Rhinacanthus nasutus Protects Cultured Neuronal Cells against Hypoxia Induced Cell Death
Abstract
:1. Introduction
2. Results and Discussion
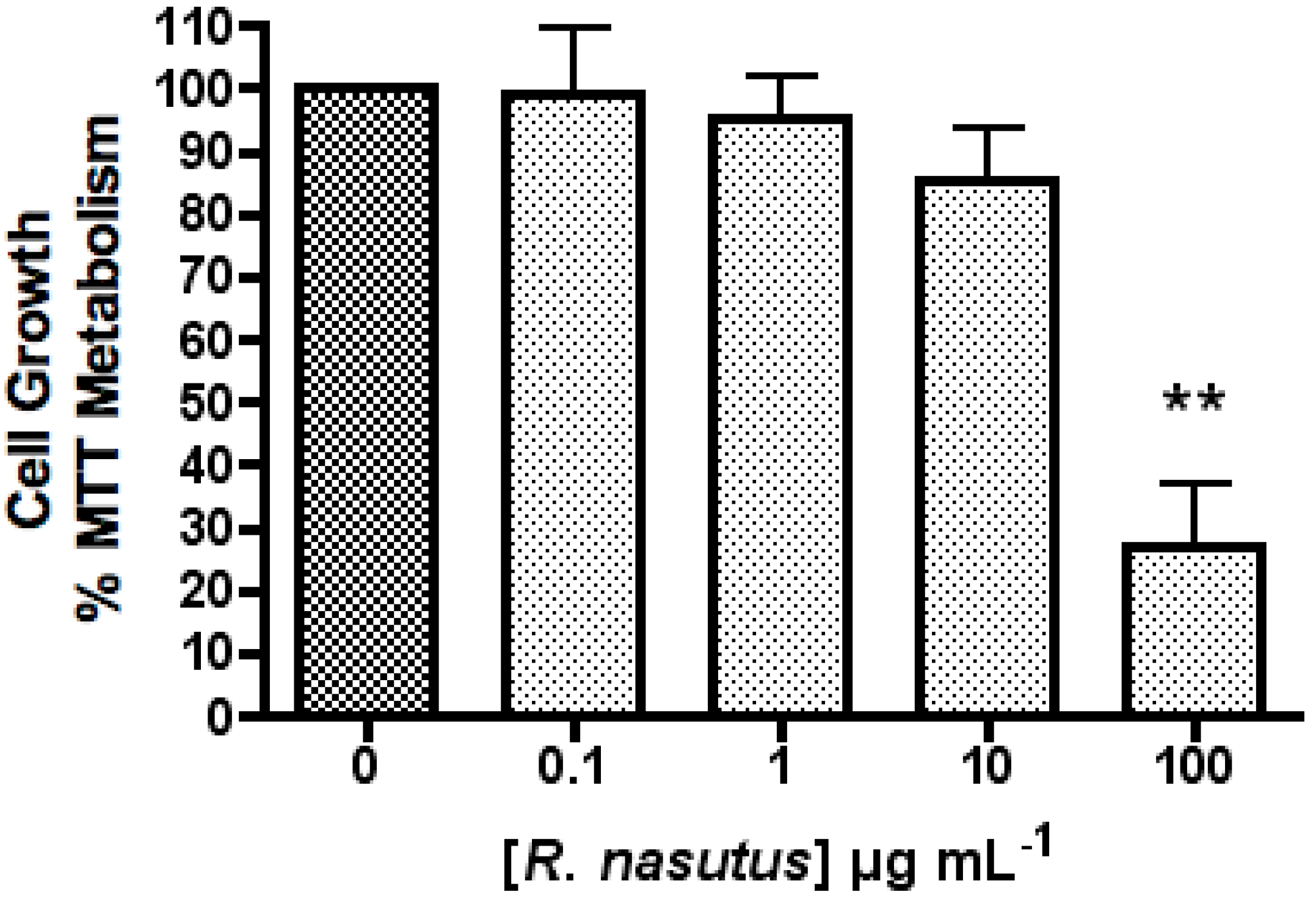
The Protective Effect of R. nasutus against Cell Death Induced by Hypoxia and Reoxygenation
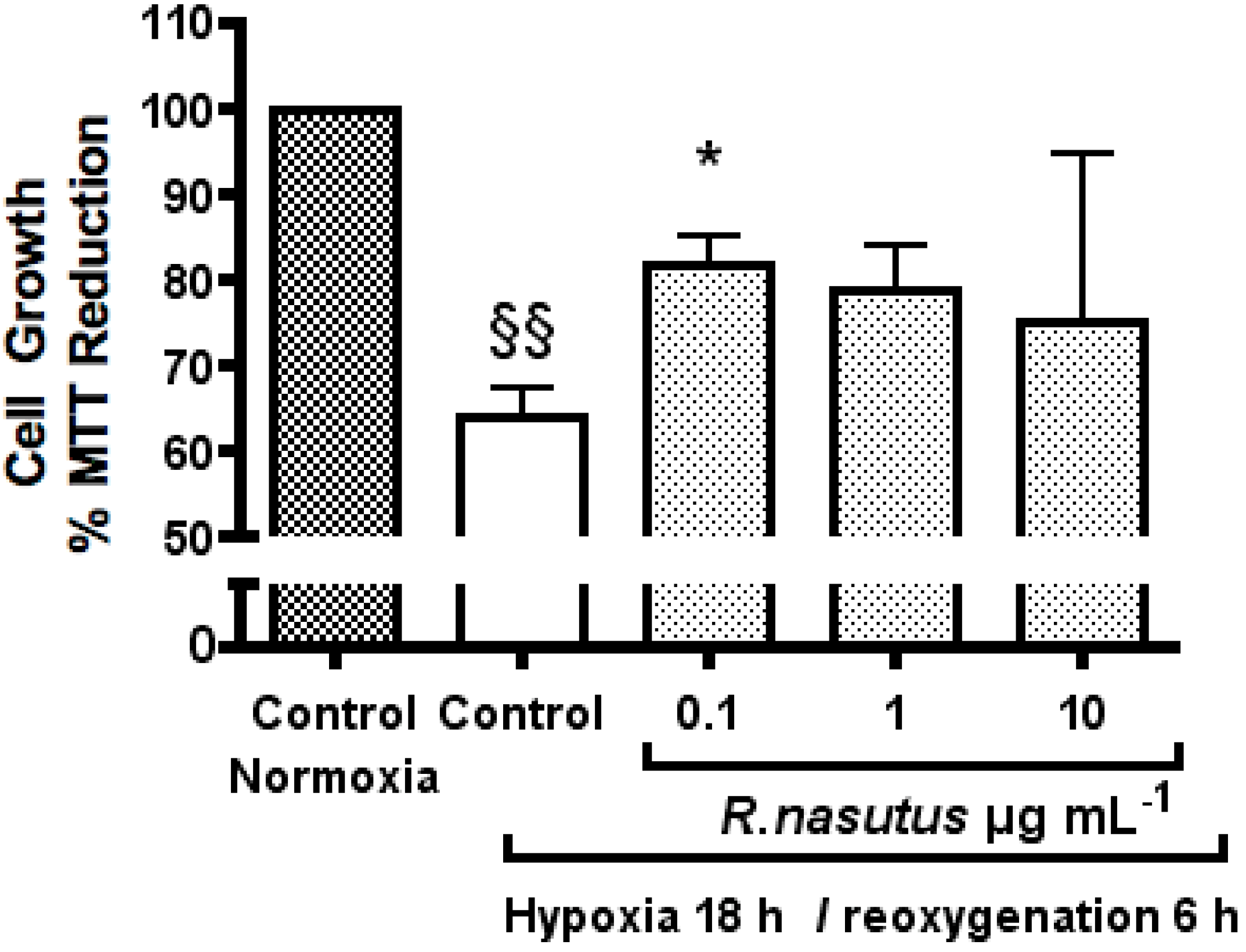
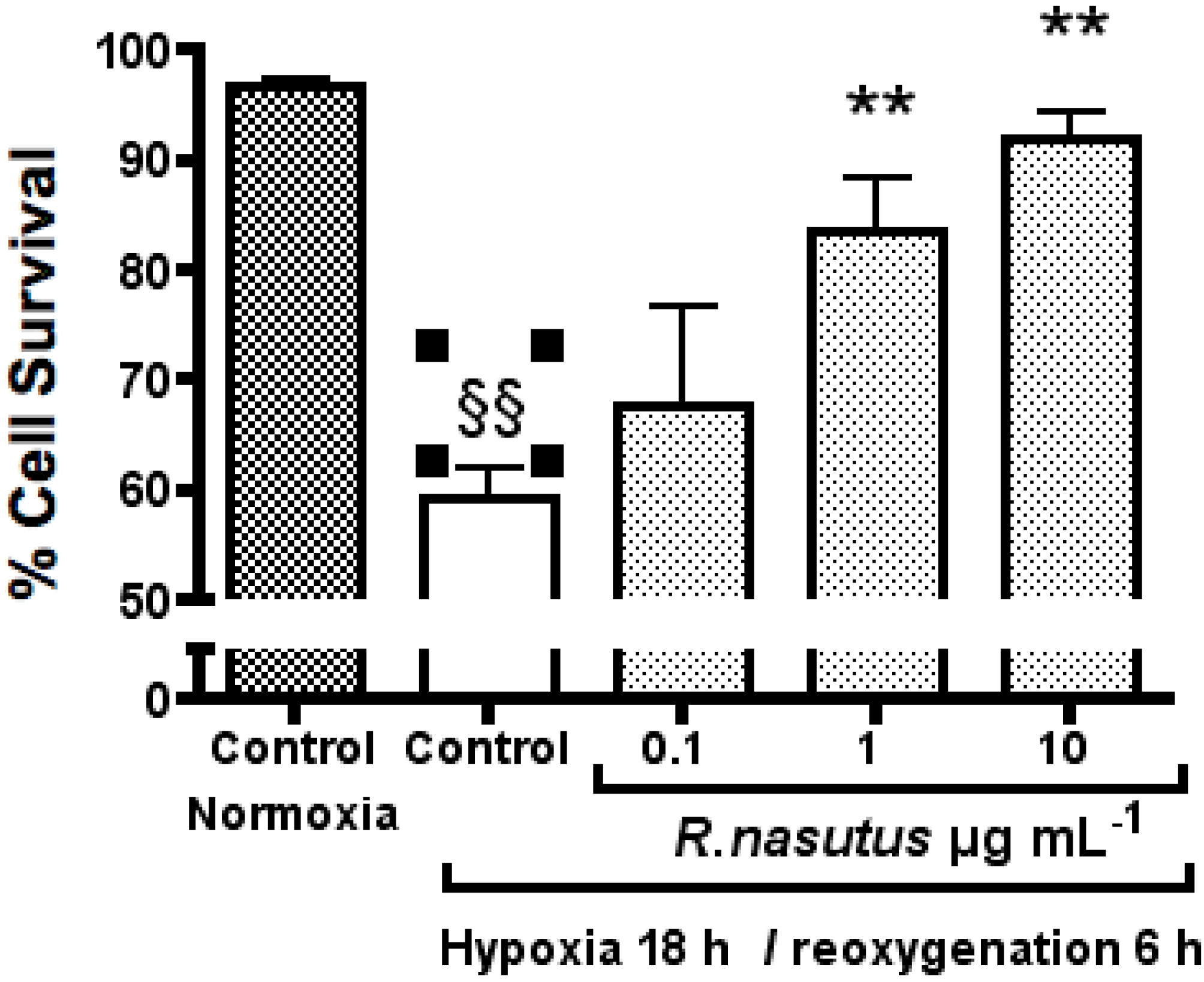
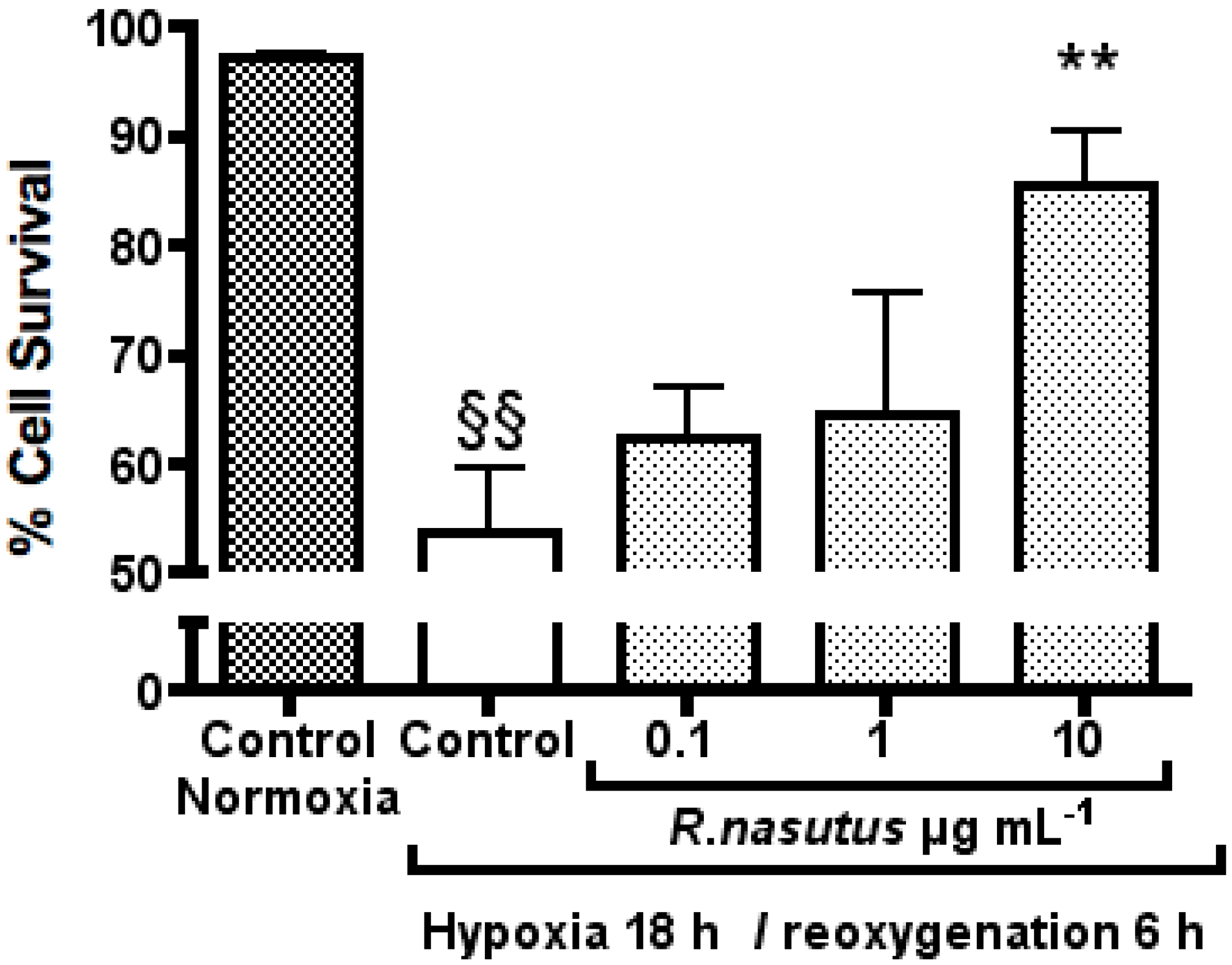
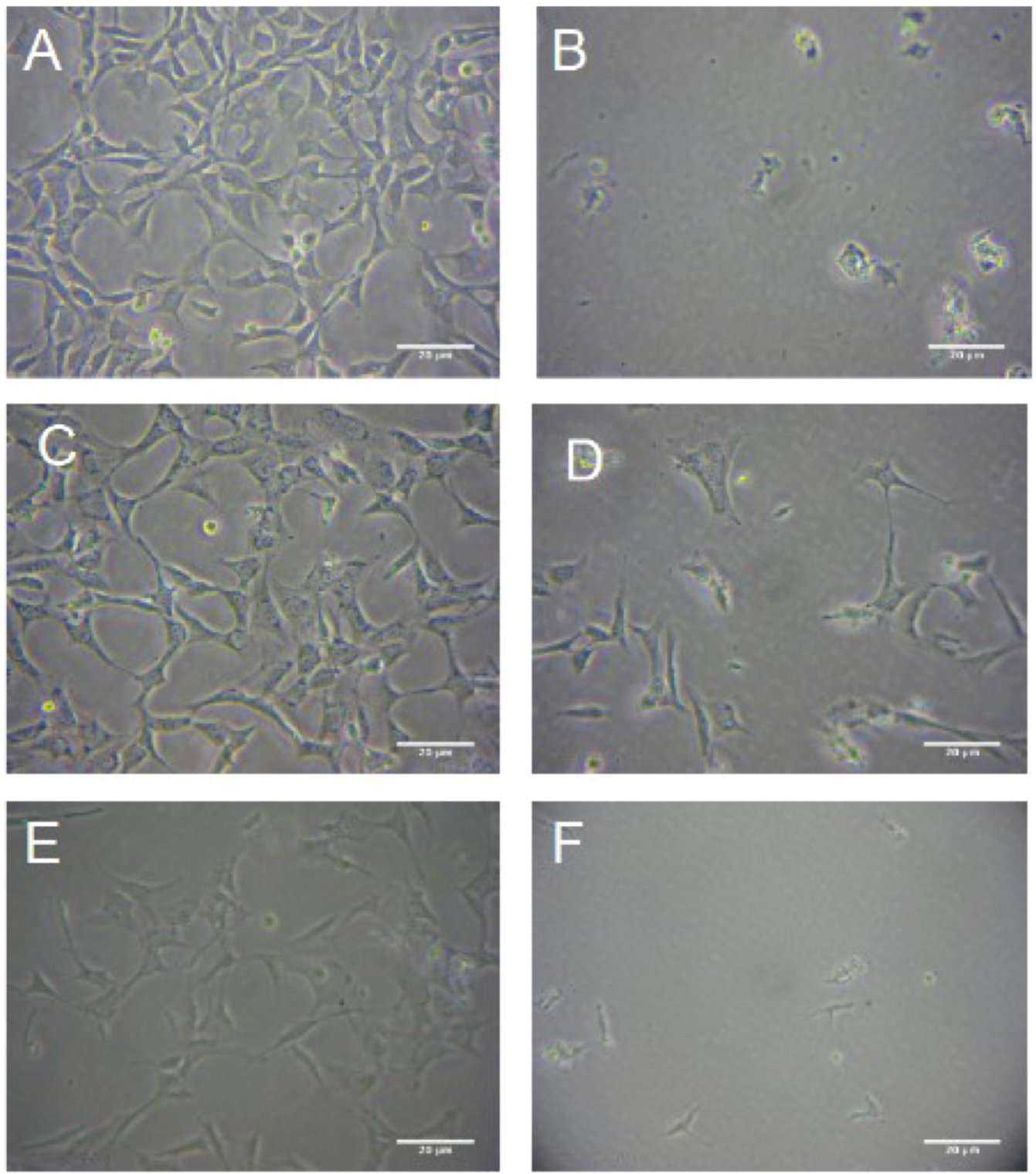
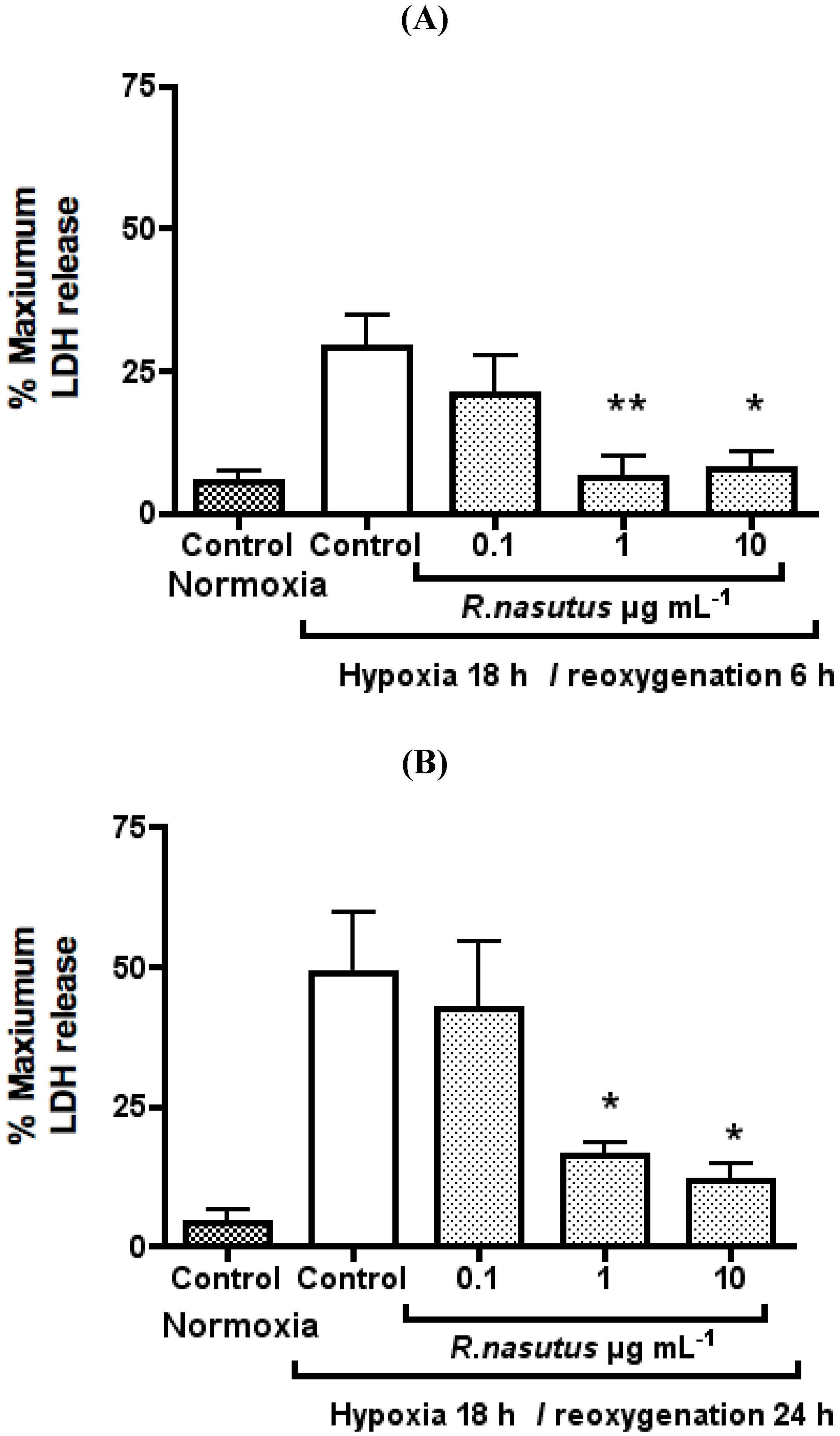
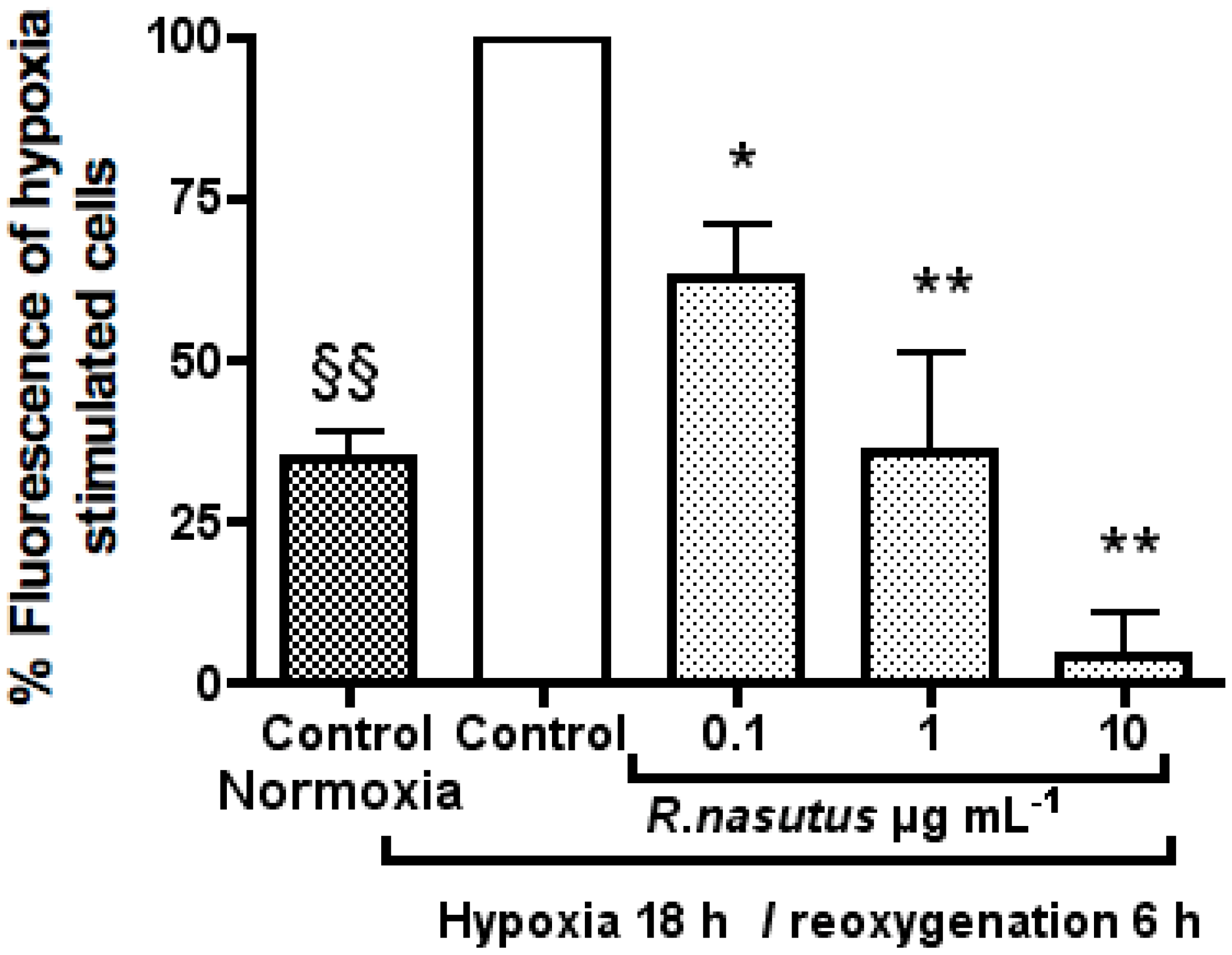
3. Experimental
3.1. Cell Culture
3.2. Plant Material
3.3. Hypoxia and Reoxygenation

3.4. MTT Assay
3.5. Trypan Blue Exclusion assay
3.6. LDH Assay
3.7. H2DCFDA Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Shimada, S.; Hirabayashi, M.; Ishige, K.; Kosuge, Y.; Kihara, T.; Ito, Y. Activation of dopamine D4 receptors is protective against hypoxia/reoxygenation-induced cell death in HT22 cells. J. Pharmacol. Sci. 2010, 114, 217–224. [Google Scholar]
- Hartman, P.; Ponder, R.; Lo, H.H.; Ishii, N. Mitochondrial oxidative stress can lead to nuclear hypermutability. Mech. Ageing Dev. 2004, 125, 417–420. [Google Scholar]
- Jefferson, J.A.; Simoni, J.; Escudero, E.; Hurtado, M.E.; Swenson, E.R.; Wesson, D.E.; Schreiner, G.F.; Schoene, R.B.; Johnson, R.J.; Hurtado, A. Increased oxidative stress following acute and chronic high altitude exposure. High Alt. Med. Biol. 2004, 5, 61–69. [Google Scholar]
