Exogenous Application of Biostimulants and Synthetic Growth Promoters Improved the Productivity and Grain Quality of Quinoa Linked with Enhanced Photosynthetic Pigments and Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
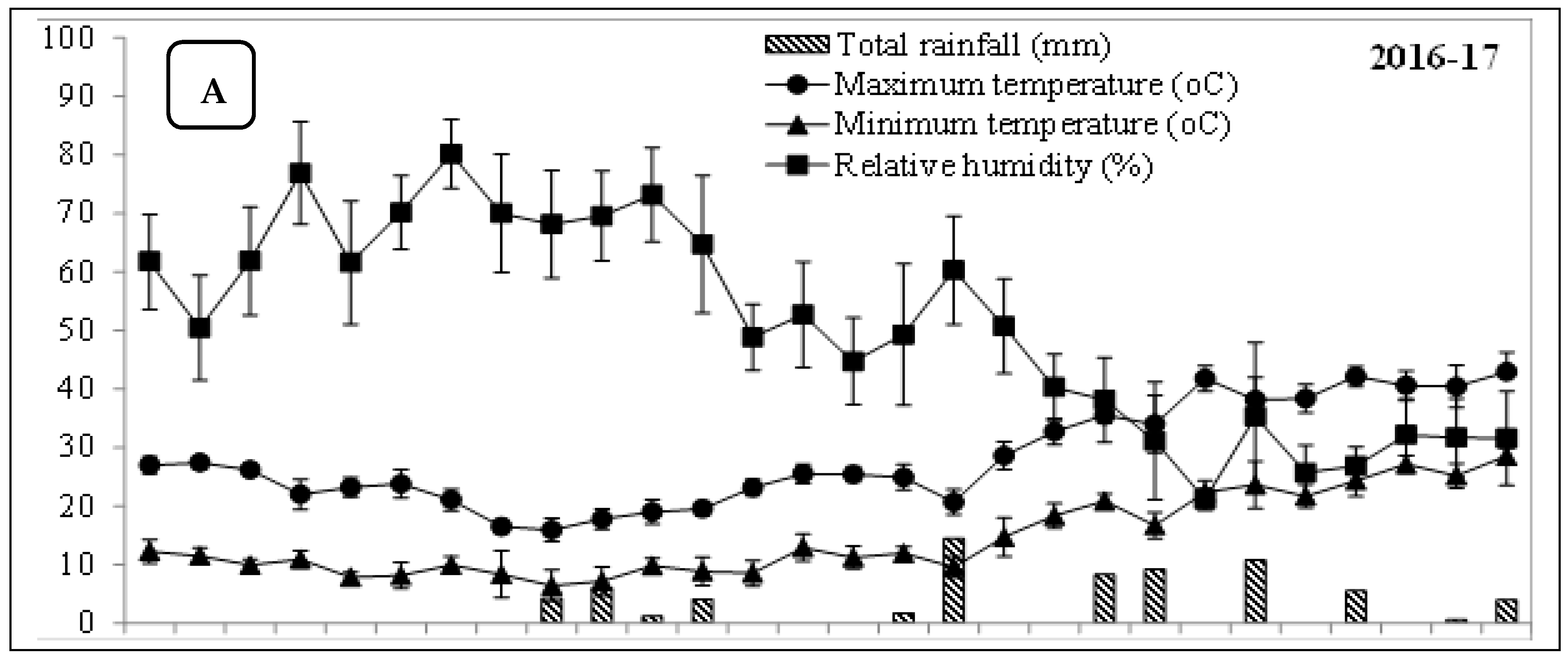
2.1. Experimental Particulars
2.2. Treatment Plan and Implementation
