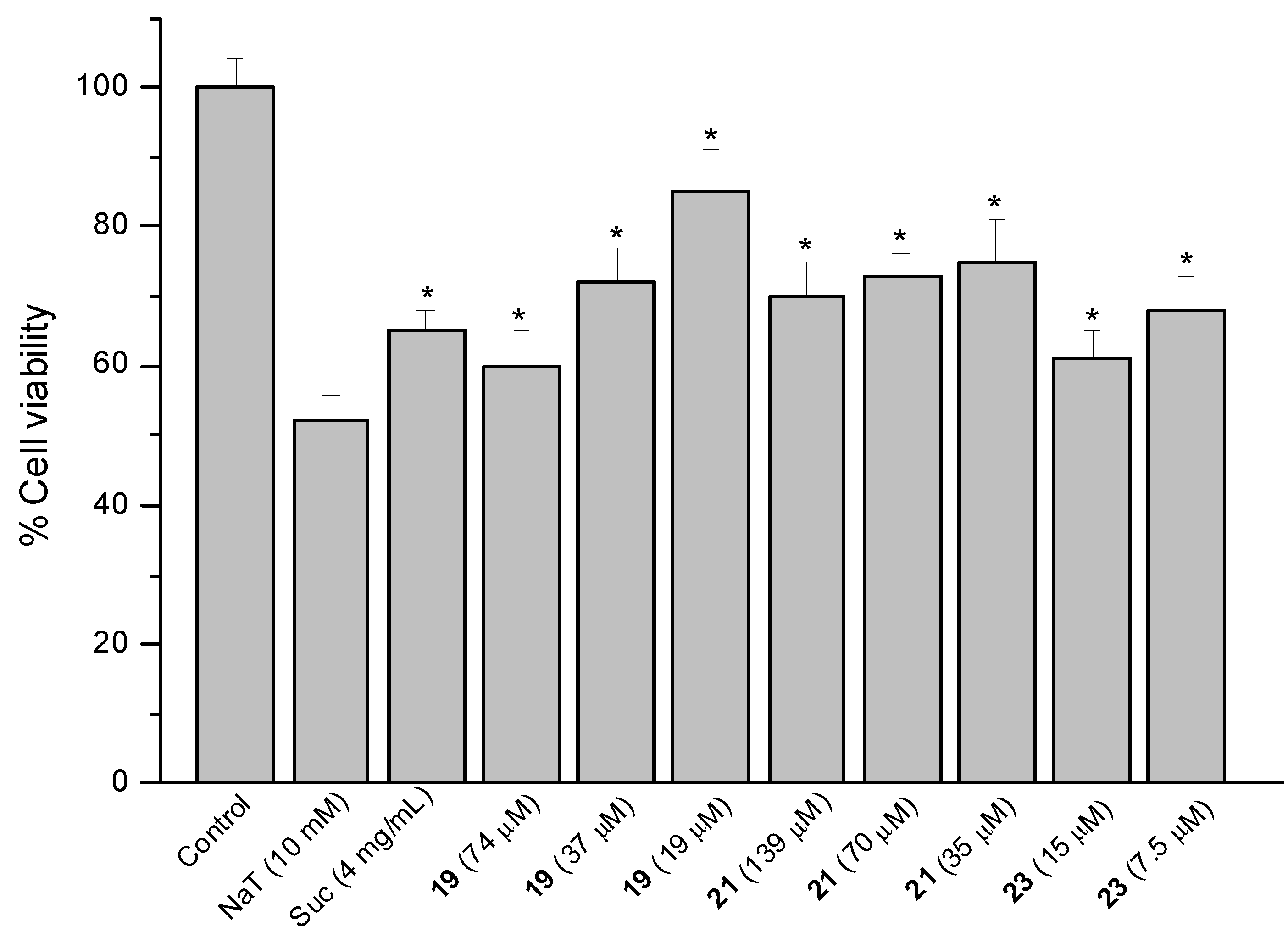
2.5. Effect on Prostaglandin E2 (PGE2) Content
| Compound | Concentration (µM) | PGE2 (pg/mL) |
|---|---|---|
| Control | - | 52 ± 5 |
| Indomethacin a | 100 | Not detected |
| Lapachol (1) | 286 | 132 ± 12 * |
| 143 | 117 ± 10 * | |
| Lapachoyl junicedrate methyl ester (8) | 500 | 787 ± 56 * |
| 250 | 272 ± 30 * | |
| Dihydroprenyl lapachol junicedrate (9) | 12.5 | 309 ± 29 * |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | 323 | 465 ± 39 * |
| 162 | 306 ± 32 * | |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | 500 | 372 ± 25 * |
| 250 | 279 ± 20 * | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 221 | 156 ± 13 * |
| 111 | 123 ± 9 * | |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | 85 | 162 ± 18 * |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 170 | 114 ± 11 * |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (24) | 250 | 123 ± 12 * |
2.6. Effect on Cellular Reduced Glutathione (GSH) Content
| Compound | Concentration (µM) | GSH (nmol/106 cells) |
|---|---|---|
| Control | - | 13.6 ± 0.7 |
| NAC a | 750 | * 17.7 ± 1.4 |
| Lapachol(1) | 143 | 14.1 ± 0.8 |
| 72 | 13.9 ± 0.9 | |
| Dihydroprenyl lapachol(2) | 73 | 13.8 ± 0.7 |
| 37 | 13.6 ± 0.7 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachol (3) | 22 | 13.0 ± 0.5 |
| 11 | 14.3 ± 0.7 | |
| Junicedric acid (4) | 96 | * 17.2 ± 1.0 |
| 48 | * 18.4 ± 1.1 | |
| Δ8(9) Junicedric acid (5) | 166 | 14.1 ± 0.6 |
| 83 | 13.6 ± 0.5 | |
| 17β-Dihydrojunicedric acid (6) | 71 | ** 21.7 ± 1.3 |
| 37 | * 19.5 ± 0.9 | |
| Lapachoyl junicedrate (7) | 36 | * 16.7 ± 0.7 |
| 18 | 16.1 ± 0.9 | |
| Lapachoyl junicedrate methyl ester (8) | 250 | * 16.8 ± 0.9 |
| 125 | * 16.2 ± 0.6 | |
| Dihydroprenyl lapachol junicedrate (9) | 12 | * 15.8 ± 0.6 |
| 6 | * 15.6 ± 0.6 | |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | 165 | 13.0 ± 0.7 |
| 83 | 13.4 ± 0.7 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate (11) | 16 | 13.2 ± 0.5 |
| 8 | 13.1 ± 0.6 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (12) | 250 | 13.9 ± 0.8 |
| 125 | 13.7 ± 1.0 | |
| Lapachoyl 17β-dihydrojunicedrate (13) | 88 | 11.4 ± 0.6 |
| 44 | 11.9 ± 0.4 | |
| Lapachoyl 17β-dihydrojunicedrate methyl ester (14) | 229 | 12.7 ± 0.4 |
| 115 | 14.4 ± 0.5 | |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | 250 | * 18.4 ± 1.2 |
| 125 | * 17.8 ± 0.9 | |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16) | 160 | 14.1 ± 0.6 |
| 80 | 14.9 ± 0.7 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17) | 15 | ** 20.5 ± 1.0 |
| 7.5 | ** 25.8 ± 1.6 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 221 | * 17.2 ± 1.0 |
| 111 | * 17.1 ± 1.1 | |
| Lapachoyl Δ8(9) junicedrate (19) | 37 | 13.5 ± 0.5 |
| 19 | 13.1 ± 0.4 | |
| Lapachoyl Δ8(9) junicedrate methyl ester (20) | 80 | * 15.4 ± 1.3 |