- Jin, D.Q.; Lim, C.S.; Hwang, J.K.; Ha, I.; Han, J.S. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem. Biophys. Res. Commun. 2005, 331, 1264–1269. [Google Scholar]
- Kamiya, T.; Kwon, A.H.; Kanemaki, T.; Matsui, Y.; Uetsuji, S.; Okumura, T.; Kamiyama, Y. A simplified model of hypoxic injury in primary cultured rat hepatocytes. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 131–137. [Google Scholar] [CrossRef]
- Luo, H.; Huang, J.; Liao, W.G.; Huang, Q.Y.; Gao, Y.Q. The antioxidant effects of garlic saponins protect PC12 cells from hypoxia-induced damage. Br. J. Nutr. 2010, 105, 1164–1172. [Google Scholar]
- Cumming, R.C.; Schubert, D. Amyloid-beta induces disulfide bonding and aggregation of GAPDH in Alzheimer's disease. FASEB J. 2005, 19, 2060–2062. [Google Scholar]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol. Psychiatry 2000, 48, 755–765. [Google Scholar] [CrossRef]
- Sahay, A.; Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007, 10, 1110–1115. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar]
- Dranovsky, A.; Hen, R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol. Psychiatry 2006, 59, 1136–1143. [Google Scholar] [CrossRef]
- Charney, D.S.; Manji, H.K. Life stress, genes, and depression: Multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE 2004, 2004, re5. [Google Scholar] [CrossRef]
- Siripong, P.; Yahuafai, J.; Shimizu, K.; Ichikawa, K.; Yonezawa, S.; Asai, T.; Kanokmedakul, K.; Ruchirawat, S.; Oku, N. Induction of apoptosis in tumor cells by three naphthoquinone esters isolated from Thai medicinal plant: Rhinacanthus nasutus KURZ. Biol. Pharm. Bull. 2006, 29, 2070–2076. [Google Scholar] [CrossRef]
