Abstract
Diseases or complications that are caused by bone tissue damage affect millions of patients every year. Orthopedic and dental implants have become important treatment options for replacing and repairing missing or damaged parts of bones and teeth. In order to use a material in the manufacture of implants, the material must meet several requirements, such as mechanical stability, elasticity, biocompatibility, hydrophilicity, corrosion resistance, and non-toxicity. In the 1970s, a biocompatible glassy material called bioactive glass was discovered. At a later time, several glass materials with similar properties were developed. This material has a big potential to be used in formulating medical devices, but its fragility is an important disadvantage. The use of bioactive glasses in the form of coatings on metal substrates allows the combination of the mechanical hardness of the metal and the biocompatibility of the bioactive glass. In this review, an extensive study of the literature was conducted regarding the preparation methods of bioactive glass and the different techniques of coating on various substrates, such as stainless steel, titanium, and their alloys. Furthermore, the main doping agents that can be used to impart special properties to the bioactive glass coatings are described.
1. Introduction
In time, the research development in the field of biomaterials led to the improvement of materials properties as well as the discovery of new materials with more suitable properties for the aimed applications. Depending on the interaction with the human body, biomaterials can be classified into three main categories: Bioinert, bioactive, and bioresorbable materials [1].
From the beginning of modern medicine, the first materials that began to be used in order to help restore organs were the natural ones. These materials were selected, in a multidisciplinary approach, involving surgeons and engineers that studied the material interaction with the human body. The selected materials had to be tolerated by the body and to be inert, thus minimizing the formation of scar tissue at the contact of the material with the tissue, in other words to be bioinert. Taking into consideration the fact that there were no known completely inert materials, the lack of toxic response was considered a satisfactory result [2]. The Industrial Revolution, among other merits, also led to new discoveries in the field of materials, giving rise to the second generation of biomaterials. In 1969, Professor Hench et al. developed a glassy material later called “bioactive glass/bioglass” which was able to form bonds with the human bone or soft tissue without being rejected [3,4]. When the bioactive glass meets the biological tissues, a succession of chemical reactions take place, which eventually lead to stimulation of the formation of bonds between the tissue and the bioactive glass. Another important characteristic of bioactive glass is that it possesses antibacterial properties due to the local change of the pH [5,6,7,8,9,10,11,12]. This discovery led to the creation of the second generation of biomaterials that are called bioactive materials [2]. The main characteristic of bioactive materials is the ability to form bonds with the host tissue, at the interface. For example, at the site of implantation it induces the formation of strong bonds with bone tissue, due to the formation of a layer of wollastonite which turns into hydroxycarbonate apatite, that has a similar composition to mineral bone [1,13]. The third generation of biomaterials focuses on the concepts of bioactive and resorbable materials. Polymers are among the bioactive materials that present resorbable properties and can induce specific interactions with cellular integrins leading to cell proliferation, differentiation, production, and organization of the extracellular matrix [14]. Currently, the materials used for tissue regeneration are composites, bioactive glass, hybrid materials, and macroporous foams, that are designed to activate genes that stimulate the regeneration of living tissues [1,15].
As mentioned earlier, Hench et al. developed the first representatives of the bioactive glass family. At a later time, other compositions possessing similar or improved bioactive properties were discovered and studied. Bioactive glasses have mechanical properties suitable for most technical applications. The compression strength of glass can be compared to iron and is higher than concrete, the tensile strength of glass is low, but it is sufficient for most applications. The most important challenge with the bioactive glass is its fragility. Compared to steel, glass cannot withstand high local stresses, thus cracks can appear and propagate. When bioactive glass is in contact with the biological environment, interfacial layers are formed that can generate surface defects. These defects can turn into cracks that spread to the entire surface of the implant when a force is applied and subsequently leads to its destruction. Therefore, bioactive glass cannot be used in the manufacturing of load-bearing implants. However, several solutions have been developed to solve this problem. Bioactive glass can be part of a composite structure and in this way the fragility can be considerably compensated [16]. Moreover, sintered bioactive glass particles were incorporated into the pores of load-bearing metal implants [17,18,19,20,21].
Bioactive glass coatings of some metal implants are intensively studied, and the interest for them in the last 15 years has increased considerably. These coatings may behave similarly to hydroxyapatite or other calcium phosphates, thus metal coated implants may be better integrated into the bone tissue [19,22,23,24,25,26,27,28]. However, obtaining bioactive glass coatings on the surface of metal implants remains a challenge [29]. Scientists encounter problems in maintaining a bioactive glass composition due to crystallization, the transfer of unwanted ions contained in the metal core, the difficulty of matching the coefficients of expansion of the metal core, and the bioactive glass shell [5].
The discovery of bioactive glass took place more than 50 years ago. In addition, it consolidated with the bone regenerative medicine by introducing the concept that a material implanted in the body can not only form a close bond with living tissues, but can also stimulate the growth of new, healthy tissues [30].
2. The Mechanism of Bone Tissue Formation on the Surface of the Bioactive Glass
The interactions between the implant biomaterial and the physiological environment take place at the implant surface. After the insertion of an implant into the body, a series of simultaneous reactions take place between the target tissue and the surface of the implant. Bioactive glass can bind to living tissues, such as bones, and in some cases even to soft tissues. Bioactive fixation occurs when a bioactive hydroxycarbonate apatite layer forms on the surface of the implanted bioactive glass. After about 3-6 months, the connection between the bone and the bioactive glass is as strong as the natural one, which means that the healing process was successful [1].
New surface analysis techniques have been developed to study and understand the processes that take place, both in vitro and in vivo, between the surface of an implant and the biological environment. The bonding mechanism between a bioactive glass surface and bone takes place in 11 stages [31,32]. The first five steps include ionic reactions between the glass and the biological environment, which occur within the first 24 h after implantation. At the surface of the bioactive glass, there is a rapid release of soluble ionic species in the biological environment. In the first two stages, the formation of SiOH bonds and the release of Si(OH)4 takes place. In step 3, the SiOH bonds are polycondensed to form Si-O-Si hydrated silica gel. In stage 4, the adsorption of Ca2+, PO43−, and CO32+ species takes place. In step 5, hydroxycarbonate apatite is formed. In the next step, the formed layer increases the adsorption and desorption of growth factors. This significantly reduces the time required for step 7, in which macrophages prepare the implant site for tissue repair. In stage 8, the osteoblasts and stem cells are fixed. In addition, in the next stage, the differentiation and proliferation of osteoblasts on the surface of the implant takes place. Normally, on the surface of a bioinert material, these processes take place in a few weeks, but in the case of bioactive glass, all of the steps described above take place in a few tens of hours. Approximately 24–48 h after implantation, in stage 10, the production of various growth factors begins, which stimulate cell division, mitosis, and the production of extracellular matrix proteins. Shortly afterwards, in the 11th stage, this matrix is mineralized. In 6–12 days, mature osteocytes are incorporated into the collagen-hydroxycarbonate apatite matrix assuring new bone formation and osseointegration [15,31,33,34].
In vitro and in vivo tests are used to evaluate the biological response of bioactive glass coatings. When immersed in SBF, the bioactive glasses react and gradually transform into wollastonite and further into apatite. Intermediary, an apatite-wollastonite core@shell phase is obtained. This phase is a type of glass-ceramics that exhibits a multistep bioactivity mechanism by gradual transformation of wollastonite into apatite, on the surface [35]. The dissolution rate of phosphate systems can be influenced by adding various doping agents such as TiO2, CuO, and Fe2O3. Therefore, the dissolution process can take place in a few hours or it can be increased to months depending on the used modifier [35]. In vitro assays consist of immersing the samples in the SBF solution at 37 °C for 14–30 days. After 1, 7, 14, and 28 days, the morphology of the sample surface is analyzed by scanning electron microscopy. The bioactive response is proportional to the degree of formation of the hydroxyapatite layer on the sample surface [36]. In vivo tests are performed to assess the possible toxicity of the coatings. For this, the samples are implanted in animals over a period of time, for example 4, 12 or 24 weeks. Thereafter, the animals are euthanized, and the samples are extracted and analyzed. Usually, mice [37,38], rats [39,40], rabbits [41,42], dogs [43], and sheep [20] are used.
3. Methods for Obtaining Bioactive Glass
To obtain bioactive glass of any composition, two techniques are mainly used: The melting process of the components and the sol-gel technique. The first method is an older process of obtaining glass of any composition, which consists of mixing the precursors and melting them at high temperatures, followed by cooling and grinding the obtained glass [42,44,45,46,47]. This method is still applied today to obtain bioactive glass or other types of glass [39,40,48,49,50,51,52,53]. The sol-gel method consists of the transformation of precursors, such as tetraethyl orthosilicate, triethyl orthophosphate, and calcium nitrate into a colloidal solution (gel), followed by solvent removal by heating, then crushing of the obtained glass [54,55,56,57,58,59,60]. This can eliminate certain disadvantages that are present in the first process. The sol-gel process allows the attainment of bioactive glass with different compositions and biological properties [48,61]. Furthermore, using the sol-gel technique, bioactive glasses with different porosities can be obtained [62,63].
Physical properties of bioactive glass highly depend on the preparation technique. The melting method or the sol-gel process can produce amorphous powders, which undergo various changes as a result of heat treatments. At lower temperatures, the main crystalline phase that occurs in molten powders is Na2CaSi2O6, due to its high stability. In fact, at higher temperatures of about 900 °C, this crystalline phase with traces of Na2Ca4(PO4)2SiO4 is also present, except for the cristobalite traces present in the glass resulting from the sol-gel process.
Furthermore, when the sol-gel method is used to prepare bioactive glass, various nanostructures can be obtained, depending on the used catalyst. Sodium calcium silicate (Na2Ca2Si3O9) is formed when the reaction is catalyzed by HNO3. When HCl is used, wollastonite (CaSiO3) is the main crystalline phase [64].
The sintering behavior of the 45S5 melt was characterized by three different steps. Initially, a glassy transition takes place, which involves a densification of the material. Then, at higher temperatures, crystallization of Na2CaSi2O6 takes place, which impedes the process of shrinkage of the material. Finally, a densification takes place again, corresponding to the second glass transition, and at 1100 °C, when the maximum density is reached, the process is completed.
