A Facile Synthesis of Highly Functionalized 4-Arylcoumarins via Kostanecki Reactions Mediated by DBU
Abstract
:1. Introduction
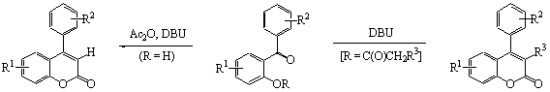
2. Results and Discussion
| Entry a,b | 1 | R1 | R2 | Conditions | 2 or 3 (% yield) c | Lit. |
|---|---|---|---|---|---|---|
| 1 | 1a | H | H | KOAc, 150 °C, 4 h | 2a (94) | |
| 2 | KOAc, 190 °C, 72 h | 3a (71) | ||||
| 3 | piperidine, 150 °C, 72 h | 2a (95) | ||||
| 4 | DABCO, MeCN, reflux, 20 h | 3a (17) d | ||||
| 5 | DBN, MeCN, reflux, 20 h | 3a (48) d | ||||
| 6 | DBU, MeCN, reflux, 8 h | 3a (86) | ||||
| 7 | DBU, MeCN, rt, 20 h | 3a (82) | [14,16] | |||
| 8 | 1b | 5-CH3 | H | DBU, MeCN, rt, 10 h | 3b (89) | [14] |
| 9 | 1c | 5-F | 4’-Cl | DBU, MeCN, rt, 30 h | 3c (82) | - |
| 10 | 1d | 4,6-(CH3)2 | 4’-CH3 | DBU, MeCN, rt, 20 h | 3d (53) | [14] |
| Entry | 4 | Time (h) | 5 | Yield (%) | ||
|---|---|---|---|---|---|---|
| 1 | 4a | 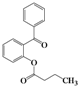 | 72 | 5a | 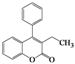 | 47 |
| 2 | 4b | 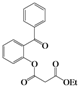 | 72 | 5bb | 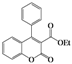 | 38 |
| 3 | 4c | 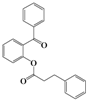 | 72 | 5c |  | 48 |
| 4 | 4d | 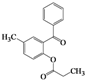 | 20 | 5d | 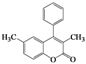 | 43 |
| 5 | 4e | 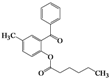 | 40 | 5e |  | 39 |
| 6 | 4f | 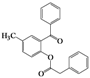 | 2 | 5f | 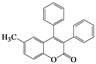 | 60 |
| 7 | 4g | 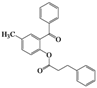 | 20 | 5g | 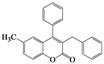 | 48 |
| 8 | 4h | 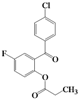 | 3 | 5h | 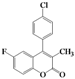 | 40 |
| 9 | 4i | 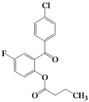 | 5 | 5i |  | 44 |
| 10 | 4j |  | 6 | 5j | 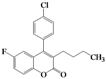 | 35 |
| 11 | 4k |  | 2 | 5k | 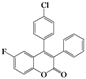 | 50 |
| 12 | 4l | 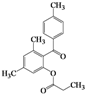 | 20 | 5l |  | 42 |
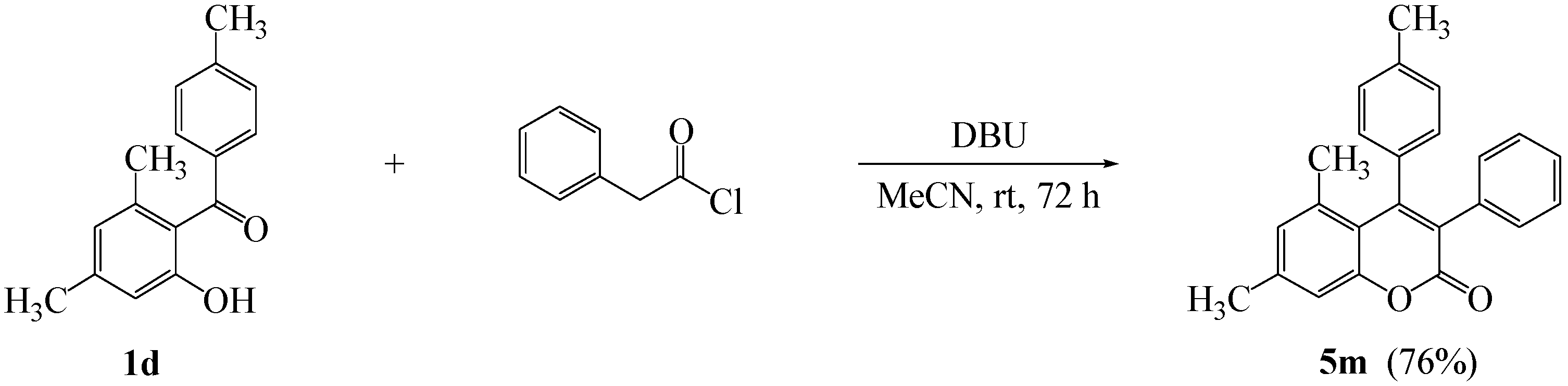
3. Experimental
3.1. General
3.2. Typical Procedure for the Synthesis of 4-Phenyl-2H-chromen-2-one (3a)
3.3. Typical Procedure for the Synthesis of 3,6-Dimethyl-4-phenyl-2H-chromen-2-one (5d)
3.4. Typical Procedure for the Synthesis of 5,7-Dimethyl-3-phenyl-4-(4’-methylphenyl)-2H-chromen-2-one (5m)
4. Conclusions
Acknowledgments
References and Notes
- Riveiro, M.E.; De Kimpe, N.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar]
- Bailly, C.; Bal, C.; Barbier, P.; Combes, S.; Finet, J.-P.; Hildebrand, M.-P.; Peyrot, V.; Wattez, N. Synthesis and biological evaluation of 4-arylcoumarin analogues of combretastatins. J. Med. Chem. 2003, 46, 5437–5444. [Google Scholar]
- Yeh, J.-Y.; Coumar, M.S.; Horng, J.-T.; Shiao, H.-Y.; Kuo, F.-M.; Lee, H.-L.; Chen, I.-C.; Chang, C.-W.; Tang, W.-F.; Tseng, S.-N.; et al. Anti-influenza drug discovery: Structure-activity relationship and mechanistic insight into novel angelicin derivatives. J. Med. Chem. 2010, 53, 1519–1533. [Google Scholar] [CrossRef]