2.3. Estimation of Leaf Physiological and Biochemical Attributes
2.4. Estimation of Seed Quality Attributes and Root Shoot Minerals
2.5. Measurement of Growth and Yield Parameters
2.6. Statistical Analysis
3. Results
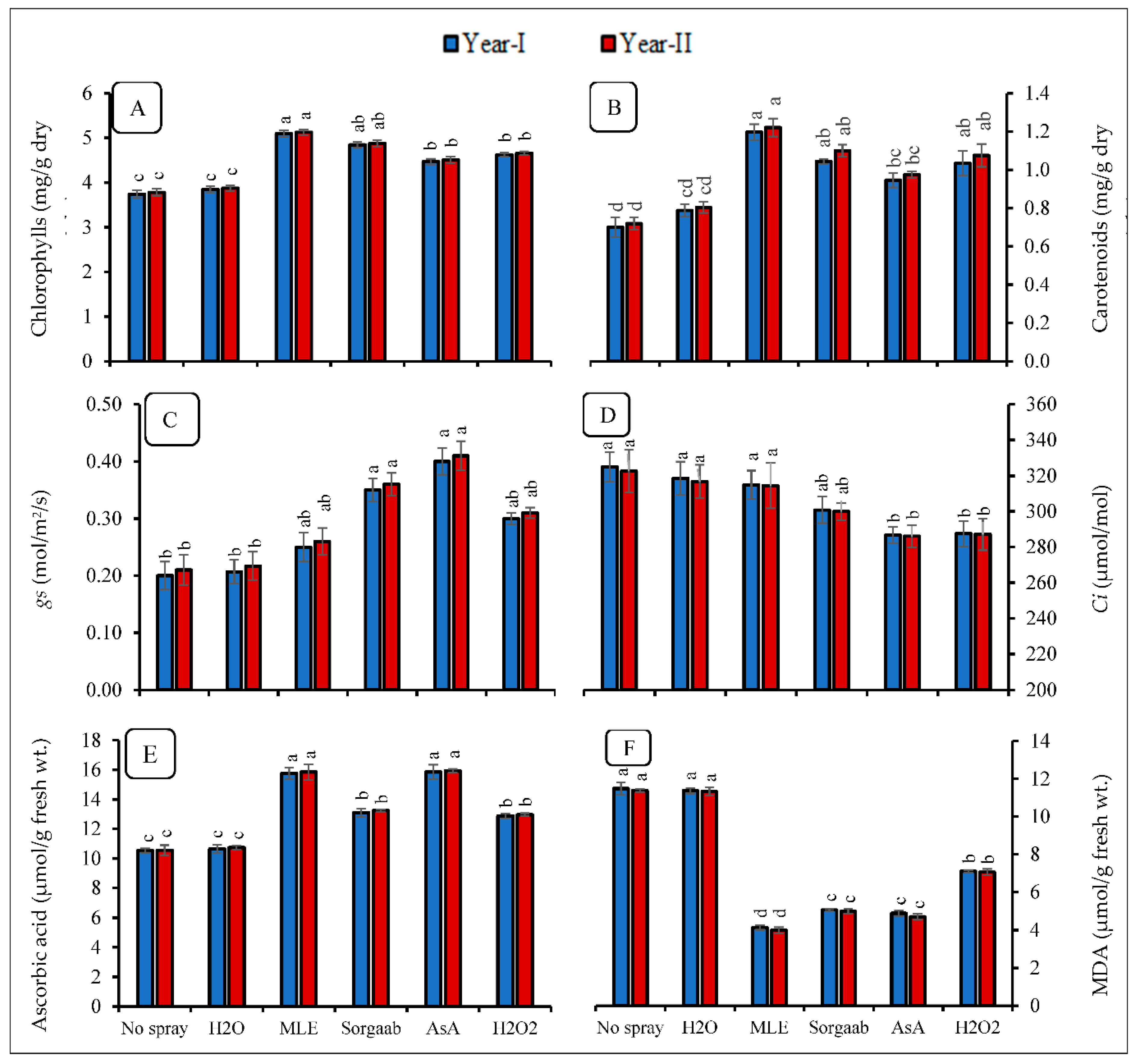
3.1. Photosynthetic Pigments and Gas Exchange Attributes
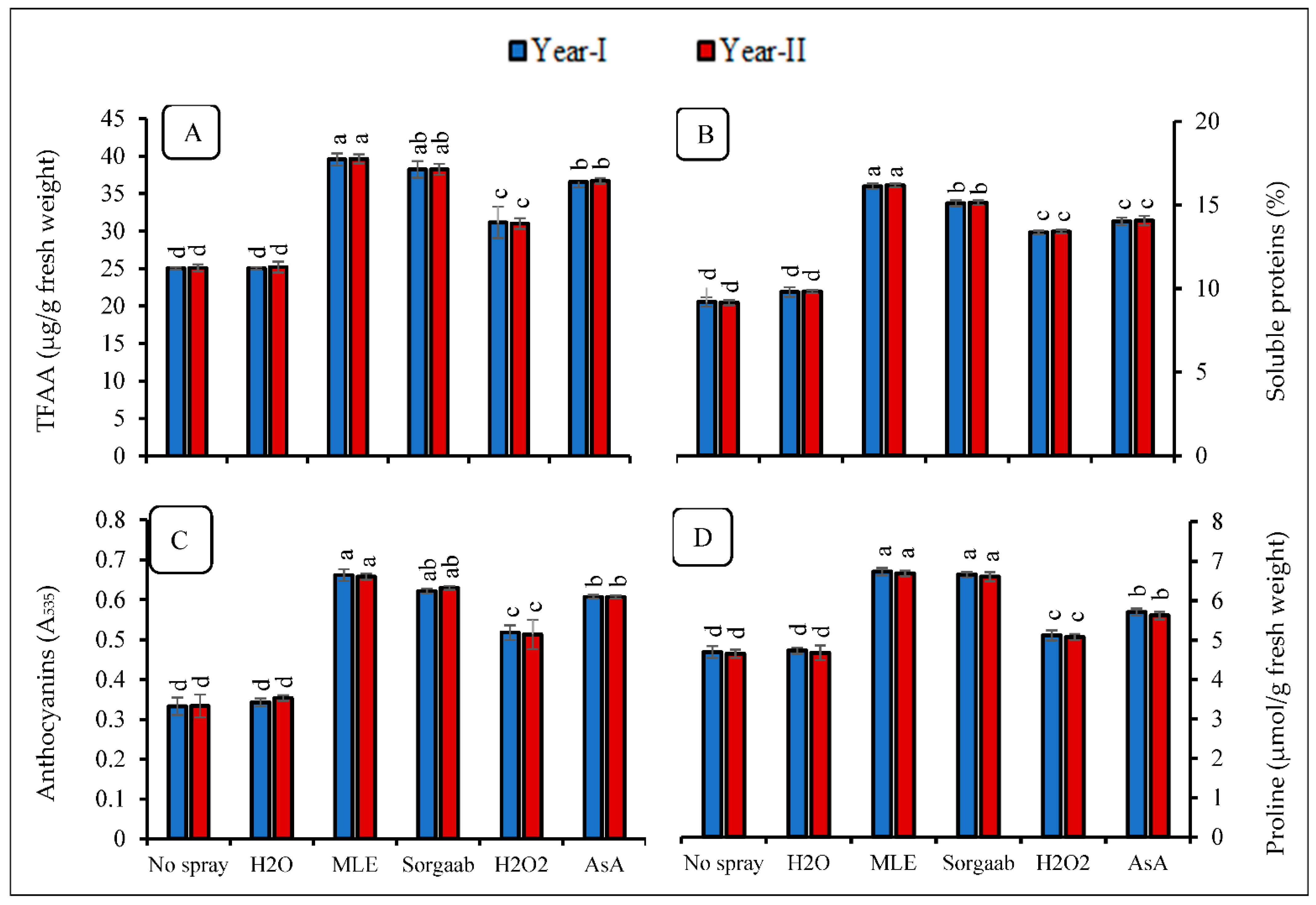