In order to completely remove the traces of raw materials and to have a better control of the carbonation process, the powder resulting from the sol-gel process requires a calcination step. During this process, a partial crystallization occurs, which limits the densification of the 45S5 bioactive glass. This is an important disadvantage for the use of 45S5 resulting from the sol-gel process for the production of bioactive substrates [65,66].
4. Bioactive Glass Deposition Methods
Bone-surface interactions and osseointegration play an important role for the long-term application of the implant in vivo. Osseointegration is correlated with the longevity and biocompatibility of a biomaterial. This can be adjusted by changing the surface properties of the implant through coating it with a biomaterial. Therefore, surfaces with the desired properties can be obtained, such as hardness, wetting capacity, and roughness. In turn, these properties adjust interfacial interactions with the cells surrounding the implant. As mentioned earlier, bioactive glass possesses excellent properties for rapid recovery and osseointegration. Obtaining bioactive glass coatings on metal implants makes it possible to combine the mechanical hardness of metals and the bioactivity of bioactive glass.
High quality coatings are difficult to obtain. The main aspects that must be taken into consideration are the surface topography, mechanical properties, and crystallinity. In order to accelerate bone formation, the presence of amorphous phases is preferred due to the higher solubility in the aqueous medium. However, this can increase the risk of failure due to the low stability of the newly formed bone, especially due to the low adhesion of the new bone onto the core implant. Therefore, the control of coating crystallinity is very important when designing a coated implant.
The bone cell adherence and proliferation are highly influenced by the surface topography. Cell attachment is more likely to take place on a rough, textured surface, but, at the same time, the coating adherence is weakened. Therefore, a balance must be maintained. When the implant is used under load conditions, a high adhesion degree of coating on the substrate, high hardness, and toughness are the main mechanical properties that must be accomplished by the coatings performed [67].
There are several techniques that can be used in order to obtain these coatings, which are generally classified into two categories: Physical and chemical. This chapter will briefly describe the most used coating processes.
4.1. Enameling
Enameling is a process used for many centuries for coating metals with glass. In this procedure, a suspension of glass powder is applied on a metal surface, followed by a heat treatment. This coating process is simple and inexpensive, and coatings of different thicknesses can be obtained [68]. In the case of bioactive glass, containing 45% silicon oxide, which facilitates bone binding, the attainment of stable and resistant coatings on metal implants through this procedure remains a challenge. In addition, due to the low level of silicon oxide, metal ions such as Al, Fe Ni, Co, Mo, Cr, Ta, and Ti can pass through the crystal lattice, reducing or completely inhibiting the bioactivity of the bioactive glass. Another problem when performing coatings with bioactive glass by enameling is the partial crystallization of the bioactive glass. In addition, the difference in the coefficients of expansion of the bioactive glass and metals further complicates the coating process. Following the heat treatment, the oxidation of the titanium surface is observed. The oxides formed reduce the bonding strength of the bioactive glass coatings to the substrate. An intermediate layer between the titanium and glass can be applied to solve this problem.
A good result for enameling metallic scaffolds with bioactive glass was obtained by applying a layer of silicon oxide-rich glass intermediate layer with a coefficient of expansion close to the substrate [69]. Improvement of bioactive glass adhesion to a scaffold of Ti-6Al-4V was studied and a bioactive glass with a high content of boron and titanium oxide was obtained. Boron oxide reduces the coefficient of expansion and lowers the softening temperature of the bioactive glass. In addition, titanium dioxide leads to the controlled formation of chemical bonds, which increase the adhesion of the bioactive glass layer to the metal substrate [70,71]. The intermediate layer does not influence the bioactivity of the final layer.
Very good coating results were obtained by enameling with bioactive glass on metal and metal oxide substrates, such as Vitallium and Co-Cr alloy [72,73,74,75], alumina [76,77,78,79,80,81], zirconia [82,83], titanium, and alloys [43,84,85,86,87,88,89]. The thickness of the obtained layer varies between 25 and 60 µm. A very good adhesion is explained by the formation of a thin layer (100 nm) of chromium oxide during the coating process [90]. Moreover, studies were performed on 316L stainless steel substrates that were covered with phosphate-free bioactive glass coatings (PFBG) [45].
4.2. Thermal Spraying
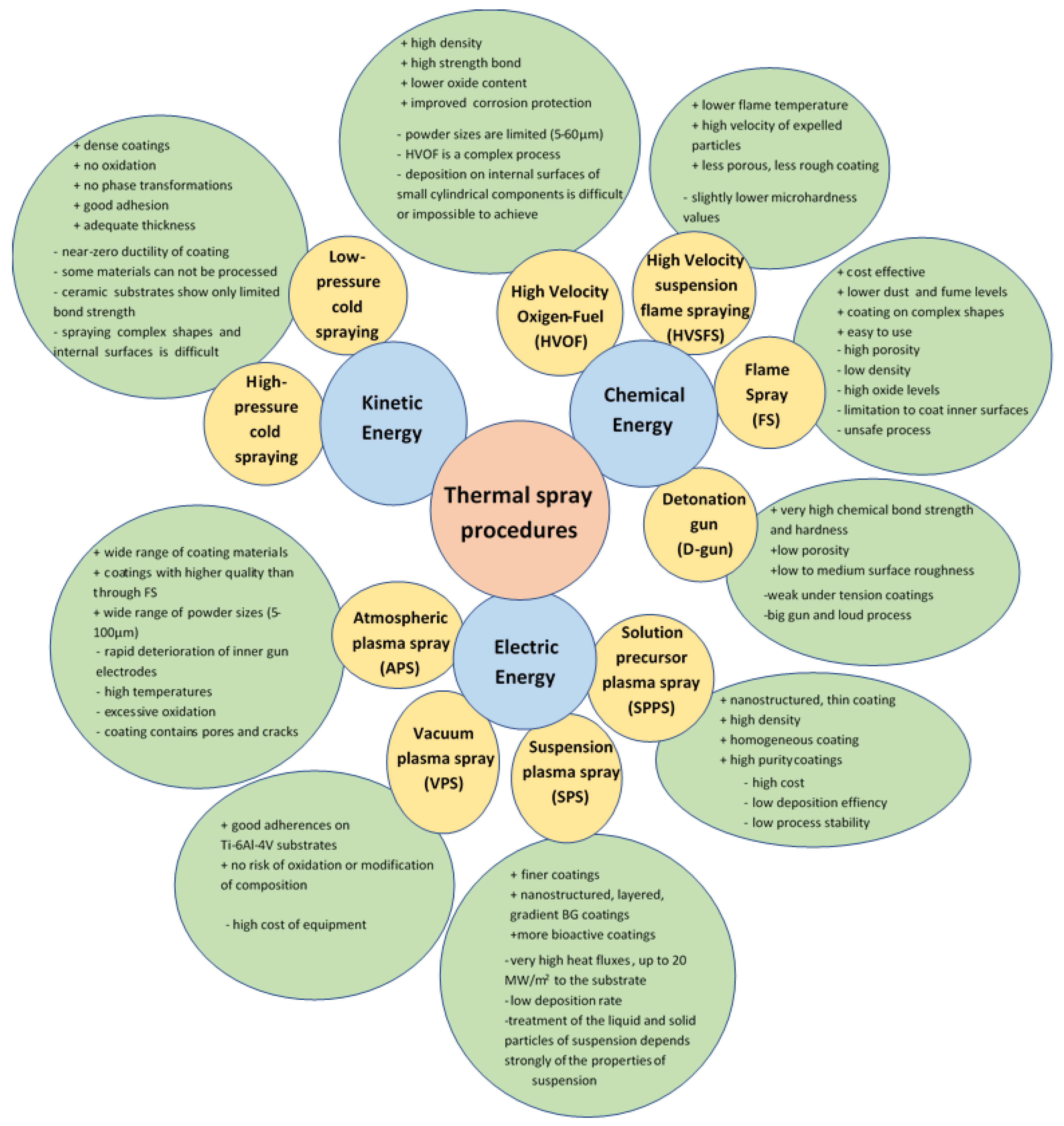
Thermal spraying is a coating process also used in the biomedical industry, due to the possibility of obtaining coatings with a controlled chemical structure on implants of different shapes. The thermal spraying process has been used industrially for more than 50 years to coat metals. In the last century, this technique was mainly used to cover biomedical devices with hydroxyapatite [91]. The process consists of using a chemical, kinetic or electrical energy source to accelerate and heat the materials to be deposited, used in a powder form. The coating materials are softened, partially or completely melted and deposited on metal surfaces, such as implants. The properties of the obtained coatings depend on the kinetic and thermal energy used in the coating process. Thermal energy is used to melt or soften particles, and kinetic energy to accelerate and impregnate particles on the surface of the device. The control of these parameters allows the attainment of resistant coatings with the desired properties. The thickness of the coatings obtained by thermal spraying varies between 50 µm and 2 mm [2]. Several thermal coating processes are currently available, and are illustrated in Figure 1.
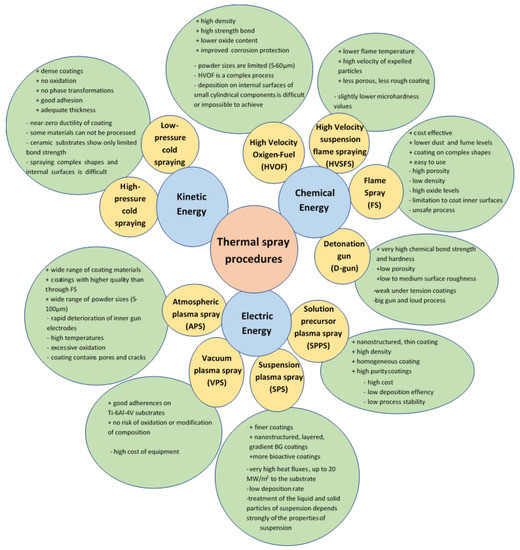
Figure 1.
Thermal spray deposition technique and its characteristics [91,92,93,94,95,96].