- Pierson, J.-T.; Dumètre, A.; Hutter, S.; Delmas, F.; Laget, M.; Finet, J.-P.; Azas, N.; Combes, S. Synthesis and antiprotozoal activity of 4-arylcoumarins. Eur. J. Med. Chem. 2010, 45, 864–869. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of coumarins from the roots of ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar]
- Zhao, P.-L.; Wang, L.; Zu, X.-L.; Huang, X.; Zhau, C.-G.; Wu, J.-W.; Yang, G.-F. Subnanomolar inhibitor of cytochrome bc1 complex designed by optimizing interaction with conformationally flexible residues. J. Am. Chem. Soc. 2010, 132, 185–194. [Google Scholar]
- Teichert, J.F.; Feringa, B.L. Catalytic asymmetric conjugate reaction of Grignard reagents to coumarins-synthesis of versatile chiral building blocks. Chem. Commun. 2011, 47, 2679–2681. [Google Scholar] [CrossRef]
- Key, J.A.; Kho, S.; Timerghazin, Q.K.; Brown, A.; Cairo, C.W. Photophysical characterization of triazole-substituted coumarin fluorophores. Dyes Pigm. 2009, 82, 196–203. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, J.; Gao, J.; Han, L.; Li, Y.; Sheng, W. The one-pot synthesis and fluorimetric study of 3-(2′-benzothiazolyl)coumarins. Dyes Pigm. 2010, 86, 123–128. [Google Scholar] [CrossRef]
- Stoyanov, E.V.; Mezger, J. Pechmann reaction promoted by boron trifluoride dihydrate. Molecules 2005, 10, 762–766 and many references cited therein. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Lakishmi, G.; Damu, V.; Rao, Y.K.; Rao, C.V. A solvent-free synthesis of coumarins via Pechmann condensation using heterogeneous catalyst. J. Mol. Cat. A: Chem. 2006, 255, 49–52. [Google Scholar] [CrossRef]
- Tyagi, B.; Mishira, M.K.; Jasra, R.V. Microwave-assisted solvent free synthesis of hydroxy derivatives of 4-methyl coumarin using nano-crystalline sulfated-zirconia catalyst. J. Mol. Cat. A: Chem. 2008, 286, 41–46. [Google Scholar] [CrossRef]
- Potdar, M.K.; Rasalkar, M.S.; Mohile, S.S.; Salunkhe, M.M. Convenient and efficient protocols for coumarin synthesis via Pechmann condensation in neutral ionic liquids. J. Mol. Cat. A: Chem. 2005, 235, 249–252. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Venkatesh, V. Jadhav, D.N. Palladium-catalyzed synthesis of 4-arylcoumarins using triarylbismuth compounds as atom-efficient multicoupling organometallic nucleophiles. Eur. J. Org. Chem. 2010, 3945–3955. [Google Scholar]
- Ganina, O.G.; Daras, E.; Bourgarel-Rey, V.; Peyrot, V.; Andresyuk, A.N.; Finet, J.-P.; Fedorov, A.Y.; Beletskaya, I.P.; Combes, S. Synthesis and biological evaluation of polymethoxylated 4-heteroarylcoumarins as tubulin assembly inhibitor. Bioorg. Med. Chem. 2008, 16, 8806–8812. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Fathi, R.; Yang, Z. Palladium-catalyzed cross-coupling reactions of 4-tosylcoumarin and arylboronic acids: Synthesis of 4-arylcoumarin compounds. Tetrahedron Lett. 2002, 43, 4395–4397. [Google Scholar]
- Battistuzzi, G.; Cacchi, S.; Salve, I.D.; Fabrizi, G.; Parisi, L.M. Synthesis of coumarins in a molten n-Bu4NOAc/n-Bu4NBr mixture through a domino Heck reaction/cyclization process. Adv. Synth. Catal. 2005, 347, 308–312. [Google Scholar]