| 40 | * 16.2 ± 1.0 | |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | 70 | * 18.1 ± 1.1 |
| 35 | 13.3 ± 0.7 | |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 85 | 14.2 ± 0.6 |
| 43 | 13.6 ± 0.9 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23) | 7.3 | * 17.1 ± 1.3 |
| 3.6 | 12.6 ± 0.8 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester(24) | 250 | * 18.0 ± 0.9 |
| 125 | * 18.7 ± 1.0 |
2.7. Inhibition of Lipoperoxidation
| Compound | % Inhibition of the lipoperoxidation a |
|---|---|
| Lapachol (1) | 38 |
| Dihydroprenyl lapachol (2) | IC50 25.4 ± 2.4 µg/mL |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachol (3) | IC50 33.3 ± 2.3 µg/mL |
| Junicedric acid (4) | 25 |
| Δ8(9) Junicedric acid (5) | IC50 43.8 ± 2.8 µg/mL |
| 17β-dihydrojunicedric acid (6) | 30 |
| Lapachoyl junicedrate (7) | 10 |
| Lapachoyl junicedrate methyl ester (8) | IC50 81.4 ± 11.0 µg/mL |
| Dihydroprenyl lapachol junicedrate (9) | IC50 40.1 ± 5.3 µg/mL |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | IC50 99.4 ± 9.1 µg/mL |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate (11) | 44 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (12) | 37 |
| Lapachoyl 17β-dihydrojunicedrate (13) | IC50 61.1 ± 5.9 µg/mL |
| Lapachoyl 17β-dihydrojunicedrate methyl ester (14) | IC50 19.7 ± 1.4 µg/mL |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | IC50 25.3 ± 4.1 µg/mL |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16) | 46 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17) | 48 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 28 |
| Lapachoyl Δ8(9) junicedrate (19) | 49 |
| Lapachoyl Δ8(9) junicedrate methyl ester (20) | IC50 58.1 ± 4.1 µg/mL |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | Nd |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 46 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23) | IC50 59.5 ± 4.3 µg/mL |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (24) | 27 |
| Catechin b | IC50 75.4 ± 6.0 µg/mL |
3. Experimental
3.1. General Experimental Procedures
3.2. General Procedure for the Synthesis of Hybrid Compounds
3.2.1. Quinones
3.2.2. Diterpenes
3.2.3. Hybrid Compounds
 +2.35 (c 0.085 CHCl3); IR νmax (film) 2957, 2931, 2854, 1725, 1675, 1463, 1386, 1287, 1074, 998 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.75 (2H, m, quinone H-6 and H-7), 2.74 (1H, dd, J = 16, 4 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.42 (1H, m, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.27 (3H, s, H-18), 1.13 (3H, d, J = 6.8 Hz, H-16), 0.93 (6H, d, J = 6.4 Hz, H-4' and H-5'), 0.90 (3H, d, J = 6.4 Hz, H-17), 0.84 (3H, s, H-20); 13C-NMR (CDCl3): 39.61 (C-1), 19.43 (C-2), 37.45 (C-3), 45.46 (C-4), 52.82 (C-5), 23.00 (C-6), 34.94 (C-7), 29.00 (C-8), 57.64 (C-9), 38.84 (C-10), 18.84 (C-11), 35.12 (C-12), 30.79 (C-13), 40.72 (C-14), 170.29 (C-15), 20.10 (C-16), 15.17 (C-17), 28.04 (C-18), 178.08 (C-19), 14.89 (C-20); Quinone: 173.42, 184.49 (C-1 and C-4), 151.04 (C-2), 140.09 (C-3), 130.89, 132.08 (C-4a, C-8a), 126.61, 126.50 (C-5 and C-8), 133.95, 133.72 (C-6 and C-7), 22.40 (C-1'), 37.00 (C-2'), 28.34 (C-3'), 22.28 (C-4'), 22.28 (C-5'); EIMS m/z 576 [M]+ (2), 293 (17), 244 (20), 243 (20), 242 (100), 227 (27), 123 (28), 109 (14), 95 (10), 81 (11), 55 (11).
+2.35 (c 0.085 CHCl3); IR νmax (film) 2957, 2931, 2854, 1725, 1675, 1463, 1386, 1287, 1074, 998 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.75 (2H, m, quinone H-6 and H-7), 2.74 (1H, dd, J = 16, 4 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.42 (1H, m, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.27 (3H, s, H-18), 1.13 (3H, d, J = 6.8 Hz, H-16), 0.93 (6H, d, J = 6.4 Hz, H-4' and H-5'), 0.90 (3H, d, J = 6.4 Hz, H-17), 0.84 (3H, s, H-20); 13C-NMR (CDCl3): 39.61 (C-1), 19.43 (C-2), 37.45 (C-3), 45.46 (C-4), 52.82 (C-5), 23.00 (C-6), 34.94 (C-7), 29.00 (C-8), 57.64 (C-9), 38.84 (C-10), 18.84 (C-11), 35.12 (C-12), 30.79 (C-13), 40.72 (C-14), 170.29 (C-15), 20.10 (C-16), 15.17 (C-17), 28.04 (C-18), 178.08 (C-19), 14.89 (C-20); Quinone: 173.42, 184.49 (C-1 and C-4), 151.04 (C-2), 140.09 (C-3), 130.89, 132.08 (C-4a, C-8a), 126.61, 126.50 (C-5 and C-8), 133.95, 133.72 (C-6 and C-7), 22.40 (C-1'), 37.00 (C-2'), 28.34 (C-3'), 22.28 (C-4'), 22.28 (C-5'); EIMS m/z 576 [M]+ (2), 293 (17), 244 (20), 243 (20), 242 (100), 227 (27), 123 (28), 109 (14), 95 (10), 81 (11), 55 (11). +20 (c 0.056, CHCl3); IR νmax (film) 2951, 2868, 2844, 1772, 1725, 1679, 1463, 1380, 1300, 1151, 1081, 941, 712 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.74 (2H, m, quinone H-6 and H-7), 3.65 (3H, s, OCH3), 2.75 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.44 (1H, dd, J = 15.2, 8.8 Hz, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.19 (3H, s, H-18), 1.13 (3H, d, J = 6.4 Hz, H-16), 0.95 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 7.6 Hz, H-17), 0.70 (3H, s, H-20); 13C-NMR (CDCl3): 40.23 (C-1), 19.47 (C-2), 38.55 (C-3), 44.31 (C-4), 53.31 (C-5), 23.43 (C-6), 35.35 (C-7), 29.66 (C-8), 57.89 (C-9), 39.08 (C-10), 19.28 (C-11), 35.62 (C-12), 31.27 (C-13), 41.18 (C-14), 170.79 (C-15), 20.57 (C-16), 15.34 (C-17), 29.26 (C-18), 178.51 (C-19), 14.68 (C-20), 51.55 (OCH3); Quinone: 173.26, 184.95 (C-1 and C-4), 151.5 (C-2), 140.54 (C-3), 131.32, 132.52 (C-4a, C-8a), 127.06, 126.95 (C-5 and C-8), 134.41, 134.18 (C-6 and C-7), 22.86 (C-1'), 37.90 (C-2'), 28.79 (C-3'), 22.74 (C-4'), 22.74 (C-5'); molecular formula: C36H50O6 (578.3607). m/z 596.3947 [M+NH4]+, error: 0.20 ppm with the empirical formula C36H54NO6; m/z 601.3499 [M+Na]+, error: 0.08 ppm with the empirical formula C36H50O6Na.