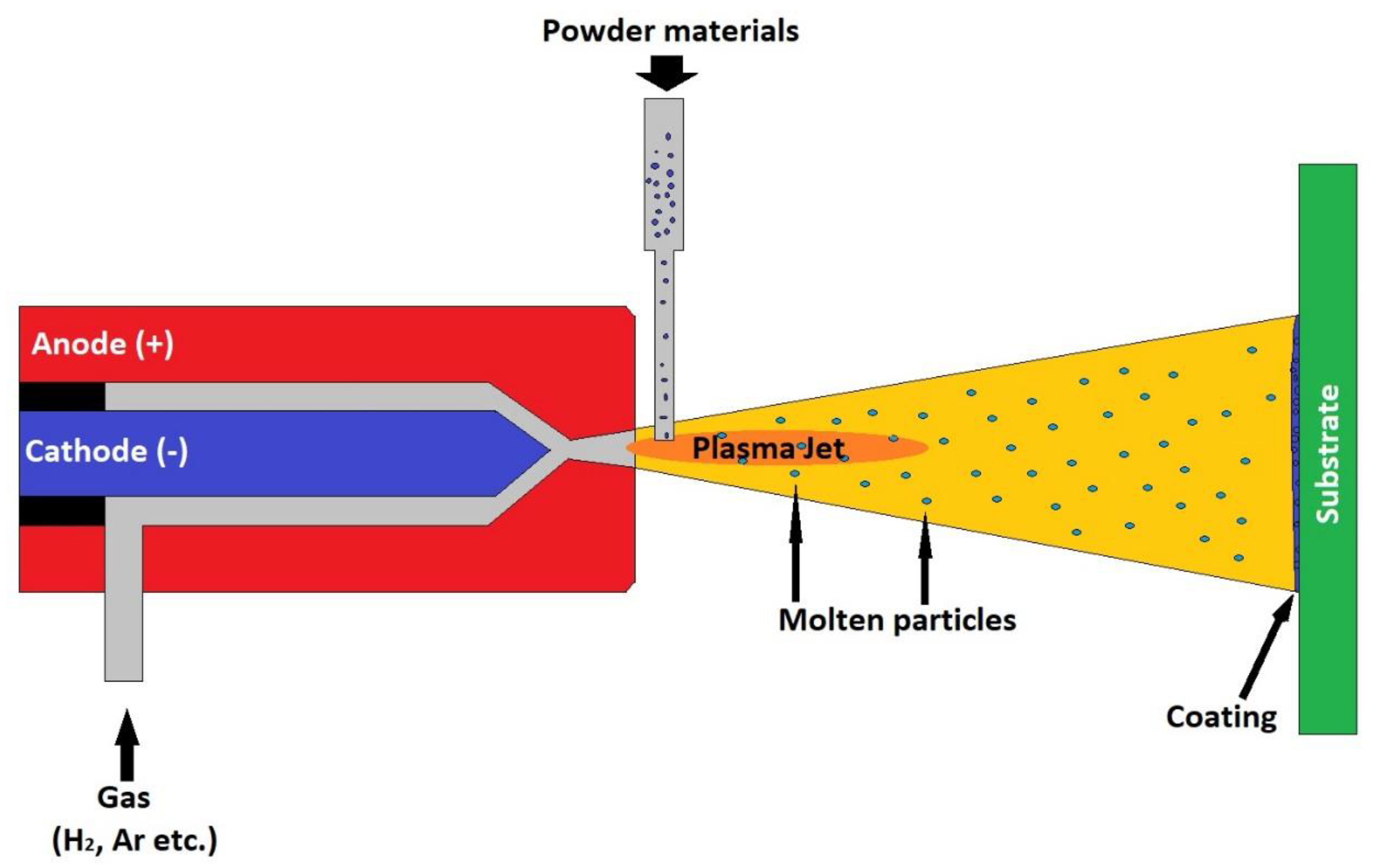
The atmospheric plasma spraying (APS) process is one of the most widely used processes for obtaining bioactive coatings. Numerous examples of manufacturing and clinical testing of coatings obtained by this procedure are described in the literature. The APS coating device consists of a gun containing a copper anode and a tungsten cathode. A stream of non-reactive gas passes through this gun, for example, nitrogen, hydrogen, helium or argon. The gas is ionized as it passes through the electric arc formed between the two electrodes connected to an electric current source. The temperature of the formed plasma reaches values of 10,000–25,000 °C. The substance to be deposited, in powder form, is introduced into the formed plasma. The particles are heated and expelled to the substrate at speeds of about 80–300 ms. Figure 2 shows the plasma spray coating process. This process allows the coverage of large areas of different shapes [91].
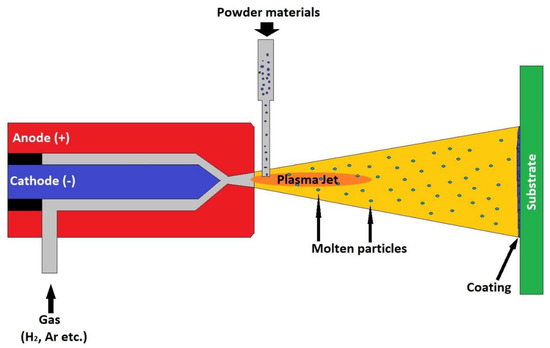
Figure 2.
Plasma spray coating process.
The atmospheric plasma spray process is used to coat implants with bioactive glass. In order to obtain resistant bioactive glass coatings with the desired biological properties, the process parameters must be chosen with great caution. For example, in order to maintain the amorphous structure of bioactive glass coatings, parameters such as the gas flow and electric arc current must be adjusted in a certain way to minimize the heating of the glass powder. Moreover, in order to obtain bioactive glass coatings, parameters such as the flow rate of glass particles, the distance to the substrate, and its temperature must be carefully adjusted. Partial or total crystallization of bioactive glass can change the mechanical and chemical properties of the coating. The rapid cooling of the substrate after coating favors the formation of amorphous glass coatings. However, the formation of a quantity of crystalline phase is beneficial and determines the ability to form glass (GFA) [97].
In this case, it was observed that the distance and the morphology of the feed particles influence the final properties of the coating. In the APS process, the separation distance and the morphology of the particle influence the amount of kinetic and thermal energy transmitted to the particle before impact with the substrate and the degree of crystallinity of the obtained coating. The optimum separation distance is between 60 and 140 mm, and the size of the glass particles 63–200 µm.
The bioactive glass coatings obtained by the APS process consist of partially and totally molten particles, pores, and cracks. These cracks appear as a result of the cooling of the formed coating. The degree of adhesion of the coatings obtained by this method varies between 6–41 MPa. To increase adhesion, some researchers have resorted to the use of an Al2O3/TiO2 intermediate layer. They found that this layer significantly increases the coating-substrate adhesion and decreases the stress discrepancy between the substrate and cooling coating, thus reducing the degree of cracking that appears on the coating. Other researchers have resorted to the heat treatment of the bio-device after coating. However, this operation must be performed very carefully to avoid changing the structure of the coating. By the APS technique, bioactive glass coatings were obtained on pure titanium substrates [98,99,100,101,102], Ti-6Al-4V alloy [103,104,105,106,107,108], stainless steel AISI 304 [109,110] and AISI 316 [97,111], Co-Cr-Mo alloy [112], etc.
Bioactive glass coatings can also be obtained by vacuum plasma spraying (VPS). The technique is similar to APS, the difference here is that the process takes place in a vacuumed chamber, where air is removed using a vacuum pump and replaced with argon. The work pressure is around 100 mbar. Coatings with a good adherence on the Ti-6Al-4V substrate can be obtained using this technique, without risk of oxidation or modification of the initial bioactive glass composition [91,113].
Another alternative to obtain bioactive glass coatings is the use of suspension plasma spray (SPS). The difference in this case is that the powder is fed as an aqueous suspension in the plasma jet. The solvent is removed by evaporation, and the solid particles melt partially or completely and are accelerated to the substrate. The main advantage of this method is that finer coatings are obtained due to the high fluidity of the coating material. Through this procedure, nanostructured, layered, and gradient bioactive glass coatings can be prepared. The coatings are porous, but compared to the classical method, their size is nanometric. In addition, the number of pores in the coatings obtained by SPS is higher than in the case of applying the classical procedure, and the resulting coatings are more active in SBF.
The most modern technique of thermal spray coating is solution precursor plasma spraying (SPPS). Using this method, nanostructured, thin, and homogeneous coatings can be obtained. The use of precursor solutions allows the attainment of high purity coatings. This technique has been successfully applied to obtain 45S5 type bioactive glass coatings [114]. The use of nitric acid as a catalyst leads to the formation of a dense coating with a high degree of adhesion. In addition, with the help of this procedure, doping agents can be easily introduced, thus improving the properties of the obtained coatings [91,113].
Another method that can be used to obtain bioactive glass coatings is high velocity suspension flame spraying (HVSFS). In this case, a mixture of combustible gases is ignited into a special chamber, and the energy generated by the explosion heats and expels the powdered material to be applied, with supersonic velocity to the target. The energy generated by the combustion of the gas is sufficient to partially or completely melt the solid particles. The advantage of this method over SPS is the lower flame temperature than the plasma arc and, at the same time, a higher velocity of the expelled particles. This procedure results in a less porous and less rough coating compared to the SPS method. Bioactive glass coatings using this method were obtained on pure titanium substrates [115] or stainless steel AISI 304 [116].
The most inexpensive and simple coating procedure is flame spraying (FS). The process involves burning a mixture of gases, such as acetylene/oxygen, hydrogen/oxygen, and propane/oxygen. The powder is fed into the resulting flame where it melts. The speed of particle expulsion is much lower than in the HVOF and HVSFS processes. Porous and composite coatings can be obtained using this procedure [117].
If the coatings are obtained by thermal spraying, scanning electron microscopy is essential in the evaluation of the microstructure of the coatings, while surface roughness is especially analyzed using white light scanning interferometry [116].