3.2. Metabolomics
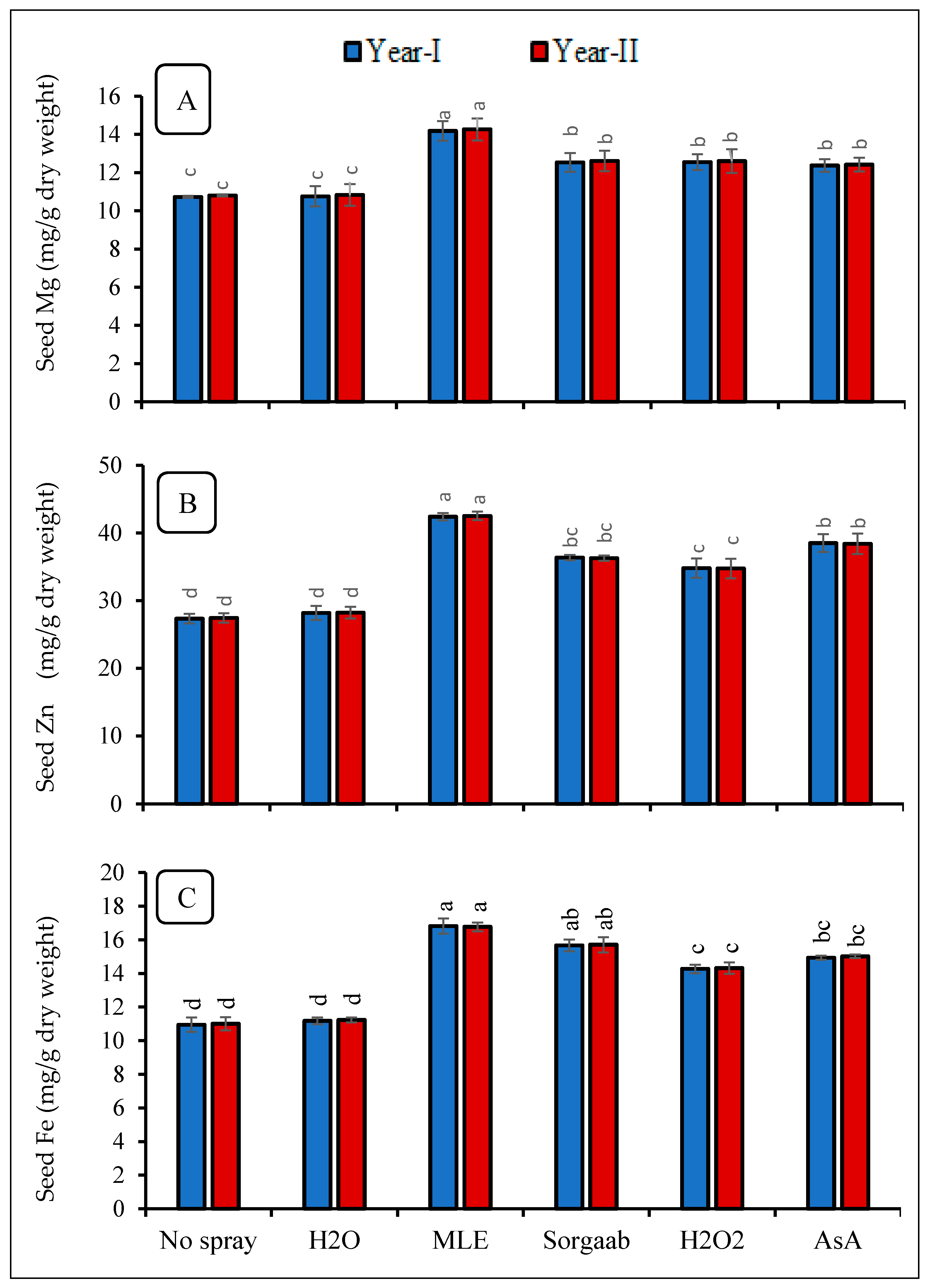
3.3. Mineral Nutrients
3.4. Growth Parameters
3.5. Grain Yield and Quality Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Quinoa Production 2010–2019. 2021. Available online: https://www.statista.com/statistics/486442/global-quinoa-production/ (accessed on 7 November 2021).