+20 (c 0.056, CHCl3); IR νmax (film) 2951, 2868, 2844, 1772, 1725, 1679, 1463, 1380, 1300, 1151, 1081, 941, 712 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.74 (2H, m, quinone H-6 and H-7), 3.65 (3H, s, OCH3), 2.75 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.44 (1H, dd, J = 15.2, 8.8 Hz, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.19 (3H, s, H-18), 1.13 (3H, d, J = 6.4 Hz, H-16), 0.95 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 7.6 Hz, H-17), 0.70 (3H, s, H-20); 13C-NMR (CDCl3): 40.23 (C-1), 19.47 (C-2), 38.55 (C-3), 44.31 (C-4), 53.31 (C-5), 23.43 (C-6), 35.35 (C-7), 29.66 (C-8), 57.89 (C-9), 39.08 (C-10), 19.28 (C-11), 35.62 (C-12), 31.27 (C-13), 41.18 (C-14), 170.79 (C-15), 20.57 (C-16), 15.34 (C-17), 29.26 (C-18), 178.51 (C-19), 14.68 (C-20), 51.55 (OCH3); Quinone: 173.26, 184.95 (C-1 and C-4), 151.5 (C-2), 140.54 (C-3), 131.32, 132.52 (C-4a, C-8a), 127.06, 126.95 (C-5 and C-8), 134.41, 134.18 (C-6 and C-7), 22.86 (C-1'), 37.90 (C-2'), 28.79 (C-3'), 22.74 (C-4'), 22.74 (C-5'); molecular formula: C36H50O6 (578.3607). m/z 596.3947 [M+NH4]+, error: 0.20 ppm with the empirical formula C36H54NO6; m/z 601.3499 [M+Na]+, error: 0.08 ppm with the empirical formula C36H50O6Na. +23 (c 0.065, CHCl3); IR νmax (film) 2959, 1769, 1699, 1662, 1625, 1549, 1467, 1455, 1382, 1269, 1205, 1077, 913, 755 cm−1; 1H-NMR (CDCl3): δ 2.68 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.40 (2H, m, quinone H-5 and H-8), 2.28 (1H, m, H-14 β), 1.70 (2H, m, quinone H-6 and H-7), 1.59 (2H, m, H-1'), 1.48 (2H, m, H-2'), 1.25 (3H, s, H-18), 1.10 (3H, d, J = 6.8 Hz, H-16), 0.92 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 6.4 Hz, H-17), 0.80 (3H, s, H-20); 13C-NMR (CDCl3): 40.14 (C-1), 19.22 (C-2), 38.31 (C-3), 44.20 (C-4), 53.34 (C-5), 23.41 (C-6), 35.36 (C-7), 29.67 (C-8), 57.86 (C-9), 39.30 (C-10), 18.33 (C-11), 35.60 (C-12), 31.24 (C-13), 41.13 (C-14), 170.97 (C-15), 20.51 (C-16), 15.36 (C-17), 29.45 (C-18), 180.70 (C-19), 14.79 (C-20); Quinone: 184.36, 187.37 (C-1 and C-4), 149.02 (C-2), 141.29 (C-3), 137.32, 143.20 (C-4a, C-8a), 23.22, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.41 (C-1'), 37.90 (C-2'), 28.67 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H52O6 (568.3764). m/z 586.4086 [M+NH4]+, error: 0.20 ppm with the empirical formula C35H56NO6.
+23 (c 0.065, CHCl3); IR νmax (film) 2959, 1769, 1699, 1662, 1625, 1549, 1467, 1455, 1382, 1269, 1205, 1077, 913, 755 cm−1; 1H-NMR (CDCl3): δ 2.68 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.40 (2H, m, quinone H-5 and H-8), 2.28 (1H, m, H-14 β), 1.70 (2H, m, quinone H-6 and H-7), 1.59 (2H, m, H-1'), 1.48 (2H, m, H-2'), 1.25 (3H, s, H-18), 1.10 (3H, d, J = 6.8 Hz, H-16), 0.92 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 6.4 Hz, H-17), 0.80 (3H, s, H-20); 13C-NMR (CDCl3): 40.14 (C-1), 19.22 (C-2), 38.31 (C-3), 44.20 (C-4), 53.34 (C-5), 23.41 (C-6), 35.36 (C-7), 29.67 (C-8), 57.86 (C-9), 39.30 (C-10), 18.33 (C-11), 35.60 (C-12), 31.24 (C-13), 41.13 (C-14), 170.97 (C-15), 20.51 (C-16), 15.36 (C-17), 29.45 (C-18), 180.70 (C-19), 14.79 (C-20); Quinone: 184.36, 187.37 (C-1 and C-4), 149.02 (C-2), 141.29 (C-3), 137.32, 143.20 (C-4a, C-8a), 23.22, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.41 (C-1'), 37.90 (C-2'), 28.67 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H52O6 (568.3764). m/z 586.4086 [M+NH4]+, error: 0.20 ppm with the empirical formula C35H56NO6. +43 (c 0.014 g/100 mL CHCl3); IR νmax (film) 2956, 2846, 1772, 1729, 