- Siripong, P.; Yahuafai, J.; Shimizu, K.; Ichikawa, K.; Yonezawa, S.; Asai, T.; Kanokmedakul, K.; Ruchirawat, S.; Oku, N. Antitumor activity of liposomal naphthoquinone esters isolated from Thai medicinal plant: Rhinacanthus nasutus KURZ. Biol. Pharm. Bull. 2006, 29, 2279–2283. [Google Scholar] [CrossRef]
- Thirumurugan, R.S.; Kavimani, S.; Srivastava, R.S. Antitumour activity of rhinacanthone against Dalton's ascitic lymphoma. Biol. Pharm. Bull. 2000, 23, 1438–1440. [Google Scholar] [CrossRef]
- Blignaut, E.; Patton, L.L.; Nittayananta, W.; Ramirez-Amador, V.; Ranganathan, K.; Chattopadhyay, A. (A3) HIV Phenotypes, oral lesions, and management of HIV-related disease. Adv. Dent. Res. 2006, 19, 122–129. [Google Scholar] [CrossRef]
- Sendl, A.; Chen, J.L.; Jolad, S.D.; Stoddart, C.; Rozhon, E.; Kernan, M.; Nanakorn, W.; Balick, M. Two new naphthoquinones with antiviral activity from Rhinacanthus nasutus. J. Nat. Prod. 1996, 59, 808–811. [Google Scholar] [CrossRef]
- Punturee, K.; Wild, C.P.; Kasinrerk, W.; Vinitketkumnuen, U. Immunomodulatory activities of Centella asiatica and Rhinacanthus nasutus extracts. Asian Pac. J. Cancer Prev. 2005, 6, 396–400. [Google Scholar]
- Thongrakard, V.; Tencomnao, T. Modulatory effects of Thai medicinal plant extract on proinflammatory cytokines-induced apoptosis in human keratinocyte HaCaT cells. African J. Biotechnol. 2010, 9, 4999–5003. [Google Scholar]