4.3. Sol-Gel Deposition Technique
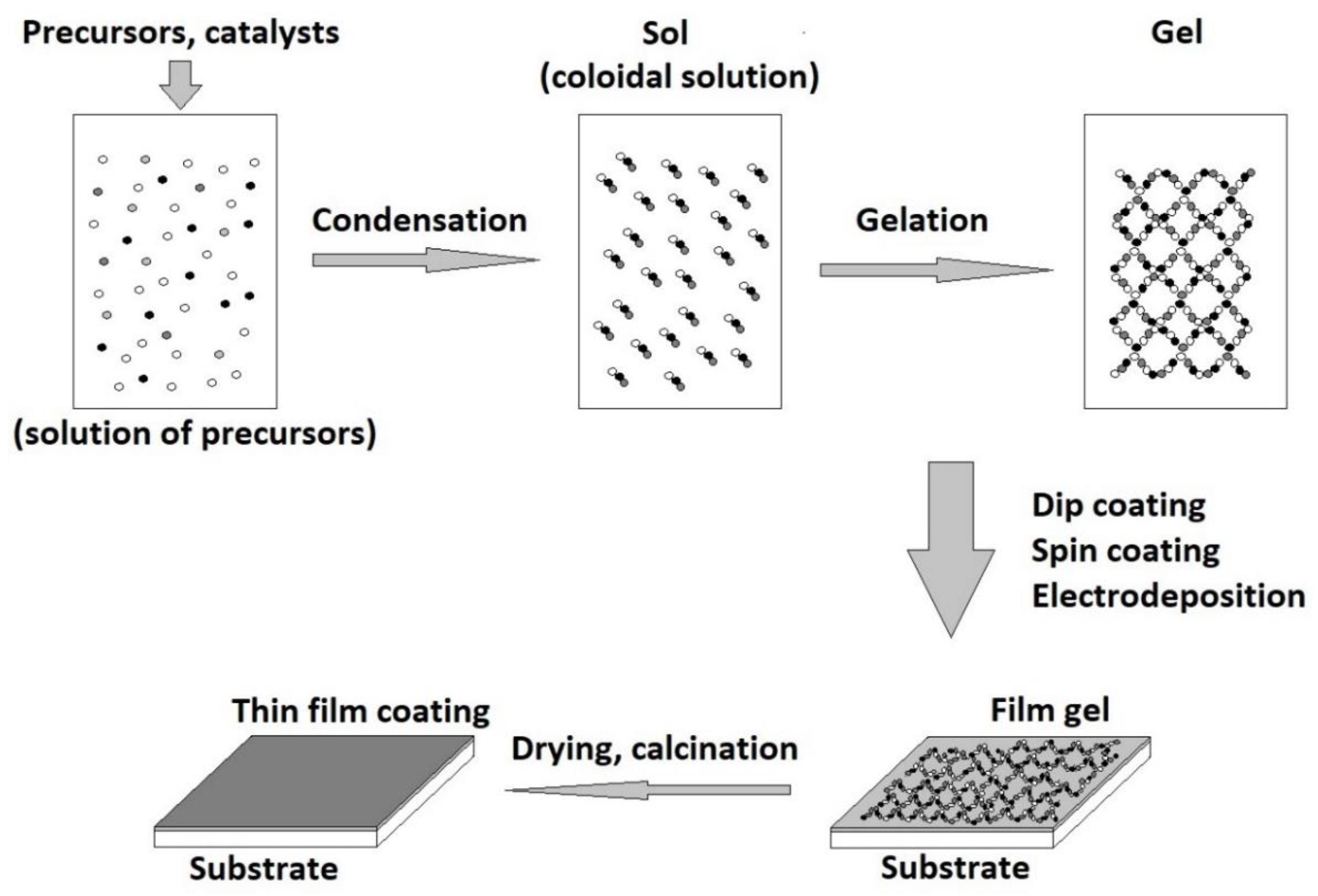
One of the most used coating techniques for bioactive glass deposition is the sol-gel method. By applying this technique, homogeneous glass with a controlled composition can be obtained [118,119]. Moreover, the glass obtained by the sol-gel technique shows bioactivity in a larger range than the melting method [120,121]. This technique is very versatile when it comes to obtaining bioactive glass coatings, the elasticity and viscosity of the coating gel can be adjusted according to the substrate to be coated [122]. The sol-gel method is a chemical method of glass preparation, at low temperature. The procedure consists of dissolving the glass precursors, usually metal alkoxides and nitrates, in a solvent. After conducting the hydrolysis and polycondensation reactions, a gel is obtained. To solidify the gel, it is dried by removing the solvent and then the densification is achieved by heat treatment at 600–900 °C [123]. In addition, due to the way the glass is synthesized by this technique, complex elements can be introduced in its structure, which offer special characteristics to the obtained glass. For example, nanoparticles, mesoporous agents, and antibacterial agents can be introduced [41,124]. The sol-gel method is successfully used for the preparation of a wide range of bioactive glass with a porous microstructure having a large specific surface area. This allows the absorption of proteins and the cell adhesion on the obtained bioactive glass surfaces [125].
During the gelation step of the sol-gel method, several coating techniques can be applied, such as (Figure 3) electrodeposition, dip coating, and spin coating. The choice of the appropriate technique is made depending on the shape of the substrate and the characteristics of the desired coating. For example, in the case of spin coating, the substrate must be flat, and the resulting coating thickness is around 40 nm to 10 mm. The thickness of the coating depends on the sol-gel viscosity, rotation speed, and time [126]. Using this technique, uniform multilayer structures such as bioactive glass/zirconium titanate were obtained. The thickness and roughness of the multilayer coatings increase nonlinearly depending on the number of layers applied. A special perspective is presented by a class of nanocomposite coatings composed of bioactive and inert components [127]. Therefore, the bioactivity and bioavailability of the coating can be combined with the corrosion and wear resistance of the implant [128].
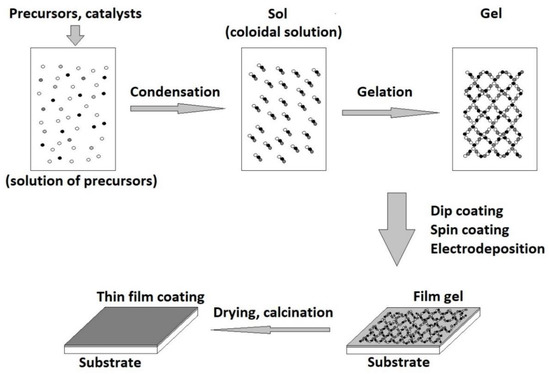
Figure 3.
Sol-gel coating technique.
In the case of electrodeposition, the substrate must conduct electricity and its shape can be complex. The thickness of the obtained coating varies between 1 and 100 µm and depends on the nature of the material as well as on the deposition parameters, such as time and electric charge.
Immersion deposition is the most versatile deposition technique used for the sol-gel derived glass coatings. The object to be coated is immersed in sol-gel. Depending on the desired coating thickness, the extraction speed and sol-gel viscosity are adjusted [129]. This technique can be applied for 3D objects and porous 3D structures. Moreover, the deposition can be made in a vacuum in order to ensure the penetration of the sol-gel into the pores of the substrate surface.
Using the methods described in this chapter, bioactive glass coatings were obtained on substrates of stainless steel [130,131,132,133,134,135,136], magnesium and its alloys [137,138,139,140], PET [141], titanium [36,142,143,144], and alloys such as Ti-6Al-4V [145,146] and NiTi [147], silica [148], etc. These alloys possess a high strength, low elastic module, and good biocompatibility [49]. A few of these materials were tested in vitro and in vivo and the formation of hydroxycarbonate apatite was observed on the surface of implants coated with bioactive glass [149].
In addition to coatings, the sol-gel method allows the attainment of bioactive glass based biodegradable polymers. These polymers can be used to make surgical implants that decompose in vivo and do not require a subsequent operation to remove them [37].
The coatings obtained are characterized by several methods. To determine the particle distribution, homogeneity, and integrity of the coating, the scanning electron microscopy is used. The composition of the coatings is determined by X-ray diffraction. Electrochemical tests are performed using the electrochemical impedance spectroscopy [135] and potentiodynamic polarization in order to assess the corrosion resistance. The bond strength (MPa) of the coatings can be determined by the tensile bond test [65,138].
4.4. Radio Frequency Magnetron Sputtering
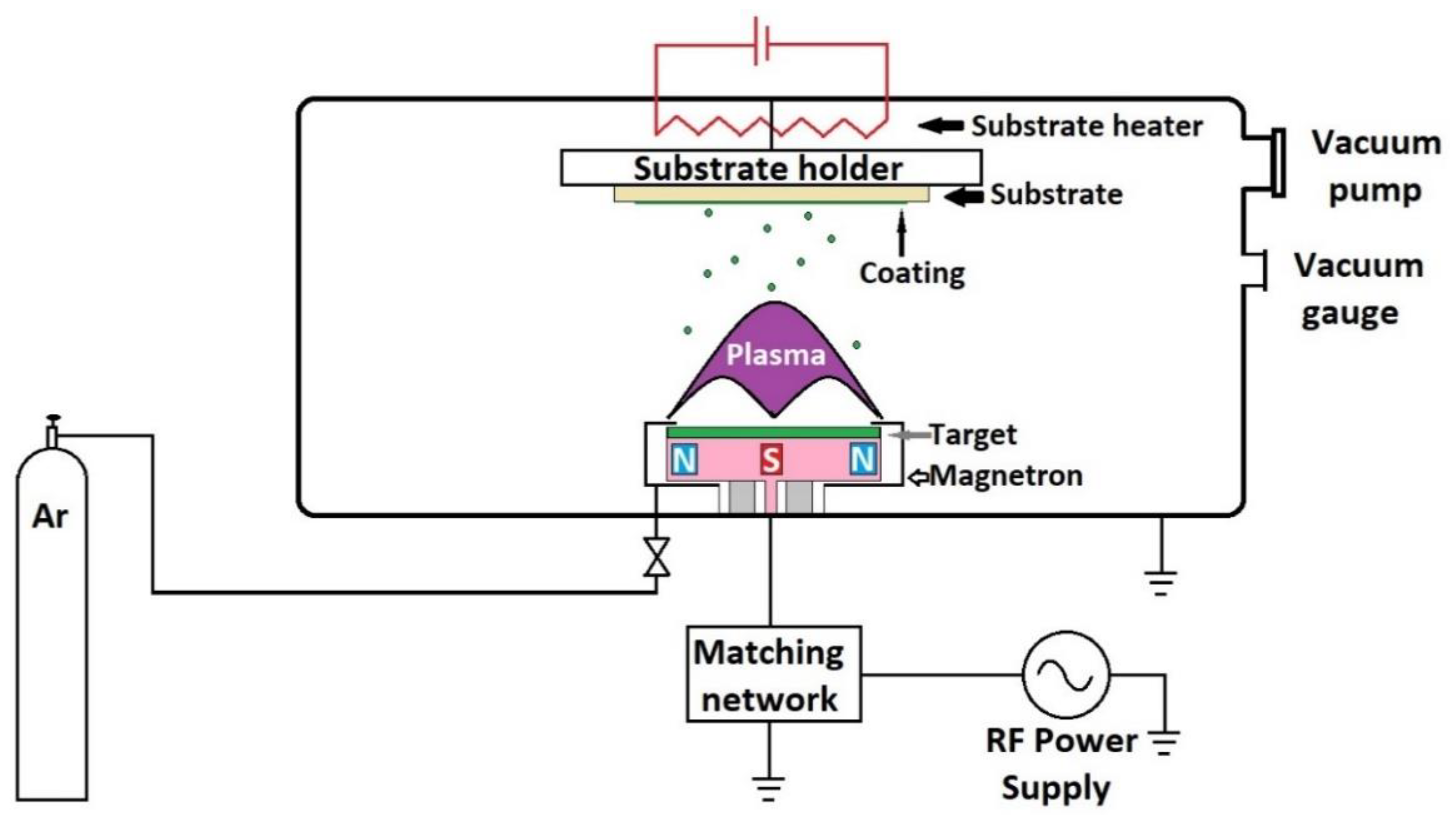
Radio frequency magnetron sputtering (RF-MS) is a low-pressure technique that allows the deposition of uniform coatings of controlled thickness. The method is mainly used to obtain thin coatings with a high degree of adhesion. Coatings are obtained at low working pressures, lower substrate temperatures, and have a high degree of purity and uniformity [150]. During the sputtering process, a target of bioactive glass is bombarded with plasma ions, including reactive oxygen ions, that eventually lead to the formation of the coating. As the reactive gas flow increases, the target is poisoned, the rate of spray erosion decreases, and the deposition process decreases. The deposition rate, structure, thickness, composition, and biomineralization capacity depend on the deposition pressure. The deposition rate decreases with the increasing working pressure, and this can be explained by the fact that, as the pressure increases, the particles to be sputtered on the substrate suffer collisions during the movement from the target to the substrate, and some are returned to the target [113].