1662, 1464, 1452, 1385, 1208, 1193, 1151, 1077, 910 cm−1; 1H-NMR (CDCl3): 21 δ 3.57 (3H, s, OCH3), 2.60 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.31 (2H, m, quinone H-5 and H-8), 2.30 (1H, m, H-14 β), 2.07 (1H, d, J = 12.4 Hz, H-13), 1.62 (2H, m, quinone H-6 and H-7), 1.53 (2H, m, H-1'), 1.10 (3H, s, H-18), 1.01 (3H, d, J = 6.8 Hz, H-16), 0.84 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.82 (3H, d, J = 7.2 Hz, H-17), 0.60 (3H, s, H-20); 13C-NMR (CDCl3): 40.22 (C-1), 19.46 (C-2), 38.55 (C-3), 44.32 (C-4), 53.31 (C-5), 23.42 (C-6), 35.34 (C-7), 29.65 (C-8), 57.89 (C-9), 39.08 (C-10), 19.27 (C-11), 35.62 (C-12), 31.26 (C-13), 41.13 (C-14), 170.97 (C-15), 20.52 (C-16), 15.33 (C-17), 29.26 (C-18), 178.53 (C-19), 14.66 (C-20), 51.54 (OCH3); Quinone: 180.73, 187.37 (C-1 and C-4), 149.03 (C-2), 141.30 (C-3), 137.33, 143.21 (C-4a, C-8a), 23.07, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.23 (C-1'), 37.91 (C-2'), 28.68 (C-3'), 22.70 (C-4'), 22.70 (C-5'); molecular formula: C36H54O6 (582.3920). m/z 600.4242 [M+NH4]+, error: 2.85 ppm with the empirical formula C36H58NO6.
+43 (c 0.014 g/100 mL CHCl3); IR νmax (film) 2956, 2846, 1772, 1729, 1662, 1464, 1452, 1385, 1208, 1193, 1151, 1077, 910 cm−1; 1H-NMR (CDCl3): 21 δ 3.57 (3H, s, OCH3), 2.60 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.31 (2H, m, quinone H-5 and H-8), 2.30 (1H, m, H-14 β), 2.07 (1H, d, J = 12.4 Hz, H-13), 1.62 (2H, m, quinone H-6 and H-7), 1.53 (2H, m, H-1'), 1.10 (3H, s, H-18), 1.01 (3H, d, J = 6.8 Hz, H-16), 0.84 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.82 (3H, d, J = 7.2 Hz, H-17), 0.60 (3H, s, H-20); 13C-NMR (CDCl3): 40.22 (C-1), 19.46 (C-2), 38.55 (C-3), 44.32 (C-4), 53.31 (C-5), 23.42 (C-6), 35.34 (C-7), 29.65 (C-8), 57.89 (C-9), 39.08 (C-10), 19.27 (C-11), 35.62 (C-12), 31.26 (C-13), 41.13 (C-14), 170.97 (C-15), 20.52 (C-16), 15.33 (C-17), 29.26 (C-18), 178.53 (C-19), 14.66 (C-20), 51.54 (OCH3); Quinone: 180.73, 187.37 (C-1 and C-4), 149.03 (C-2), 141.30 (C-3), 137.33, 143.21 (C-4a, C-8a), 23.07, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.23 (C-1'), 37.91 (C-2'), 28.68 (C-3'), 22.70 (C-4'), 22.70 (C-5'); molecular formula: C36H54O6 (582.3920). m/z 600.4242 [M+NH4]+, error: 2.85 ppm with the empirical formula C36H58NO6. +31 (c 0.036, CHCl3); IR νmax (film) 2954, 2864, 1772, 1695, 1662, 1619, 1469, 1380 1263, 1207, 1134, 1114, 1077, 911 cm−1; 1H-NMR (CDCl3): δ 2.61 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.41 (4H, m, quinone H-5 and H-8), 2.40–2.35 (1H, m, 14 β), 2.40-2.33 (2H, m, H-1'), 2.08 (1H, m, H-13), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.30 (2H, m, H-2'), 1.24 (3H, s, H-18), 1.08 (3H, d, J = 6.6 Hz, H-16), 0.89 (3H, d, J = 6.6 Hz, H-4'), 0.89 (3H, d, J = 6.6, H-5'), 0.85 (3H, s, H-20), 13C-NMR (CDCl3): 37.22 (C-1), 19.89 (C-2), 37.38 (C-3), 44.18 (C-4), 53.92 (C-5), 21.45 (C-6), 37.16 (C-7), 127.18 (C-8), 139.35 (C-9), 40.13 (C-10), 26.13 (C-11), 34.62 (C-12), 31.75 (C-13), 41.35 (C-14), 170.87 (C-15), 19.88 (C-16), 20.11 (C-17), 29.04 (C-18), 180.68 (C-19), 18.29 (C-20); Quinone: 184.73, 187.36 (C-1 and C-4), 149.01 (C-2), 141.29 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.86 (C-5 and C-8), 21.45, 21.11 (C-6 and C-7), 22.28 (C-1'), 37.49 (C-2'), 28.66 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H50O6 (566.3607), m/z 567.3687 [M+H]+, error: 1.11 ppm with the empirical formula C35H51O6; m/z 584.3944 [M+NH4]+, error: 0.23 ppm with the empirical formula C35H54NO6.