- Upendra, R.M.; Sreenivasulu, M; Chengaiah, B.; Ravikrishna, D.; Jaganmohan, R.K.; Sangeetha, K.; Chetty, C.M. Rhinacanthus nasutus (LINN.) kurz: A comprahensive review. Int. J. Pharm. Res. Dev. 2010, 2. article no.-7. [Google Scholar]
- Punturee, K.; Wild, C.P.; Vinitketkumneun, U. Thai medicinal plants modulate nitric oxide and tumor necrosis factor-alpha in J774.2 mouse macrophages. J. Ethnopharmacol. 2004, 95, 183–189. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S.K. Nitric oxide and major depression. Nitric Oxide 2011, 24, 125–131. [Google Scholar] [CrossRef]
- Shyamal, S.; Latha, P.G.; Suja, S.R.; Shine, V.J.; Anuja, G.I.; Sini, S.; Pradeep, S.; Shikha, P.; Rajasekharan, S. Hepatoprotective effect of three herbal extracts on aflatoxin B1-intoxicated rat liver. Singapore Med. J. 2010, 51, 326–331. [Google Scholar]
- Kodama, O.; Ichikawa, H.; Akatsuka, T.; Santisopasri, V.; Kato, A.; Hayashi, Y. Isolation and identification of an antifungal naphthopyran derivative from Rhinacanthus nasutus. J. Nat. Prod. 1993, 56, 292–294. [Google Scholar] [CrossRef]
- Wu, T.-S.; Tien, H.-J.; Yeh, M.-Y.; Lee, K.-H. solation and cytotoxicity of rhinacanthin-A and -B, two; naphthoquinones, from Rhinacanthus nasutus. Phytochemistry 1988, 27, 3787–3788. [Google Scholar]
- Wu, T.-S.; Yang, C.-C.; Wu, P.-L.; Liu, L.-K. A quinol and steroids from the leaves and stems of Rhinacanthus nasutu. Phytochemistry 1995, 40, 1247–1249. [Google Scholar] [CrossRef]