Figure 4 shows the schematic representation of the spray installation. Practically, it consists of a vacuum chamber connected to a vacuum pump, for example, turbo-molecular or diffusion pump, in order to achieve the desired low-pressure values. The pressure value is measured using a vacuum gauge. Inert or reactive gases (Ar, O2, N2, etc.) may be introduced into the chamber. The target, in the form of a disk, made from the material to be deposited, is placed at the top of a device called magnetron. The magnetron is connected to a radio frequency source, usually 13.56 MHz, but it can also work at other frequencies. The radio frequency ionizes the gas molecules, forming plasma that is concentrated in a small area on the target due to the magnets inside the magnetron. The ionized gas hits the target and expels the material from which it is made. During the deposition process, the magnetron heats up and that can lead to damage to the magnets. These magnets are cooled from the outside with water. The substrate is located on the opposite side of the magnetron. For a more uniform deposition, the substrate can be electrically heated and rotated by means of a motor. The distance between the target and the substrate can be adjusted and this is an important parameter that influences the coating characteristics.
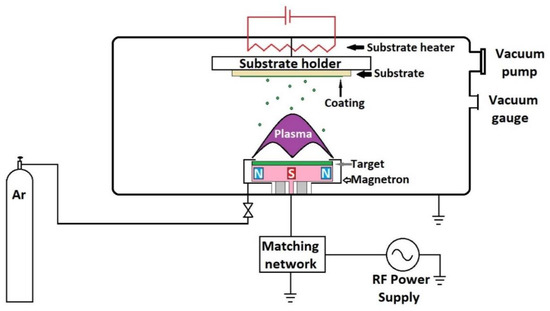
Figure 4.
Schematic representation of radio frequency magnetron sputtering unit.
In the literature, several bioactive glass coatings with 45S5 [151], 58S [150], bioactive glass with low content of silicon [152,153] or sodium [154,155] performed on Ti [152,153,155,156,157,158], Ti-6Al-4V [44,150,151,154,159,160,161], and silicon [44] were reported. These coatings were used in order to increase the bioavailability of dental implants [154] and bioactive scaffolds [150].
The morphology of the surfaces obtained by sputtering is usually analyzed by atomic force microscopy (AFM) or by scanning electron microscopy (SEM) [156]. The surface composition is analyzed by X-ray energy dispersion spectroscopy. Spectroscopic ellipsometry (SE) is used to determine the thickness and refractive index, and X-ray diffraction is used to evaluate the degree of crystallinity [44].
4.5. Laser Cladding
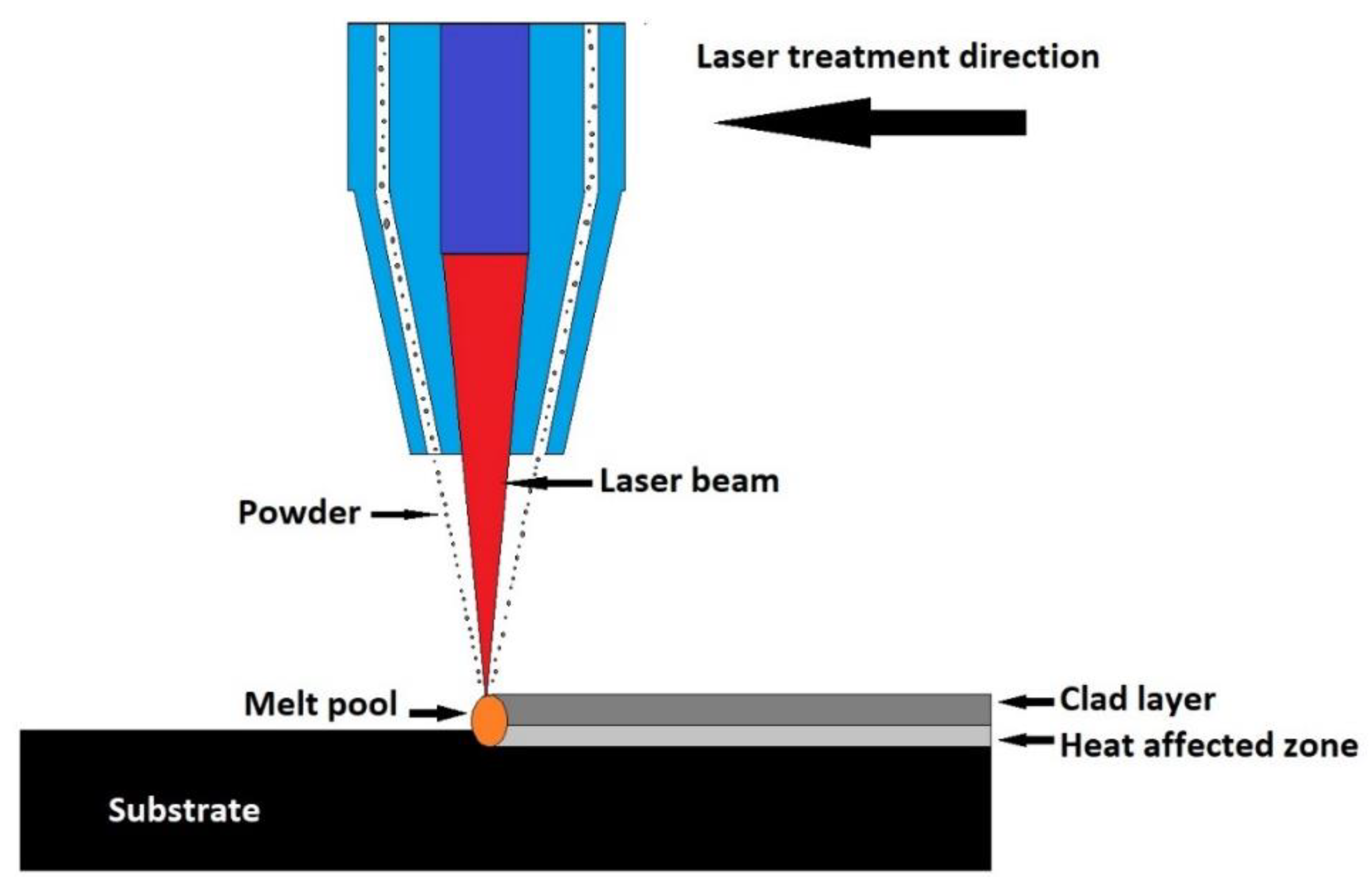
Laser cladding is a coating technique in which different types of materials are linked together using laser intercession. The covering material is fed in a powder form on the substrate surface and melted using laser, eventually forming the coating, as represented in Figure 5. Several changes to this coating technique are currently being developed. In an industrial installation, the powder is fed through a nozzle and the laser beam is directed towards the powder, melts it, and forms the coating [2,118].
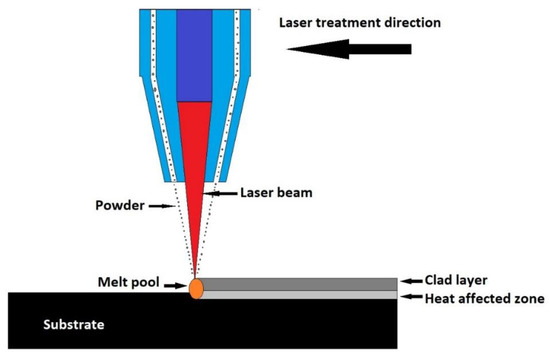
Figure 5.
Schematic representation of laser cladding technique.
The powder or paste material can also be deposited on the surface of the substrate, then melted with a laser beam [162]. Bioactive glass coatings obtained using this technique were applied to Ti-6Al-4V alloy substrates [163,164]. In addition to the 45S5 bioactive glass, S520 type bioactive glass coatings were obtained. This glass has a smoother wetting angle-temperature dependence and has been used as a precursor for bioactive coatings. A Nd:YAG laser was used to melt the material. The process resulted in a uniform coating with crystalline calcium silicate content. This coating shows bioactivity in vitro, while being immersed in the SBF solution [165].
Laser cladding is a flexible technique that allows the coating of plane, but also curved shape scaffolds with bioactive glass. The laser cladding technique has been successfully applied for the deposition of bioactive glass coatings on acetabular alumina/zirconium ceramic cups. The coatings obtained can be porous or pore-free depending on the conditions of the deposition process. The coatings showed a high degree of adhesion to the ceramic substrate and good in vitro bioactivity. Studies performed by immersion in SBF showed the formation of an apatite layer on the implant [166].