+31 (c 0.036, CHCl3); IR νmax (film) 2954, 2864, 1772, 1695, 1662, 1619, 1469, 1380 1263, 1207, 1134, 1114, 1077, 911 cm−1; 1H-NMR (CDCl3): δ 2.61 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.41 (4H, m, quinone H-5 and H-8), 2.40–2.35 (1H, m, 14 β), 2.40-2.33 (2H, m, H-1'), 2.08 (1H, m, H-13), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.30 (2H, m, H-2'), 1.24 (3H, s, H-18), 1.08 (3H, d, J = 6.6 Hz, H-16), 0.89 (3H, d, J = 6.6 Hz, H-4'), 0.89 (3H, d, J = 6.6, H-5'), 0.85 (3H, s, H-20), 13C-NMR (CDCl3): 37.22 (C-1), 19.89 (C-2), 37.38 (C-3), 44.18 (C-4), 53.92 (C-5), 21.45 (C-6), 37.16 (C-7), 127.18 (C-8), 139.35 (C-9), 40.13 (C-10), 26.13 (C-11), 34.62 (C-12), 31.75 (C-13), 41.35 (C-14), 170.87 (C-15), 19.88 (C-16), 20.11 (C-17), 29.04 (C-18), 180.68 (C-19), 18.29 (C-20); Quinone: 184.73, 187.36 (C-1 and C-4), 149.01 (C-2), 141.29 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.86 (C-5 and C-8), 21.45, 21.11 (C-6 and C-7), 22.28 (C-1'), 37.49 (C-2'), 28.66 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H50O6 (566.3607), m/z 567.3687 [M+H]+, error: 1.11 ppm with the empirical formula C35H51O6; m/z 584.3944 [M+NH4]+, error: 0.23 ppm with the empirical formula C35H54NO6. +22 (c 0.027, CHCl3); IR νmax (film) 2953, 2868, 1769, 1726, 1659, 1470, 1449, 1379, 1138, 1081, 916, 761 cm−1; 1H-NMR (CDCl3): δ 3.61 (3H, s, OCH3), 2.76 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.62 (1H, dd, J = 15.3, 8.1 Hz, H-14 β), 2.06 (1H, m, H-13), 2.40 (4H, m, quinone H-5 and H-8), 2.40-2.33 (2H, m, H-1'), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.27 (2H, m, H-2'), 1.18 (3H, s, H-18), 1.07 (3H, d, J = 6.8 Hz, H-16), 0.89 (3H, d, J = 6.4 Hz, H-4'), 0.89 (3H, d, J = 6.4, H-5'), 0.75 (3H, s, H-20), 13C-NMR (CDCl3): 37.63 (C-1), 19.98 (C-2), 37.89 (C-3), 44.28 (C-4), 53.93 (C-5), 21.25 (C-6), 37.62 (C-7), 127.18 (C-8), 139.42 (C-9), 39.95 (C-10), 26.12 (C-11), 34.71 (C-12), 31.75 (C-13), 41.36 (C-14), 170.88 (C-15), 19.89 (C-16), 20.14 (C-17), 28.86 (C-18), 178.55 (C-19), 18.14 (C-20), 51.51 (OCH3); Quinone: 180.69, 187.36 (C-1 and C-4), 149.00 (C-2), 141.28 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.71 (C-5 and C-8), 21.44, 21.44 (C-6 and C-7), 22.28 (C-1'), 38.13 (C-2'), 28.66 (C-3'), 22.84 (C-4'), 22.84 (C-5'); molecular formula: C36H52O6 (580.3764). m/z 581.3832 [M+H]+, error: 0.65 ppm with the empirical formula C36H53O6; m/z 598.4102 [M+NH4]+, error: 0.13 ppm with the empirical formula C36H56NO6.