- Wu, T.S.; Hsu, H.C.; Wu, P.L.; Leu, Y.L.; Chan, Y.Y.; Chern, C.Y.; Yeh, M.Y.; Tien, H.J. Naphthoquinone esters from the root of Rhinacanthus nasutus. Chem. Pharm. Bull. (Tokyo) 1998, 46, 413–418. [Google Scholar] [CrossRef]
- Nirmaladevi, R.; Padma, P.R.; Kavitha, D. Analysis of the methanolic extract of the leaves of Rhinacanthus nasutus. J. Med. Plant Res. 2010, 4, 1554–1560. [Google Scholar]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Tetsuka, T.; Baier, L.D.; Morrison, A.R. Antioxidants inhibit interleukin-1-induced cyclooxygenase and nitric-oxide synthase expression in rat mesangial cells. Evidence for post-transcriptional regulation. J. Biol. Chem. 1996, 271, 11689–11693. [Google Scholar] [CrossRef]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Kimura, Y.; Dargusch, R.; Schubert, D.; Kimura, H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox. Signal. 2006, 8, 661–670. [Google Scholar]
- Suh, H.W.; Kang, S.; Kwon, K.S. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol. Cell. Biochem. 2007, 298, 187–194. [Google Scholar] [CrossRef]
- Sucontphunt, A.; De-Eknamkul, W.; Nimmannit, U.; Dan Dimitrijevich, S.; Gracy, R.W. Protection of HT22 neuronal cells against glutamate toxicity mediated by the antioxidant activity of Pueraria candollei var. mirifica extracts. J. Nat. Med. 2011, 65, 1–8. [Google Scholar] [CrossRef]
- Jamarkattel-Pandit, N.; Pandit, N.R.; Kim, M.Y.; Park, S.H.; Kim, K.S.; Choi, H.; Kim, H.; Bu, Y. Neuroprotective effect of defatted sesame seeds extract against in vitro and in vivo ischemic neuronal damage. Planta Med. 2010, 76, 20–26. [Google Scholar] [CrossRef]