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Pal, M. Chenopodium: Seed protein, fractionation and amino acid composition. Int. J. Food Sci. Nutr. 1998, 49, 271–275. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic variability and heritability of selected traits during different cuttings of vegetable Chenopodium. Ind. J. Genet. Plant Breed. 2003, 63, 359–360. [Google Scholar]
- James, A.L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266–273. [Google Scholar] [CrossRef]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The worldwide potential of quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Wang, X.; Iqbal, S.; Hafeez, M.B.; Khan, S.; Raza, A.; Iqbal, J.; Maqbool, M.M.; Fiaz, S.; Qazi, M.A.; et al. Effect of Water Stress on Grain Yield and Physiological Characters of Quinoa Genotypes. Agronomy 2021, 11, 1934. [Google Scholar] [CrossRef]
- Iqbal, H.; Yaning, C.; Waqas, M.; Rehman, H.; Shareef, M.; Iqbal, S. Hydrogen peroxide application improves quinoa performance by affecting physiological and biochemical mechanisms under water-deficit conditions. J. Agron. Crop Sci. 2018, 204, 541–553. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Nawaz, M.; Hussain, I.; Foidl, N. Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2020, 129, 74–81. [Google Scholar] [CrossRef]
- Brazales-Cevallos, D.K.; Romero-Contreras, Y.J.; Vences-Guzmán, M.A.; Torres, M.; Aviles-Baltazar, N.Y.; Sohlenkamp, C.; Serrano, M. Transcriptional characterization of the biostimulant effect of Moringa oleifera leaf extracts using Arabidopsis thaliana as a model. S. Afr. J. Bot. 2022, 144, 250–256. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwinska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef] [Green Version]
- Tanga, M.; Lewu, F.B.; Oyedeji, A.O.; Oyedeji, O.O. Yield and morphological characteristics of Burdock (Arctium lappa L.) in response to mineral fertilizer application. Asian J. Agric. Biol. 2020, 8, 511–518. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Waraczewska, Z.; Budka, A. The Influence of Bio-Stimulants and Foliar Fertilizers on Yield, Plant Features, and the Level of Soil Biochemical Activity in White Lupine (Lupinus albus L.) Cultivation. Agronomy 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Campobenedetto, C.; Grange, E.; Mannino, G.; van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A Biostimulant Seed Treatment Improved Heat Stress Tolerance During Cucumber Seed Germination by Acting on the Antioxidant System and Glyoxylate Cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Sobarzo-Bernal, O.; Gómez-Merino, F.C.; Alcántar-González, G.; Saucedo-Veloz, C.; Trejo-Téllez, L.I. Biostimulant Effects of Cerium on Seed Germination and Initial Growth of Tomato Seedlings. Agronomy 2021, 11, 1525. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Ammar, G.A.G.; Ammar, G. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Mutale-joan, C.; Rachidi, F.; Mohamed, H.A.; Mernissi, N.E.; Aasfar, A.; Barakate, M.; Mohammed, D.; Sbabou, L.; Arroussi, H.E. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 2021, 1–17. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane-1-carboxylate deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic Responses of Maize Shoots and Roots Elicited by Combinatorial Seed Treatments With Microbial and Non-microbial Biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Maignan, V.; Bernay, B.; Géliot, P.; Avice, J.-C. Biostimulant Effects of Glutacetine R and Its Derived Formulations Mixed With N Fertilizer on Post-heading N Uptake and Remobilization, Seed Yield, and Grain Quality in Winter Wheat. Front. Plant Sci. 2020, 11, 607615. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The Application of a Plant Biostimulant Based on Seaweed and Yeast Extract Improved Tomato Fruit Development and Quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Laurent, E.-A.; Ahmed, N.; Durieu, C.; Grieu, P.; Lamaze, T. Marine and fungal biostimulants improve grain yield, nitrogen absorption and allocation in durum wheat plants. J. Agric. Sci. 2020, 158, 279–287. [Google Scholar] [CrossRef]
- Hassanein, Y.Z.; Abdel-Rahman, S.S.A.; Soliman, W.S.; Salaheldin, S. Growth, yield, and quality of roselle (Hibiscus sabdariffa L.) plants as affected by nano zinc and bio-stimulant treatments. Hortic. Environ. Biotechnol. 2021, 53, 397–403. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesus, A.; Brockman, H.; Munne-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Shareef, H.J. Organic fertilizer modulates IAA and ABA levels and biochemical reactions of date palm Phoenix dactylifera L. Hillawi cultivar under salinity conditions. Asian J. Agric. Biol. 2020, 8, 24–30. [Google Scholar] [CrossRef]