The multilayer application of the bioactive glass in the form of drops allows the attainment of stable coatings, without cracks. Laser cladding allows the preparation of bioactive glass coatings in situ, on metal surfaces, with a well-controlled microstructure and composition, with high adhesion to the substrate [149].
It has been found that through varying the wavelength of the laser used, the depth of penetration can be controlled. Therefore, a laser beam with a shorter wavelength is more strongly absorbed by the material, which means that it penetrates to a shallower depth and scatters the deposited material less. In the case of longer wavelengths, the absorption of the beam decreases, and it penetrates the material better, leading to the phenomenon of overheating and evaporation [162].
4.6. Pulsed Laser Ablation and Deposition
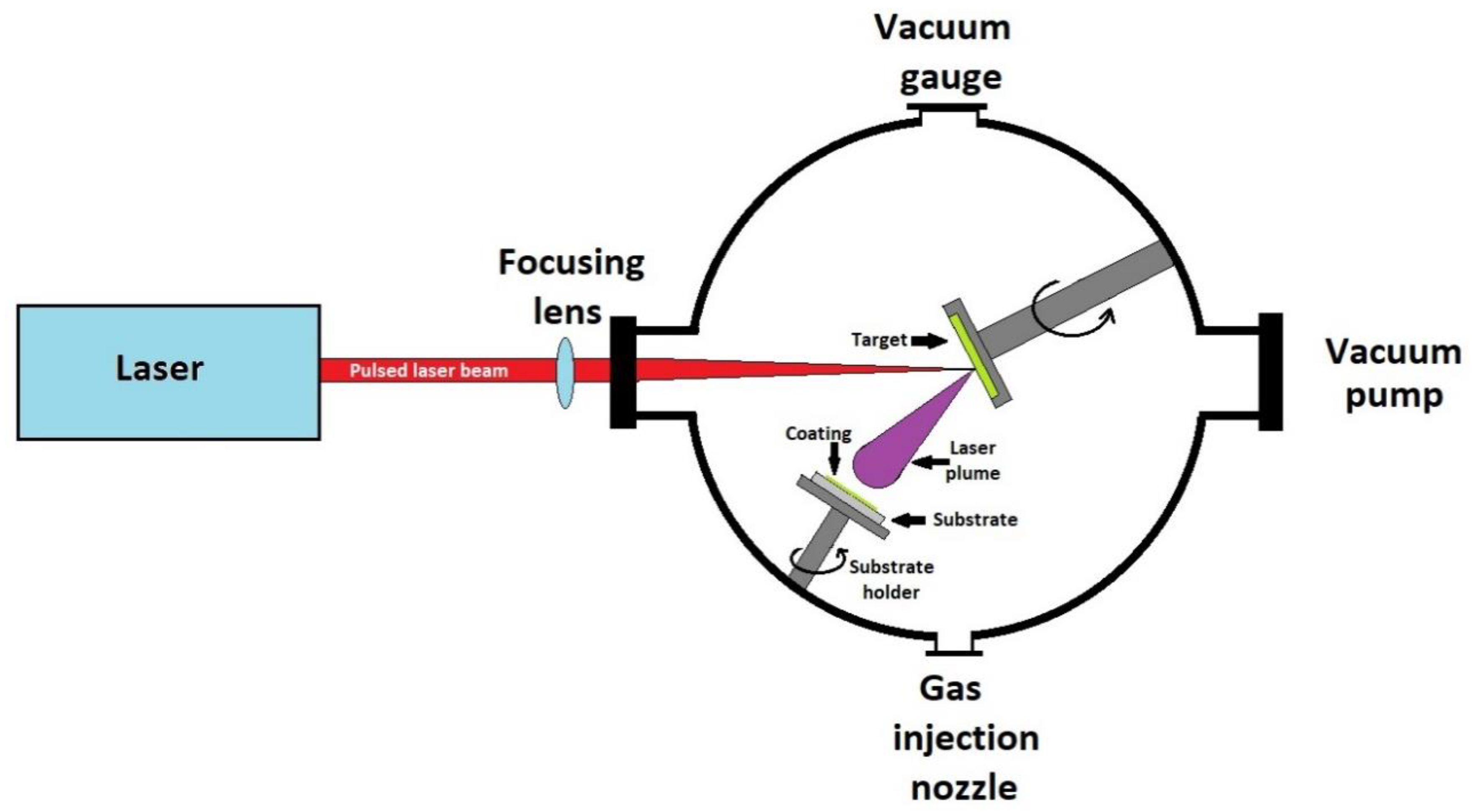
Currently, the technique of pulsed laser ablation and deposition (PLD) is widely used for the deposition of thin films in the form of single or multi-layer. Laser pulse deposition has several advantages, such as deposition of materials with high melting point, stoichiometric transfer of target material, and lack of contamination. In addition, if the deposition process takes place in the atmosphere of a reactive gas, during ablation, reactions can take place between the target material and the present gas, leading to the formation of new compounds. This process is called reactive pulsed laser deposition (RPLD) and allows a fine control of the properties of the deposited coatings. The process is of particular interest in the deposition of silicon-based bioactive materials.
The deposition process using pulsed laser consists of ablation of the target material using a pulsed laser beam. Following irradiation of the target with the laser beam, a plasma cloud appears which transfers the target material to the substrate in the form of a thin film. A schematic representation of a pulsed laser deposition apparatus is shown in Figure 6.
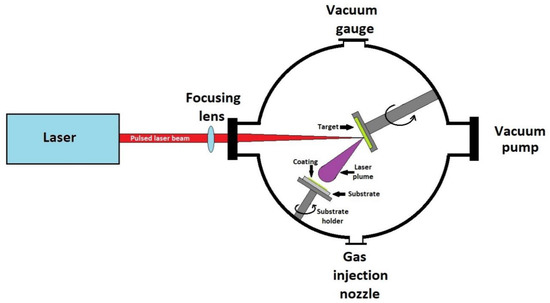
Figure 6.
Schematic representation of a pulsed laser deposition apparatus.
The apparatus consists of a sealed chamber, vacuumed using a pump system. Inside the chamber is a rotating target with the material to be deposited and a heated place for the substrate to be covered. The laser used for ablation is of the excimer type, the target is made of suitable bioactive glass. Deposition takes place at reduced pressures of 10–4 Pa [167].
The parameters that influence the properties of the obtained films are the substrate temperature, the type of gas present in the vacuum chamber, the laser wavelength, energy density, the target properties, and the deposition rate. The partial pressure of the reactive gas plays a special role in the growth and binding of bioactive glass films to the substrate [168]. If only argon is present in the deposition chamber, a significant decrease in the deposition rate is observed [169].
Using pulsed laser deposition, bioactive glass coatings were obtained on scaffolds made of pure titanium [170,171,172] and its alloys [173,174,175,176], stainless steel [177], magnesium [178], and silicon [144,178].
The characterization of the obtained coatings is performed by specific techniques. For example, scanning electron [174] and energy dispersive spectroscopy EDS [176] are used to characterize the coating morphology. X-ray diffraction is used to study the crystallinity of the obtained coatings [171]. Functional groups are highlighted by FTIR analysis, and the degree of adhesion of the coating is evaluated by the scratch tester method [179].
4.7. Electrophoretic Deposition
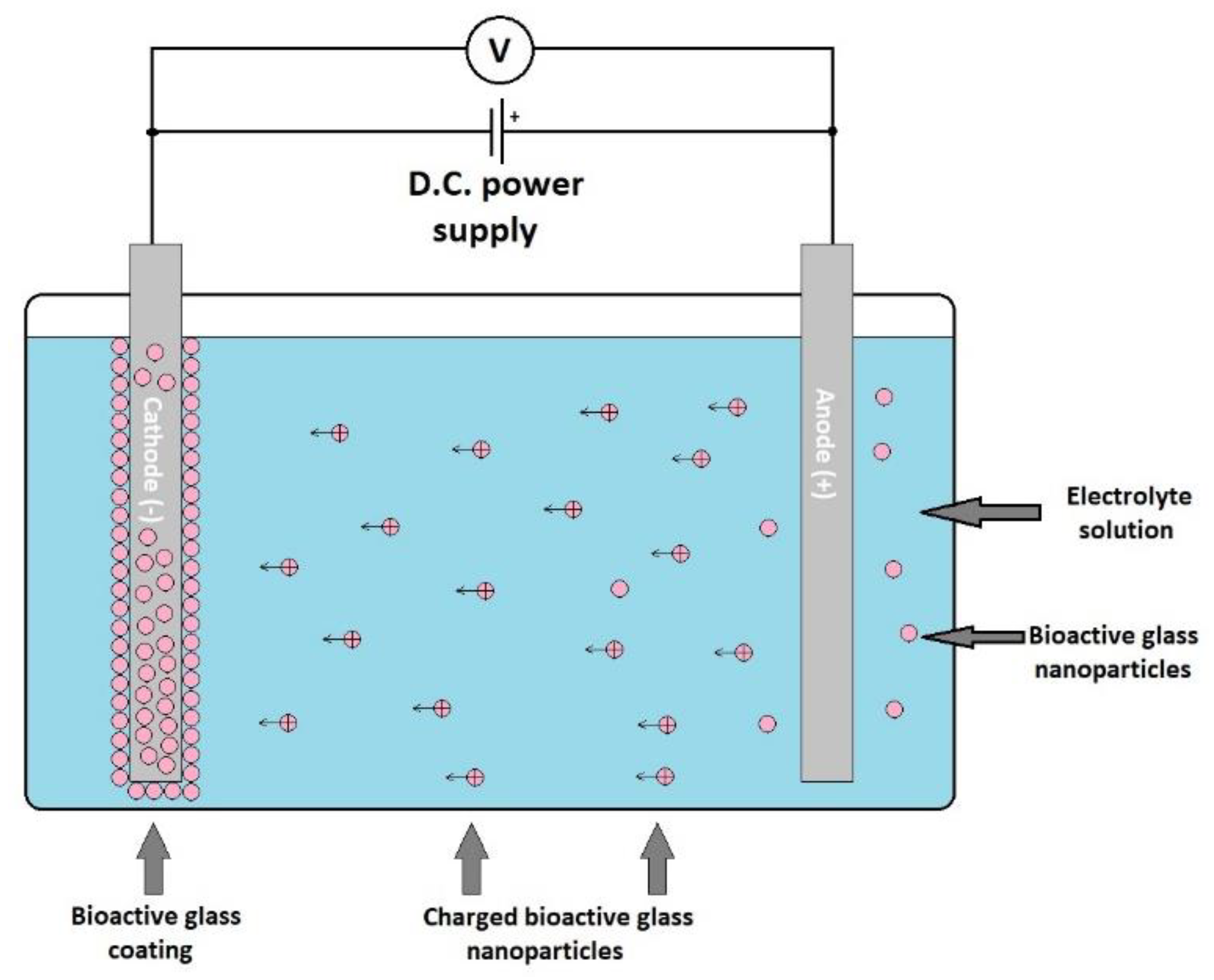
Electrophoretic deposition (ELD) is an electrochemical method that consists of applying an electric field between two electrodes that are immersed into a deposition chamber ELD filled with a suspension of the material to be deposited [122]. The electrically charged particles are attracted to the electrode with the opposite electric charge, thus resulting in coating (Figure 7). This resulting layer is then subjected to a heat treatment at the appropriate temperature. The heat treatment results in a stable coverage. This coating process is very versatile and flexible, it can be applied to obtain ceramic, glass, polymer, and metal coatings using the appropriate material suspension. Stable suspensions of different materials are used for deposition, and the substrate to be coated must conduct electricity or be previously covered with a conductive layer.