- Nath, R.; Probert, A., Jr.; McGinnis, K.M.; Wang, K.K. Evidence for activation of caspase-3-like protease in excitotoxin- and hypoxia/hypoglycemia-injured neurons. J. Neurochem. 1998, 71, 186–195. [Google Scholar]
- Krishnamurthy, R.G.; Senut, M.C.; Zemke, D.; Min, J.; Frenkel, M.B.; Greenberg, E.J.; Yu, S.W.; Ahn, N.; Goudreau, J.; Kassab, M.; et al. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J. Neurosci. Res. 2009, 87, 2541–2550. [Google Scholar] [CrossRef]
- Gotoh, A.; Sakaeda, T.; Kimura, T.; Shirakawa, T.; Wada, Y.; Wada, A.; Kimachi, T.; Takemoto, Y.; Iida, A.; Iwakawa, S.; et al. Antiproliferative activity of Rhinacanthus nasutus (L.) Kurz extracts and the active moiety, Rhinacanthin C. Biol. Pharm. Bull. 2004, 27, 1070–1074. [Google Scholar]
- Siripong, P.; Kanokmedakul, K.; Piyaviriyagul, S.; Yahusfai, J.; Chanpai, R.; Ruchirawat, S.; Oku, N. Antiproliferative naphthoquinone esters from Rhinacanthus nasutus Kurz. roots on various cancer cells. J. Trad. Med. 2006, 23, 166–172. [Google Scholar]
- Tamatani, M.; Mitsuda, N.; Matsuzaki, H.; Okado, H.; Miyake, S.; Vitek, M.P.; Yamaguchi, A.; Tohyama, M. A pathway of neuronal apoptosis induced by hypoxia/reoxygenation: Roles of nuclear factor-kappaB and Bcl-2. J. Neurochem. 2000, 75, 683–693. [Google Scholar]
- Gonzalez, G.; Celedon, G.; Sandoval, M.; Gonzalez, G.E.; Ferrer, V.; Astete, R.; Behn, C. Hypobaric hypoxia-reoxygenation diminishes band 3 protein functions in human erythrocytes. Pflugers Arch. 2002, 445, 337–341. [Google Scholar]