- Makawita, G.I.P.S.; Wickramasinghe, I.; Wijesekara, I. Using brown seaweed as a biofertilizer in the crop management industry and assessing the nutrient upliftment of crops. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Hussain, M.U.; Saleem, M.F.; Hafeez, M.B.; Khan, S.; Hussain, S.; Ahmad, N.; Ramzan, Y.; Nadeem, M. Impact of soil applied humic acid, zinc and boron supplementation on the growth, yield and zinc translocation in winter wheat. Asian J. Agric. Biol. 2022. [Google Scholar] [CrossRef]
- Anwar, Z.; Basharat, Z.; Hafeez, M.B.; Khan, S.; Zahra, N.; Rafique, Z.; Maqsood, M. Biofortification of Maize with Zinc and Iron not only Enhances Crop Growth but also Improves Grain Quality. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Tabaxi, I.; Zisi, C.; Karydogianni, S.; Folina, A.E.; Kakabouki, I.; Kalivas, A.; Bilalis, D. Effect of organic fertilization on quality and yield of oriental tobacco (Nicotiana tabacum L.) under Mediterranean conditions. Asian J. Agric. Biol 2021. [Google Scholar] [CrossRef]
- Rashid, N.; Khan, S.; Wahid, A.; Basra, S.M.A.; Alwahibi, M.S.; Jacobsen, S.-E. Impact of natural and synthetic growth enhancers on the productivity and yield of quinoa (Chenopodium quinoa willd.) cultivated under normal and late sown circumstances. J. Agron. Crop Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Phiri, C.; Mbewe, D.N. Influence of Moringa oleifera leaf extracts on germination and seedling survival of three common legumes. Int. J. Agric. Biol. 2010, 12, 315. [Google Scholar]
- Toscano, S.; Ferrante, A.; Branca, F.; Romano, D. Enhancing the Quality of Two Species of Baby Leaves Sprayed with Moringa Leaf Extract as Biostimulant. Agronomy 2021, 11, 1399. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.-N.A.; Abd El-Razek, U.A.; Abd El-Hakim, A.F.; Hasham, M.M.A.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Growth, Yield, Quality, and Phytochemical Behavior of Three Cultivars of Quinoa in Response to Moringa and Azolla Extracts under Organic Farming Conditions. Agronomy 2021, 11, 2186. [Google Scholar] [CrossRef]
- Yasmeen, A.; Basra, S.M.A.; Farooq, M.; Rehman, H.; Hussain, N.; Athar, H.R. Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 2013, 69, 225–233. [Google Scholar] [CrossRef]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar]
- Alsaadawi, I.S.; Dayan, F.E. Potentials and prospects of sorghum allelopathy in agroecosystems. Allelopath. J. 2009, 24, 255–270. [Google Scholar]
- Cheema, Z.A.; Khaliq, A. Use of sorghum allelopathic properties to control weeds in irrigated wheat in a semi arid region of Punjab. Agric. Ecosyst. Environ. 2000, 79, 105–112. [Google Scholar] [CrossRef]
- Wazir, I.; Sadiq, M.; Baloch, M.S.; Awan, I.U.; Khan, E.A.; Shah, I.H.; Nadim, M.A.; Khakwani, A.A.; Bakhsh, I. Application of bioherbicide alternatives for chemical weed control in rice. Pak. J. Weed Sci. Res. 2011, 17, 245–252. [Google Scholar]
- Farooq, O.; Ali, M.; Sarwar, N.; Rehman, A.; Iqbal, M.M.; Naz, T.; Asghar, M.; Ehsan, F.; Nasir, M.; Hussain, Q.M.; et al. Foliar applied brassica water extract improves the seedling development of wheat and chickpea. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Rehman, A.; Hassan, F.; Qamar, R.; Rehman, A.U. Application of plant growth promoters on sugarcane (Saccharum officinarum L.) budchip under subtropical conditions. Asian J. Agric. Biol. 2021, 2, 202003202. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Z.; Huang, R. Regulation of ascorbic acid synthesis in plants. Plant Signal. Behav. 2013, 8, e24536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Guo, Y.K.; Lin, S.H.; Fang, Y.Y.; Bai, J.G. Hydrogen peroxide pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat-stressed cucumber leaves. Sci. Hortic. 2010, 126, 20–26. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerny, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Nawaz, A.; Chaudhary, M.A.M.; Rehman, A. Foliage-applied sodium nitroprusside and hydrogen peroxide improves resistance against terminal drought in bread wheat. J. Agron. Crop Sci. 2017, 203, 473–482. [Google Scholar] [CrossRef]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Rizwan, M.; Ali, S.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci. Rep. 2018, 8, 17086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M.Z.; Basra, S.M.A.; Hafeez, M.B.; Khan, S.; Nazeer, S.; Iqbal, S.; Saddiq, M.S.; Zahra, N. Adaptability and yield potential of new quinoa lines under agro-ecological conditions of Faisalabad-Pakistan. Asian J. Agric. Biol. 2021, 2, 202005301. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Wahid, A. Screening of moringa landraces for leaf extract as biostimulant in wheat. Int. J. Agric. Biol. 2017, 19, 999–1006. [Google Scholar] [CrossRef]