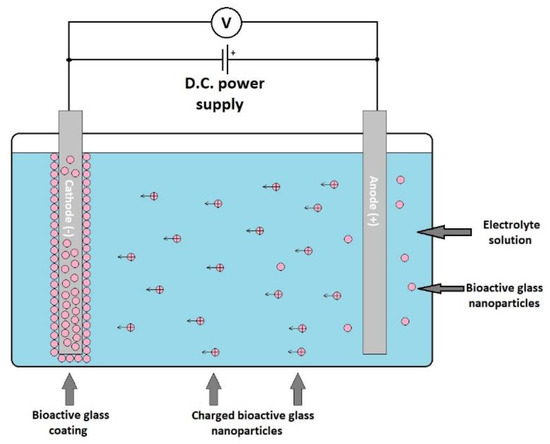
Figure 7.
Schematic representation of an electrophoretic deposition process.
The coating properties can be adjusted through varying the deposition parameters as well as the characteristics of the electrolyte solution. The most important parameters are those related to the deposition parameters: The distance between the electrodes, the applied electric field between the electrodes, the time, the deposition temperature, and pH. The studies revealed that the optimal deposition speed of bioactive glass is obtained when an electric field of about 5 V/cm is applied for 7 days, while a pH value of 7 is used [180]. Compared to the other deposition techniques, ELD allows an easier adjustment of the coating thickness.
In the literature, several studies are presented that used the ELD method to obtain coatings with bioactive glass or composites of glass with hydroxyapatite [181,182], zirconium oxide [18,183], carbon nanotubes [184,185], hyaluronic acid [186], chitosan [187,188,189,190], and alginate [133] on metallic substrates such as titanium [181,182,190,191], titanium alloy Ti-6Al-4V [18,183,189], stainless steel AISI 304 and AISI 316 [133,180,184,185,187,188,191,192,193], and magnesium [191,194]. For thicknesses up to 50 μm, microwave sintering can be applied, which has proven to be a suitable technique for obtaining coatings without cracks. Polyether ether ketone (PEEK)/bioactive glass coatings have proven to be bioactive coatings with a high degree of elasticity [193].
To characterize the morphology of the coatings obtained by this technique, scanning electron microscopy can be used. A coating thickness gauge is used to determine the thickness of the coatings, for example, Electrometer 456. The quantitative elemental analysis is performed using X-ray dispersion spectrometry. The crystal structure is determined by X-ray diffraction analysis [190,193]. The degree of adhesion of the coating to the substrate is a very important parameter, which can be measured using the tape test.
4.8. Other Bioactive Glass Coating Techniques
In this chapter, some newer deposition techniques are described, in which the application is not yet sufficiently studied, but have a potential for research and industrial application.
CoBlastTM is an easy process that can be performed at room temperature, using dry chemical means for coating reactive metals such as titanium and its alloys, cobalt—chromium, aluminum, and some stainless steels, with doping materials. These metals/alloys are commonly used in the manufacture of implants. CoBlastTM consists of a mechanical microsanding process that, unlike sandblasting, which is a process used to increase surface roughness and material removal from the surface, CoBlastTM is a method of depositing material on the surface. The resulting change leads to a textured morphology and a chemical change as a result of the incorporation of doping material that is impregnated into the metal oxide layer and does not constitute a laminate layer or a coating. Therefore, the surface cannot be detached as in the case of conventional coatings. CoBlastTM is a very frequently used process in the medical device industry that does not use solvents, thus resulting in a clean, durable, and stable coating with no environmental issues associated. Inorganic coatings can be deposited in this way to ensure very good resistance to high temperatures and wear. F. Tan et al. used this technique to deposit bioactive glass and hydroxyapatite on the surface of titanium implants and its alloys, with promising results both in vitro and in vivo. After analyzing the resulting surfaces, the authors concluded that the obtained surfaces show bioactivity, osteoconductivity, and cell adhesion and proliferation [195,196].
Pulse electron deposition (PED) is an efficient method of physical vapor deposition used to obtain nanostructured and resistant bioactive coatings, with very good control over the composition, even in the case of temperature sensitive substrates, with a low cost compared to pulse laser deposition (PLD). PED allows the film to grow without affecting the stoichiometry of the target. During the ablation process, all of the target components are expelled simultaneously, regardless of the enthalpy of evaporation, and transferred to the film, maintaining the composition of the target. Therefore, it is favored to obtain thin films composed of complex materials using a single step. D. Belluci et al. used this method to deposit two types of bioactive glass 45S5 and a recently developed bioglass BG_Ca/K (composition in mol%: 4.6 K2O; 45,6 CaO; 2.6 P2O5; 47.2 SiO2) on Ti-6Al-4V plates. The coatings obtained are nanostructured, homogeneous, hydrophilic, with a high degree of adhesion, and with a composition similar to the target [197].
Another studied technique is the drip-sedimentation method that was applied to cover porous titanium substrates with 45S5 and 1393 bioactive glass. The method consists of preparing a suspension of bioactive glass in ethanol and dripping it over the substrate, followed by evaporation of the solvent at room temperature. This procedure is repeated several times to obtain a uniform, multilayer coating. Finally, the coated samples are heat treated at 820 °C in a vacuum. Therefore, porous coatings were obtained, suitable for the manufacture of implants. This is a simple, inexpensive, easy to reproduce technique [198,199].
Researches in the field of bioactive glass coatings also studied deposition performed by plasma electrolytic oxidation (PEO), a high voltage electrolytic process that involves the generation of a plasma discharge at the metal-electrolyte interface that leads to the formation of a dense ceramic layer, without affecting the substrate surface by thermal expansion. Discharges occur when the voltage exceeds the “punching value”, usually a few hundred volts. Currently, the plasma electrolytic oxidation technique is a method of coating metals, such as magnesium, titanium, aluminum, and their alloys. Costa et al. managed to apply this deposition method to cover titanium substrates with 45S5 bioactive glass. The obtained coatings presented a complex surface topography, with improved mechanical properties and corrosion resistance. PEO-BG modulates the oral biofilm, favoring the colonization of beneficial microorganisms and reduces the pathogenic potential of the biofilm surrounding the implant. In addition, PEO-BG coatings show prolonged ion release and pH variations, leading to faster hydroxyapatite formation capacity and increased protein adsorption from blood plasma without cytotoxic effects on fibroblast cells in human gingival tissue, thus allowing the osseointegration process to take place [200].
Electrospinning is a method of producing ultrafine fibers of nanometric dimensions. The method consists of ejecting the polymer solution or the molten polymer through a needle connected to a high voltage power source. The ejected substance solidifies or coagulates as a filament. Recently, this method has been used to coat magnesium substrates with poly (ε-caprolactone) fiber/bioactive glass nanoparticles. The tests showed that the degradation of the substrate immersed in SBF was considerably reduced, and the formation of hydroxyapatite on the sample surface took place after 7 days of immersion. In addition, in vitro tests have shown that the fibroblast cells attach easily to the coating obtained [201]. In another study, the researchers used this coating method to obtain a composite coating of bioactive glass/gelatin/polycaprolactone on 316L stainless steel substrates. The SEM analysis showed that the size of the obtained fibers is 200 nm. The EIS analysis has shown that the deposited coating increases the corrosion resistance of the substrate. In vivo animal tests have shown that there is no inflammation and granulation of tissues, formation of fibrotic tissue or the appearance of a toxic effect at the site of implantation [202].