- Ye, R.; Li, N.; Han, J.; Kong, X.; Cao, R.; Rao, Z.; Zhao, G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci. Res. 2009, 64, 306–310. [Google Scholar] [CrossRef]
- Zhang, Y.; Feustel, P.J.; Kimelberg, H.K. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 1094, 200–206. [Google Scholar]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar]
- Rawal, A.K.; Muddeshwar, M.G.; Biswas, S.K. Rubia cordifolia, Fagonia cretica linn and Tinospora cordifolia exert neuroprotection by modulating the antioxidant system in rat hippocampal slices subjected to oxygen glucose deprivation. BMC Complement. Altern. Med. 2004, 4, 11. [Google Scholar] [CrossRef]
- Medling, B.D.; Bueno, R.; Chambers, C.; Neumeister, M.W. The effect of vitamin E succinate on ischemia reperfusion injury. Hand (NY) 2009. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yonetani, M.; Nakamura, H. Selective brain hypothermia protects against hypoxic-ischemic injury in newborn rats by reducing hydroxyl radical production. Kobe J. Med. Sci. 2003, 49, 83–91. [Google Scholar]
- Dhar-Mascareno, M.; Carcamo, J.M.; Golde, D.W. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic. Biol. Med. 2005, 38, 1311–1322. [Google Scholar] [CrossRef]
- Katoh, C.; Osanai, T.; Tomita, H.; Okumura, K. Brain natriuretic peptide is released from human astrocytoma cell line U373MG under hypoxia: A possible role in anti-apoptosis. J. Endocrinol. 2011, 208, 51–57. [Google Scholar]