- Bhatti, M.Q.L.; Cheema, Z.A.; Mahmood, T. Efficacy of Sorgaab as Natural Weed Inhibitor in Raya. Pak. J. Biol. Sci. 2000, 3, 1128–1130. [Google Scholar] [CrossRef] [Green Version]
- Jahangeer, A. Response of Maize (Zea mays L.) to Foliar Application of Three PLANT water Extracts. Master’s Thesis, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan, 2011. [Google Scholar]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Nawaz, M.; Rehman, H.U. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Pollut. Res. 2017, 24, 27601–27612. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzyme in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments, 2nd ed.; Goodwin, T.W., Ed.; Academic Press: Cambridge, MA, USA, 1976; Volume 2, pp. 38–165. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implication of water stress-induced changes in levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedling. Physiol. Plant. 1983, 58, 166–169. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van-Slyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–233. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal. Biochem. 1976, 72, 247–254. [Google Scholar] [CrossRef]
- Stark, D.; Wray, V. Anthocyanins. In Methods in Plant Biology, Plant Phenolics; Harborne, J.B., Ed.; Academic Press/Harcourt Brace Jovanovich: London, UK, 1989; Volume 1, pp. 32–356. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute (IRRI): Los Banos, Philippines, 1976. [Google Scholar]
- Tendon, H.L.S. (Ed.) Methods of Analysis of Soil, Plants, Water and Fertilizers; Fertilization Development and Consultation Organisation: New Delhi, India, 1993. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, M.I.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Azzedine, F.; Gherroucha, H.; Baka, M. Improvement of salt tolerance in durum wheat by ascorbic acid application. J. Stress Physiol. Biochem. 2011, 7, 27–37. [Google Scholar]
- Miyaji, T.; Kuromori, T.; Takeuchi, Y.; Yamaji, N.; Yokosho, K.; Shimazawa, A. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat. Commun. 2015, 6, 5928–6928. [Google Scholar] [CrossRef] [Green Version]
- Safdar, M.E.; Aslam, A.; Qamar, R.; Ali, A.; Javaid, M.M.; Hayyat, M.S.; Raza, A. Allelopathic effect of prickly chaff flower (Achyranthes aspera L.) used as a tool for managing noxious weeds. Asian J. Agric. Biol. 2021, 3, 202006370. [Google Scholar] [CrossRef]
- Zahid, N.; Ahmed, M.J.; Tahir, M.M.; Maqbool, M.; Shah, S.Z.A.; Hussain, S.J.; Khaliq, A.; Rehmani, M.I.A. Integrated effect of urea and poultry manure on growth, yield and postharvest quality of cucumber (Cucumis sativus L.). Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Maqbool, N.; Wahid, A.; Farooq, M.; Cheema, Z.A.; Siddique, K.H.M. Allelopathy and abiotic stress interaction in crop plants. In Allelopathy: Current Trends and Future Applications; Cheema, Z.A., Farooq, M., Wahid, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 113–143. [Google Scholar]
- Rashid, N.; Wahid, A.; Basra, S.M.A.; Arfan, M. Foliar spray of moringa leaf extract, sorgaab, hydrogen peroxide and ascorbic acid improve leaf physiological and seed quality traits of quinoa (Chenopodium quinoa) under terminal heat stress. Int. J. Agric. Biol. 2020, 23, 801–810. [Google Scholar]
- Basra, S.; Iftikhar, M.; Afzal, I. Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol. 2011, 13, 1006–1010. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.H.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 1–13. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.; Al-Qutami, M.A.; Ali, H.M. Ascorbic acid application improves salinity stress tolerance in wheat. Chiang Mai J. Sci. 2018, 45, 1296–1306. [Google Scholar]
- Zhang, X.L.; Jia, X.F.; Yu, B.; Gao, Y.; Bai, J.G. Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Sci. Hortic. 2011, 129, 656–662. [Google Scholar] [CrossRef]
- Heuer, B. Role of proline in plant response to drought and salinity. In Handbook of Plant and Crop Stress, 3rd ed.; Pessarakli, M., Ed.; Taylor and Francis Press: New York, NY, USA, 2010; pp. 213–238. [Google Scholar]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Al-Wahibi, M.S. Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J. Biol. Sci. 2015, 22, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maswada, H.F.; Abd-El-Rahman, L.A. Inducing salinity tolerance in wheat plants by hydrogen peroxide and lithovit a nano-caco3 fertilizer. J. Agric. Res. Kafr. El-Sheikh Univ. 2014, 40, 693–716. [Google Scholar]