+22 (c 0.027, CHCl3); IR νmax (film) 2953, 2868, 1769, 1726, 1659, 1470, 1449, 1379, 1138, 1081, 916, 761 cm−1; 1H-NMR (CDCl3): δ 3.61 (3H, s, OCH3), 2.76 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.62 (1H, dd, J = 15.3, 8.1 Hz, H-14 β), 2.06 (1H, m, H-13), 2.40 (4H, m, quinone H-5 and H-8), 2.40-2.33 (2H, m, H-1'), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.27 (2H, m, H-2'), 1.18 (3H, s, H-18), 1.07 (3H, d, J = 6.8 Hz, H-16), 0.89 (3H, d, J = 6.4 Hz, H-4'), 0.89 (3H, d, J = 6.4, H-5'), 0.75 (3H, s, H-20), 13C-NMR (CDCl3): 37.63 (C-1), 19.98 (C-2), 37.89 (C-3), 44.28 (C-4), 53.93 (C-5), 21.25 (C-6), 37.62 (C-7), 127.18 (C-8), 139.42 (C-9), 39.95 (C-10), 26.12 (C-11), 34.71 (C-12), 31.75 (C-13), 41.36 (C-14), 170.88 (C-15), 19.89 (C-16), 20.14 (C-17), 28.86 (C-18), 178.55 (C-19), 18.14 (C-20), 51.51 (OCH3); Quinone: 180.69, 187.36 (C-1 and C-4), 149.00 (C-2), 141.28 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.71 (C-5 and C-8), 21.44, 21.44 (C-6 and C-7), 22.28 (C-1'), 38.13 (C-2'), 28.66 (C-3'), 22.84 (C-4'), 22.84 (C-5'); molecular formula: C36H52O6 (580.3764). m/z 581.3832 [M+H]+, error: 0.65 ppm with the empirical formula C36H53O6; m/z 598.4102 [M+NH4]+, error: 0.13 ppm with the empirical formula C36H56NO6.3.3. Cell Culture
3.4. Cytotoxicity Assay
3.5. MRC-5 Fibroblast Proliferation Assay
3.6. Sodium Taurocholate-Induced Damage to AGS Cells
3.7. Determination of Prostaglandin E2 (PGE2) Content
3.8. Determination of Cellular Reduced Glutathione (GSH) Content
3.9. Inhibition of Lipoperoxidation in Erythrocyte Membranes
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schmeda-Hirschmann, G.; Astudillo, L.; Rodriguez, J.A.; Theoduloz, C.; Yañez, T. Gastroprotective effect of the Mapuche crud drug Araucaria araucana resin and its main constituents. J. Ethnopharmacol. 2005, 101, 271–276. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Papastergiou, F. Naphthoquinone derivatives and lignans from the Paraguayan crude drug “tayï pytá” (Tabebuia heptaphylla, Bignoniaceae). Z. Naturforsch. C 2003, 58, 495–501. [Google Scholar]
- Twardowschy, A.; Freitas, C.S.; Baggio, C.H.; Mayer, B.; dos Santos, A.C.; Pizzolatti, M.G.; Zacarias, A.A.; Pereira dos Santos, E.; Otuki, M.F.; Marques, M.C.A. Antiulcerogenic activity of bark extract of Tabebuia avellanedae, Lorentz ex Griseb. J. Ethnopharmacol. 2008, 118, 455–459. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Astudillo, L.; Sepulveda, B.; Rodriguez, J.A.; Theoduloz, C.; Yañez, T.; Palenzuela, J.A. Gastroprotective effect and cytotoxicity of natural and semisynthetic labdane diterpenes from Araucaria araucana. Z. Naturforsch. C 2005, 60, 511–522. [Google Scholar]
- Muregi, F.W.; Ishih, A. Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug. Develop. Res. 2010, 71, 20–32. [Google Scholar]
- Pertino, M.W.; Theoduloz, C.; Palenzuela, J.A.; Afonso, M.; Yesilada, E.; Monsalve, F.; González, P.; Droguett, D.; Schmeda-Hirschmann, G. Synthesis and pharmacological activity of diterpenylnaphthoquinone derivatives. Molecules 2011, 16, 8614–8628. [Google Scholar] [CrossRef]
- Theoduloz, C.; Carrión, I.B.; Pertino, M.W.; Schmeda-Hirschmann, G. Potential gastroprotective effect of novel cyperenoic acid/quinone derivatives in human cell cultures. Planta Med. 2012, 78, 1807–1812. [Google Scholar] [CrossRef]
- Dictionary of Natural Products [DVD]; CRC Press: Boca Raton, FL, USA, 2013.
- Miguel del Corral, J.M.; Castro, M.A.; Rodriguez, M.L.; Chamorro, P.; Cuevas, C.; Feliciano, A.S. New cytotoxic diterpenylnaphthohydroquinone derivatives obtained from a natural diterpenoid. Bioorg. Med. Chem. 2007, 15, 5760–5774. [Google Scholar] [CrossRef]
- Miguel del Corral, J.M; Castro, M.A.; Oliveira, A.B.; Gualberto, S.A.; Carmen, C.; Feliciano, A.S. New cytotoxic furoquinones obtained from terpenyl-1,4-naphthoquinones and 1,4-anthracenediones. Bioorg. Med. Chem. 2006, 14, 7231–7240. [Google Scholar] [CrossRef]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castaño, V.; Zapata, B.; del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; San Feliciano, A. Synthesis and antifungal activity of terpenyl-1,4-naphthoquinone and 1,4-anthracenedione derivatives. Eur. J. Med. Chem. 2013, 67, 19–27. [Google Scholar] [CrossRef]
- Kokoska, E.; Smith, G.; Deshpande, Y.; Wolff, A.; Miller, T. Indomethacin increasessusceptibility to injury in human gastric cells independent of PG synthesis inhibition. Am. J. Physiol. 1998, 275G, 620–628. [Google Scholar]
- Rodríguez, J.A.; Theoduloz, C.; Sánchez, M.; Razmilic, I.; Schmeda-Hirschmann, G. Gastroprotective and ulcer-healing effect of new solidagenone derivatives in human cell cultures. Life Sci. 2005, 77, 2193–205. [Google Scholar] [CrossRef]