- Abe, T.; Unno, M.; Takeuchi, H.; Kakita, T.; Katayose, Y.; Rikiyama, T.; Morikawa, T.; Suzuki, M.; Matsuno, S. A new free radical scavenger, edaravone, ameliorates oxidative liver damage due to ischemia-reperfusion in vitro and in vivo. J. Gastrointest. Surg. 2004, 8, 604–615. [Google Scholar] [CrossRef]
- Kaibori, M.; Inoue, T.; Tu, W.; Oda, M.; Kwon, A.H.; Kamiyama, Y.; Okumura, T. FK506, but not cyclosporin A, prevents mitochondrial dysfunction during hypoxia in rat hepatocyte. Life Sci. 2001, 69, 17–26. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the R. nasutus extract are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brimson, J.M.; Tencomnao, T. Rhinacanthus nasutus Protects Cultured Neuronal Cells against Hypoxia Induced Cell Death. Molecules 2011, 16, 6322-6338. https://doi.org/10.3390/molecules16086322
Brimson JM, Tencomnao T. Rhinacanthus nasutus Protects Cultured Neuronal Cells against Hypoxia Induced Cell Death. Molecules. 2011; 16(8):6322-6338. https://doi.org/10.3390/molecules16086322
Chicago/Turabian StyleBrimson, James M., and Tewin Tencomnao. 2011. "Rhinacanthus nasutus Protects Cultured Neuronal Cells against Hypoxia Induced Cell Death" Molecules 16, no. 8: 6322-6338. https://doi.org/10.3390/molecules16086322
APA StyleBrimson, J. M., & Tencomnao, T. (2011). Rhinacanthus nasutus Protects Cultured Neuronal Cells against Hypoxia Induced Cell Death. Molecules, 16(8), 6322-6338. https://doi.org/10.3390/molecules16086322