- Chaiwanon, J.; Wang, W.; Zhu, J.-Y.; Oh, E.; Wang, Z.-Y. Information integration and communication in plant growth regulation. Cell 2016, 164, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Dolatabadian, A.; Mohammad, S.A.; Asilan, K.S. Effect of ascorbic acid foliar application on yield, yield component and several morphological traits of grain corn under water deficit stress conditions. Not. Sci. Biol. 2010, 2, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.M.; Alva, A.K. Effects of Zinc and Ascorbic Acid Application on the Growth and Photosynthetic Pigments of Millet Plants Grown under Different Salinity. Agric. Sci. 2014, 5, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, A.M.; Díaz, A.; Tejada, M.; Bautista, J.; Rodríguez, B.; Santa-María, C.; Revilla, E.; Parrado, J. Enzymatic Production of an Organic Soil Biostimulant from Wheat-Condensed Distiller Solubles: Effects on Soil Biochemistry and Biodiversity. Process Biochem. 2010, 45, 1127–1133. [Google Scholar] [CrossRef]
- Yasmeen, A.; Basra, S.M.A.; Wahid, A.; Nouman, W.; Rehman, H. Exploring the potential of Moringa oleifera leaf extract (MLE) as a seed priming agent in improving wheat performance. Turk. J. Bot. 2013, 37, 512–520. [Google Scholar]
- Maheswari, U.M.; Karthik, A. Effect of foliar nutrition on growth, yield attributes and seed yield of pulse crops. Adv. Crop. Sci. Technol. 2017, 5, 278. [Google Scholar] [CrossRef]
- Shah, S.H.; Khan, E.A.; Shah, H.; Ahmad, N.; Khan, J.; Sadozai, G.U. Allelopathic sorghum water extract helps to improve yield of sunflower (Helianthus annuus L.). Pak. J. Bot. 2016, 48, 1197–1202. [Google Scholar]
- Basra, S.M.A.; Lovatt, C. Exogenous applications of Moringa oleifera leaf extract and cytokinins improve plant growth, yield and fruit quality of cherry tomato (Solanum lycopersicum). HortTechnology 2016, 26, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Basit, A.; Hafeez, M.B.; Irshad, S.; Bashir, S.; Bashir, S.; Maqbool, M.M.; Saddiq, M.S.; Hasnain, Z.; Aljuaid, B.S.; et al. Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLoS ONE 2021, 16, e0254452. [Google Scholar] [CrossRef]
- Iqbal, J.; Irshad, J.; Bashir, S.; Khan, S.; Yousaf, M.; Shah, A.N. Comparative study of water extracts of Moringa leaves and roots to improve the growth and yield of sunflower. S. Afri. J. Bot. 2020, 129, 221–224. [Google Scholar] [CrossRef]

| SOV | DF | T. Chlo | Caro | gs | Ci | AsA | MDA | TFAA | Protein | Antho |
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulants (S) | 1 | 1.76 ** | 0.208 ** | 0.039 ** | 1547.6 ** | 33.0 ** | 66.5 ** | 254.6 ** | 48.54 ** | 0.122 ** |
| Year (Y) | 1 | 0.011 NS | 0.009 NS | 0.892 NS | 10.13 NS | 0.059 NS | 0.087 NS | 0.025 NS | 0.009 NS | 0.00003 NS |
| S × Y | 1 | 0.00002 NS | 0.0018 NS | 0.043 NS | 1.03 NS | 0.002 NS | 0.0051 NS | 0.018 NS | 0.0022 NS | 0.00006 NS |
| Proline | Root P | Shoot P | Root S | Shoot S | RFW | RDW | SFW | SDW | ||
| Stimulants (S) | 1 | 5.04 ** | 186.3 ** | 211.2 ** | 40.91 ** | 188.8 ** | 45.18 ** | 15.57 ** | 405.5 ** | 482.5 ** |
| Year (Y) | 1 | 0.025 NS | 0.02 NS | 0.067 NS | 0.028 NS | 0.56 NS | 0.27 NS | 0.0013 NS | 3.1 NS | 0.032 NS |
| S × Y | 1 | 0.0004 NS | 0.0004 NS | 0.002 NS | 0.0005 NS | 0.045 NS | 0.009 NS | 0.0015 NS | 1.5 NS | 0.012 NS |
| Root L | Shoot L | PL | PW | TGW | HI | Mg | Fe | Zn | ||
| Stimulants (S) | 1 | 51.34 ** | 440 ** | 66.5 ** | 91.6 ** | 2.96 ** | 96.13 ** | 10.14 ** | 34.35 ** | 207.34 ** |
| Year (Y) | 1 | 0.17 NS | 1.58 NS | 0.034 NS | 0.036 NS | 0.0005 NS | 0.063 NS | 0.043 NS | 0.013 NS | 0.0001 NS |
| S × Y | 1 | 0.007 NS | 0.204 NS | 0.089 NS | 0.0006 NS | 0.0019 NS | 0.002 NS | 0.0003 NS | 0.003 NS | 0.013 NS |
| Treatments | Root P | Shoot P | Root S | Shoot S | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | |
| No spray | 19.40 | 19.34 | 19.37 | 30.52 | 30.55 | 30.53 | 11.12 | 11.19 | 11.15 | 21.01 | 21.02 | 21.01 |
| Water spray | 19.65 | 19.62 | 19.62 | 30.66 | 30.79 | 30.72 | 11.35 | 11.39 | 11.37 | 21.37 | 21.37 | 21.37 |
| MLE | 32.17 | 32.10 | 32.14 | 44.60 | 44.69 | 44.64 | 17.07 | 17.10 | 17.09 | 35.20 | 35.18 | 35.19 |
| Sorgaab | 29.82 | 29.78 | 29.80 | 42.36 | 42.46 | 42.41 | 16.45 | 16.52 | 16.48 | 30.86 | 30.85 | 30.85 |
| H2O2 | 22.16 | 22.11 | 22.14 | 39.86 | 39.97 | 39.92 | 15.90 | 15.96 | 15.93 | 26.05 | 26.07 | 26.06 |
| AsA | 28.63 | 28.60 | 28.61 | 38.56 | 38.64 | 38.60 | 15.11 | 15.17 | 15.14 | 29.84 | 29.84 | 29.84 |