- Ye, Y.N.; Liu, E.S.; Koo, M.W.; Li, Y.; Matsui, H.; Ch, C.H. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem. Pharmacol. 2001, 61, 1439–1448. [Google Scholar] [CrossRef]
- Romano, M.; Razandi, M.; Ivey, K. Effect of sucralfate and its components on taurocholate induced damage to rat gastric mucosal cells in tissue culture. Digest. Dis. Sci. 1990, 35, 467–476. [Google Scholar] [CrossRef]
- Ham, M.; Akiba, Y.; Takeuchi, K.; Montrose, M.H.; Kaunitz, J.D. Gastroduodenal Mucosal Defense. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L., Ed.; Academic Press: New York, USA, 2012; Volume 1, pp. 1169–1208. [Google Scholar]
- de-Faria, F.M.; Alves Almeida, A.C.; Luiz-Ferreira, A.; Dunder, R.J.; Takayama, C.; da Silva, M.S.; da Silva, M.A.; Vilegas, W.; Leite Rozza, A.; Pellizzon, C.H.; et al. Mechanisms of action underlying the gastric antiulcer activity of the Rhizophora mangle L. J. Ethnopharmacol. 2012, 139, 234–243. [Google Scholar] [CrossRef]
- Arakawa, T.; Higuchi, K.; Takashi, F. Prostaglandins in the stomach: an update. J. Clin. Gastroenterol. 1998, 27, S1–S11. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishihara, S. Role of growth factors in the repair of gastric mucosal injury. J. Gastroenterol. 2004, 39, 202–203. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Theoduloz, C.; Yáñez, T.; Becerra, J.; Schmeda-Hirschmann, G. Gastroprotective effect of the diterpene ferruginol in mice: Protection against membrane lipid peroxidation and involvement of prostaglandins. Life Sci. 2006, 78, 2503–2509. [Google Scholar] [CrossRef]
- Mutoh, H.; Hiraishi, H.; Ota, H.; Yoshida, H.; Ivey, K.; Terano, A.; Sugimoto, T. Protective role of extracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology 1990, 98, 1452–1459. [Google Scholar]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Kashima, Y.; Nakano, K.; Yoshikawa, M. Protective effects of polygodial and related compounds on ethanol-induced gastric mucosal lesions in rats: Structural requirements and mode of action. Bioorg. Med. Chem. Lett. 2002, 12, 477–482. [Google Scholar] [CrossRef]
- Kokoska, E.R.; Smith, G.S.; Deshpande, Y.; Wolff, A.B.; Rieckenberg, C.; Miller, T.A. Calcium accentuates injury induced by ethanol in human gastric cells. J. Gastro. Surg. 1999, 3, 308–318. [Google Scholar] [CrossRef]
- Smolka, A.J.; Goldenring, J.R.; Gupta, S.; Hammond, C.E. Inhibition of gastric H,K-ATPase activity and gastric epithelial cell IL-8 secretion by pyrrolizidine derivative ML 3000. BMC Gastroenterol. 2004, 10, 4–5. [Google Scholar]
- Singh, P.; Paul, K.; Holzer, W. Synthesis of pyrazole-based hybrid molecules: Search for potent multidrug resistance modulators. Bioorg. Med. Chem. 2006, 14, 5061–5071. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Multi-target drugs: Strategies and challenges for medicinal chemists. In The Practice of Medicinal Chemistry; Wermuth, C.G., Ed.; Elsevier-Academic Press: San Diego, CA, USA, 2008; pp. 549–571. [Google Scholar]
- Rodríguez, J.A.; Haun, M. Cytotoxicity of trans-dehydrocrotonin from Croton cajucara (Euphorbiaceae) on V79 cells and rat hepatocytes. Planta Med. 1999, 65, 522–526. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C.; Astudillo, L.; Feresin, G.E.; Tapia, A. Free radical scavengers and antioxidants from Peumus boldus Mol. (“Boldo”). Free Radical Res. 2003, 37, 447–452. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the starting products are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Theoduloz, C.; Bravo, I.; Pertino, M.W.; Schmeda-Hirschmann, G. Diterpenylquinone Hybrids: Synthesis and Assessment of Gastroprotective Mechanisms of Action in Human Cells. Molecules 2013, 18, 11044-11066. https://doi.org/10.3390/molecules180911044
Theoduloz C, Bravo I, Pertino MW, Schmeda-Hirschmann G. Diterpenylquinone Hybrids: Synthesis and Assessment of Gastroprotective Mechanisms of Action in Human Cells. Molecules. 2013; 18(9):11044-11066. https://doi.org/10.3390/molecules180911044
Chicago/Turabian StyleTheoduloz, Cristina, Ivanna Bravo, Mariano Walter Pertino, and Guillermo Schmeda-Hirschmann. 2013. "Diterpenylquinone Hybrids: Synthesis and Assessment of Gastroprotective Mechanisms of Action in Human Cells" Molecules 18, no. 9: 11044-11066. https://doi.org/10.3390/molecules180911044
APA StyleTheoduloz, C., Bravo, I., Pertino, M. W., & Schmeda-Hirschmann, G. (2013). Diterpenylquinone Hybrids: Synthesis and Assessment of Gastroprotective Mechanisms of Action in Human Cells. Molecules, 18(9), 11044-11066. https://doi.org/10.3390/molecules180911044