- Yamamoto, Y.; Kirai, N. Synthesis of 4-arylcoumarins via Cu-catalyzed hydroarylation with arylboronic acids. Org. Lett. 2008, 10, 5513–5516. [Google Scholar]
- Upadhyay, P.K.; Kumar, P. A novel synthesis of coumarins employing triphenyl(α-carboxymethylene)phosphorane imidazolide as a C-2 synthon. Tetrahedron Lett. 2009, 50, 236–238. [Google Scholar] [CrossRef]
- Ulubelen, A.; Kerr, R.R.; Mabry, T.J. Two new neoflavonoids and C-glycosylflavones from passiflora serratodigitata. Phytochemistry 1982, 21, 1145–1147. [Google Scholar]
- Bogdal, D. Coumarins: Fast synthesis by the Knoevenagel condensation under microwave irradiation. J. Chem. Res. (S) 1998, 468–469. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Kalinin, A.A.; Gubaidullin, A.T.; Litvinov, I.A.; Levin, Y.A. 3-Benzoylquinoxalin-2(1H)-one in the Kostanecki-Robinson reaction. Synthesis and structure of 2-oxo-4-phenylpyrano[2,3-b]quinoxaline. Chem. Heterocycl. Comp. 2003, 39, 96–100. [Google Scholar]
- Crecente-Campo, J.; Vázquez-Tato, M.P.; Seijas, J.A. Microwave-promoted, one-pot, solvent-free synthesis of 4-arylcoumarins from 2-hydroxybenzophenones. Eur. J. Org. Chem. 2010, 4130–4135. [Google Scholar]
- Cox, E.D. Action of anhydrous aluminium chloride on cresyl benzoates. J. Am. Chem. Soc. 1927, 49, 1028–1030. [Google Scholar] [CrossRef]
- Kaplan, J.P.; Raizon, B.M.; Desarmenien, M.; Feltz, P.; Headley, P.M.; Worms, P.; Lloyd, K.G.; Bartholini, G. New anticonvulsants: Schiff bases of γ-aminobutyric acid and γ-aminobutyramide. J. Med. Chem. 1980, 23, 702–704. [Google Scholar] [CrossRef]
- Schio, L.; Chatreaux, F.; Klich, M. Tosylates in palladium-catalyzed coupling reactions. Application to the synthesis of arylcoumarin inhibitors of gyrase B. Tetrahedron Lett. 2000, 41, 1543–1547. [Google Scholar]
- Königs, P.; Neumann, O.; Hackelöer, K.; Kataeva, O.; Waldvogel, S.R. Versatile one-pot synthesis of 3-alkenylcoumarins. Eur. J. Org. Chem. 2008, 343–349. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hwang, I.-T.; Lee, S.-A.; Hwang, J.-S.; Lee, K.-I. A Facile Synthesis of Highly Functionalized 4-Arylcoumarins via Kostanecki Reactions Mediated by DBU. Molecules 2011, 16, 6313-6321. https://doi.org/10.3390/molecules16086313
Hwang I-T, Lee S-A, Hwang J-S, Lee K-I. A Facile Synthesis of Highly Functionalized 4-Arylcoumarins via Kostanecki Reactions Mediated by DBU. Molecules. 2011; 16(8):6313-6321. https://doi.org/10.3390/molecules16086313
Chicago/Turabian StyleHwang, In-Taek, Sun-Ah Lee, Jin-Soo Hwang, and Kee-In Lee. 2011. "A Facile Synthesis of Highly Functionalized 4-Arylcoumarins via Kostanecki Reactions Mediated by DBU" Molecules 16, no. 8: 6313-6321. https://doi.org/10.3390/molecules16086313
APA StyleHwang, I.-T., Lee, S.-A., Hwang, J.-S., & Lee, K.-I. (2011). A Facile Synthesis of Highly Functionalized 4-Arylcoumarins via Kostanecki Reactions Mediated by DBU. Molecules, 16(8), 6313-6321. https://doi.org/10.3390/molecules16086313