| Mean (Y) | 25.31 | 25.26 | 37.76 | 37.85 | 14.50 | 14.56 | 27.39 | 27.39 | ||||
| HSD | Y = ns, FT = 2.9, Y × FT = ns | Y = ns, FT =2.8, Y × FT = ns | Y = ns, FT = 0.66, Y × FT = ns | Y = ns, FT =1.7, Y × FT = ns | ||||||||
| Treatments | Root Fresh Weight | Root Dry Weight | Shoot Fresh Weight | ||||||
| Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | |
| No spray | 19.76 | 20.06 | 19.91c | 8.08 | 8.05 | 8.07 c | 326.29 | 326.73 | 326.5 c |
| Water spray | 20.16 | 20.33 | 20.25 c | 8.70 | 8.72 | 8.71 c | 331.61 | 331.66 | 331.6 c |
| MLE | 26.75 | 26.90 | 26.82 a | 12.43 | 12.42 | 12.43 a | 489.38 | 487.13 | 488.2 a |
| Sorgaab | 25.30 | 25.50 | 25.40 b | 11.18 | 11.19 | 11.18 ab | 388.20 | 388.46 | 388.3 b |
| H2O2 | 23.26 | 23.36 | 23.31 b | 10.59 | 10.52 | 10.55 b | 363.37 | 360.53 | 361.9 b |
| AsA | 23.50 | 23.60 | 23.55 b | 9.79 | 9.79 | 9.79 bc | 482.53 | 482.33 | 482.4 a |
| Mean (Y) | 23.12 | 23.29 | 10.13 | 10.11 | 396.90 | 396.14 | |||
| HSD | Y = ns, FT = 2.5, Y × FT = ns | Y = ns, FT = 1.8, Y × FT = ns | Y = ns, FT = 28.1, Y × FT = ns | ||||||
| Treatments | Shoot Dry Weight | Root Length | Shoot Length | ||||||
| Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | |
| No spray | 63.20 | 63.30 | 63.25 d | 12.90 | 13.00 | 12.95 c | 78.93 | 79.75 | 79.34 c |
| Water spray | 64.83 | 64.94 | 64.88 d | 13.90 | 14.06 | 13.98 c | 80.36 | 81.00 | 80.68 c |
| MLE | 84.46 | 84.56 | 84.51 a | 20.56 | 20.66 | 20.61 a | 100.36 | 100.81 | 100.59 a |
| Sorgaab | 81.25 | 81.32 | 81.28 ab | 18.30 | 18.40 | 18.35 b | 95.76 | 96.16 | 95.96 ab |
| H2O2 | 71.45 | 71.55 | 71.50 c | 17.40 | 17.66 | 17.53 b | 92.93 | 93.41 | 93.17 b |
| AsA | 79.89 | 79.77 | 79.83 b | 18.40 | 18.50 | 18.45 b | 93.26 | 93.00 | 93.13 b |
| Mean (Y) | 74.18 | 74.24 | 16.91 | 17.05 | 90.27 | 90.69 | |||
| HSD | Y = ns, FT = 3.7, Y × FT = ns | Y = ns, FT = 5.9, Y × FT = ns | Y = ns, FT = 2.01, Y × FT = ns | ||||||
| Treatments | PL | PW | TSW | HI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | Year I | Year II | Mean (FT) | |
| No spray | 36.40 | 36.66 | 36.53 c | 17.13 | 17.20 | 17.16 d | 4.01 | 4.04 | 4.03 c | 25.07 | 25.14 | 25.10 c |
| Water spray | 38.20 | 38.30 | 38.25 bc | 20.00 | 20.10 | 20.05 c | 4.06 | 4.10 | 4.08 c | 25.63 | 25.70 | 25.67 c |
| MLE | 42.16 | 42.23 | 42.20 a | 27.30 | 27.35 | 27.32 a | 5.79 | 5.75 | 5.77 a | 34.71 | 34.76 | 34.73 a |
| Sorgaab | 45.33 | 45.06 | 45.20 a | 25.56 | 25.61 | 25.59 ab | 5.41 | 5.44 | 5.42 a | 32.12 | 32.17 | 32.14 ab |
| H2O2 | 44.10 | 43.73 | 43.91 ab | 23.80 | 23.85 | 23.82 b | 4.63 | 4.66 | 4.64 b | 31.36 | 31.45 | 31.40 b |
| AsA | 42.13 | 42.96 | 42.05 ab | 25.80 | 25.86 | 25.83 ab | 4.77 | 4.73 | 4.75 b | 32.94 | 33.09 | 33.02 ab |
| Mean (Y) | 41.38 | 41.32 | 23.26 | 23.33 | 4.78 | 4.79 | 30.30 | 30.39 | ||||
| HSD | Y = ns, FT =4.1, Y × FT = ns | Y = ns, FT =2.4, Y × FT = ns | Y = ns, FT = 0.53, Y × FT = ns | Y = ns, FT = 2.9, Y × FT = ns | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, N.; Khan, S.; Wahid, A.; Ibrar, D.; Hasnain, Z.; Irshad, S.; Bashir, S.; Al-Hashimi, A.; Elshikh, M.S.; Kamran, M.; et al. Exogenous Application of Biostimulants and Synthetic Growth Promoters Improved the Productivity and Grain Quality of Quinoa Linked with Enhanced Photosynthetic Pigments and Metabolomics. Agronomy 2021, 11, 2302. https://doi.org/10.3390/agronomy11112302
Rashid N, Khan S, Wahid A, Ibrar D, Hasnain Z, Irshad S, Bashir S, Al-Hashimi A, Elshikh MS, Kamran M, et al. Exogenous Application of Biostimulants and Synthetic Growth Promoters Improved the Productivity and Grain Quality of Quinoa Linked with Enhanced Photosynthetic Pigments and Metabolomics. Agronomy. 2021; 11(11):2302. https://doi.org/10.3390/agronomy11112302
Chicago/Turabian StyleRashid, Nabila, Shahbaz Khan, Abdul Wahid, Danish Ibrar, Zuhair Hasnain, Sohail Irshad, Saqib Bashir, Abdulrahman Al-Hashimi, Mohamed S Elshikh, Muhammad Kamran, and et al. 2021. "Exogenous Application of Biostimulants and Synthetic Growth Promoters Improved the Productivity and Grain Quality of Quinoa Linked with Enhanced Photosynthetic Pigments and Metabolomics" Agronomy 11, no. 11: 2302. https://doi.org/10.3390/agronomy11112302
APA StyleRashid, N., Khan, S., Wahid, A., Ibrar, D., Hasnain, Z., Irshad, S., Bashir, S., Al-Hashimi, A., Elshikh, M. S., Kamran, M., Ahmar, S., & Mora-Poblete, F. (2021). Exogenous Application of Biostimulants and Synthetic Growth Promoters Improved the Productivity and Grain Quality of Quinoa Linked with Enhanced Photosynthetic Pigments and Metabolomics. Agronomy, 11(11), 2302. https://doi.org/10.3390/agronomy11112302