5. Doping Agents
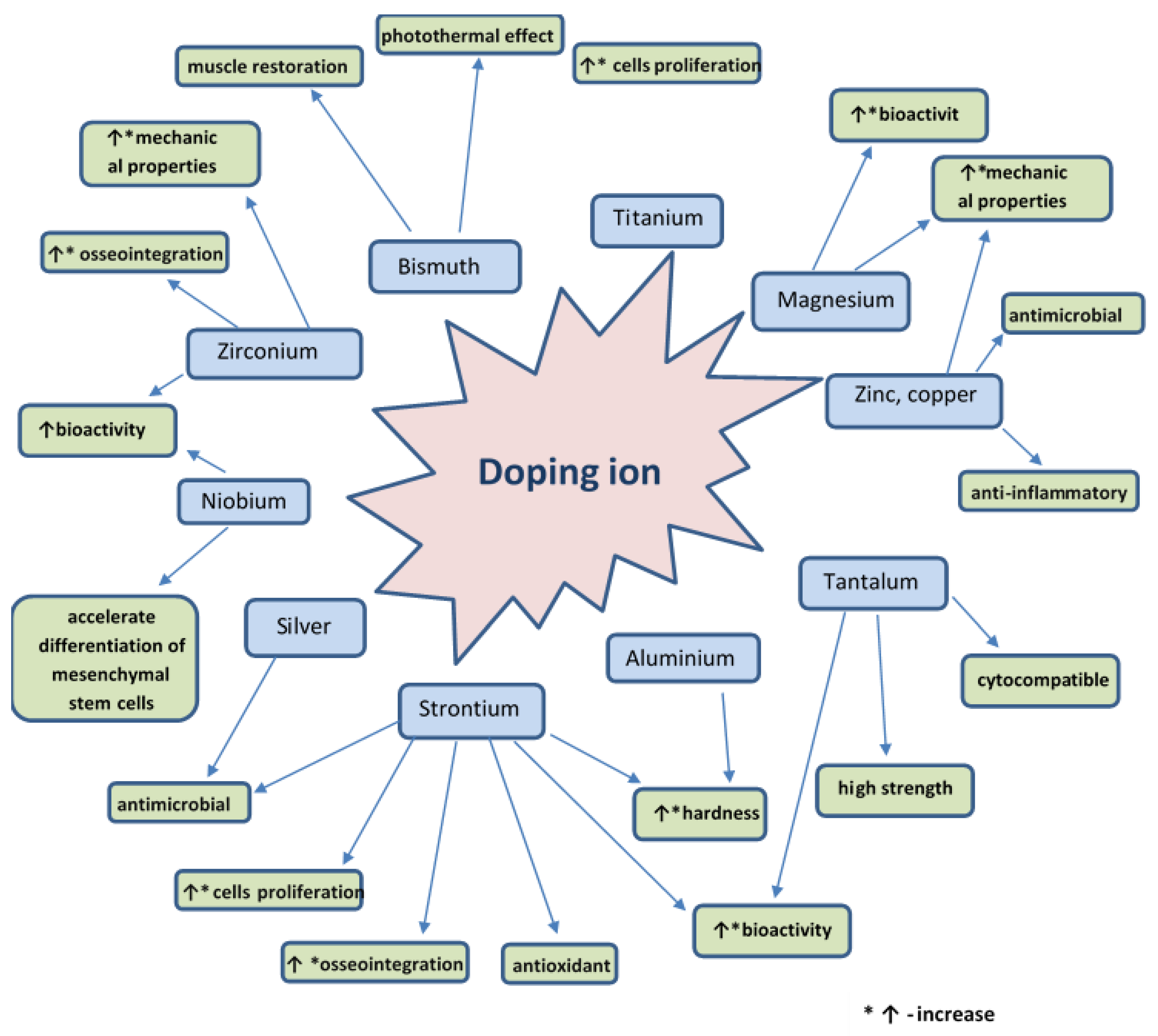
Bioactive glass is a material that can be doped with different ions in order to improve its physical, chemical, and biological properties. The doping agent is chosen according to the intended application. Figure 8 presents various doping agents and the properties they give to bioactive glass. Therefore, the bioactive glass has the required characteristics necessary for safe use.
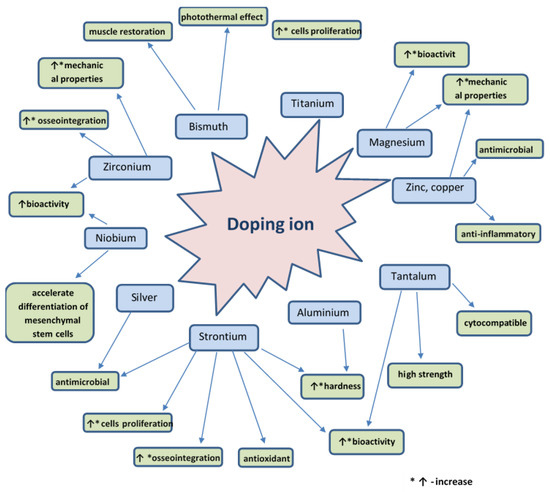
Figure 8.
Schematic representation of the properties of bioactive glass given by several doping agents.
It is proven that ions used as doping agents cannot only directly influence the behavior of cells by playing a specific biological role (e.g., genetic stimulation of osteoblast cells, induction of angiogenesis in vitro and in vivo, antibacterial and anti-inflammatory action). However, the ions can also induce changes of physical properties of bio-glass (morphology, topography, crystalline microstructure, and absorbability) [203].
Numerous examples of doping different types of bioactive glass with different ions are described in the literature. A culture of osteoblasts grown on the surface of a titanium-doped bioactive glass was found to show a higher degree of proliferation compared to the 45S5 bioactive glass, which is explained by the more controlled release of soluble components from bioactive glass. On the other hand, osteoblasts cultured on a bioactive glass surface doped with iron, fluorine or boron ions showed a weaker degree of proliferation compared to the standard 45S5 bioactive glass [204]. The addition of a small amount of silver to the 45S5 bioactive glass doped with 10% titanium gives antibacterial properties against Streptococcus mutans, Staphylococcus [205,206], and Escherichia coli [207].
Strontium ion-doped 46S6 bioactive glass has been found to stimulate the proliferation of SaOS-2, Eahy 926 endothelial cells in vitro. In vivo, Sr-doped bioactive glass exhibits good bioactivity, osseointegration, and balanced oxidation stability 60 days after surgery. Incorporation of 0.1% Sr into the bioactive glass can be an effective method for creating materials for bone regeneration with an antioxidant [26,208,209] and antimicrobial [7,210] role. Moreover, Sr ions give anticorrosive properties to the bioactive glass when it is used to coat stainless steel scaffolds [135,211]. Strontium ions present in the 45S5 bioactive glass increase the hardness of the composite material without affecting the elastic modulus of the bioactive glass [212].
Zinc and copper ions can give the anti-inflammatory properties to the bioactive glass, thus it can be used as an anti-inflammatory component to treat wounds [203]. Moreover, zinc [213] and copper [214] ions give antimicrobial properties to the bioactive glass coating [215]. Furthermore, by adding zinc, the chemical stability and mechanical properties of the bio-glass can be improved [216,217].
Magnesium ions can improve the bio-glass bioactivity. The presence of magnesium ions increases the degree of dissolution of the bioactive glass, but, at the same time, decreases the crystallization rate of hydroxyapatite on the surface [203,218]. Magnesium particles dispersed in bioactive glass improve its mechanical properties [212,219,220].
Bismuth ions allow the attainment of a biocomposite that possesses a photothermal effect, which retains its biocompatible and remineralization properties with hydroxyapatite and allows the reduction of the number of treatment cycles needed to cure bone defects. Coating with this biocomposite allows differentiation and mineralization with osteogenic cells. In addition, in vivo and in vitro experiments indicate that this coating can destroy bone tumor cells upon irradiation with NIR light, and can promote the restoration of muscle growth on these surfaces [38].
Doping of the bioactive glass with tantalum ions has led to the preparation of a cytocompatible material with osteoblast cells, with increased bioactivity and high strength [221,222,223]. Cements based on tantalum-doped bioactive glass have the appropriate rheology, hardness and radiopacity, are antibacterial, and can be used for sternal fixation [224].
Zirconium ions can be introduced to give the phosphorus-containing bioactive glass increased mechanical strength [225,226]. Zirconium is a microelement present in the human body, and its introduction into the bioactive glass can stimulate its bioactivity and osseointegration [227,228,229]. A group of researchers prepared a type of bioactive glass doped with Zr/Ho. This glass has a pronounced radiological response and clinical trials are currently done for assessing the possibility of use in brachytherapy [230].
Aluminum is an element that, when incorporated in the bioactive glass that has a high phosphorus content, increases its density and micro-hardness due to the formation of P-O-Al bonds. Moreover, it has been observed that a content of 6% Al significantly increases the bioactivity and the rate of cell proliferation [22].
Niobium ion-doped 45S5 bioactive glass has increased bioactivity [231]. Moreover, a study performed on this type of glass, revealed that an osteogenic differentiation of human mesenchymal stem cells (hMSC) was performed after 21 days on a fluoroapatite glass doped with niobium ions [232].
Prasad et al. studied the effect of Fe and Co ions addition in bioactive glass. They found that doping the bioactive glass with these ions allows the attainment of a material with magnetic properties. However, the addition of these ions to the 45S5 bioactive glass leads to a decrease in bioactivity. The formation of the hydroxycarbonate apatite layer on the surface of the doped sample immersed in SBF takes place after 14 days, compared to 3–7 days on the undoped sample. The material doped with Fe and Co ions has an improved density and compressive strength compared to the undoped material [233].
In addition to metal ions, in order to give it certain useful properties, other substances can be incorporated into the bioactive glass. For example, using 17% (mass) chitosan results in a material that tolerates osseointegration and improves the formed bone architecture. Chitosan initiates the process of mineralization and activation of cells, and the composite biomaterial obtained reduces oxidative processes and improves favorable biochemical responses [25].
Zein, a natural polymer, incorporated into the structure of bio-glass coatings allows the attainment of homogenous, antimicrobial coatings on stainless steel [192].
The use of polymethylmethacrylate allows the formation of bioceramic films, such as TiO2, Al2O3, hydroxyapatite, and bioactive glass with a very high degree of adhesion on stainless steel substrates. Moreover, this film serves as an anti-corrosion coating for the 304 and 316 stainless steels and on titanium substrates [234,235,236,237].
It is important to note that the bioactive glass-polyvinyl alcohol (PVA) composite material possesses good bioactivity, osseointegration, and oxidation stability. Combining PVA with bioactive glass is an effective method of creating antioxidant materials for bone recovery/regenerative therapy. The incorporation of ciprofloxacin into this compound decreases osseointegration and oxidation stability, but induces antimicrobial activity when the risk of infection is high [238].
6. Conclusions and Perspectives
Although bioactive glass was discovered over 50 years ago, the interest in this material is constantly growing. Properties such as bioactivity, osseointegration, and antimicrobial activity, make this material increasingly suitable for use in the field of implants. Bioactive glass can be used as bulk material, as an implant or in the form of coatings on various other materials, thus broadening its spectrum of use. Bioactive glasses are used in applications such as dental implants, orthopedics, bone grafts, and scaffolds. This review describes the methods that can be used to obtain bioactive glass coatings on various substrates, along with highlighting their relative advantages and disadvantages. Choosing the right method is closely related to the application of coatings, the desired properties, and the final cost of the bio-device. Bioactive glass properties can be modified using various doping agents. The doping agent is selected depending on the aimed application and on the desired properties. Moreover, drugs can be incorporated in the bioactive glass in order to improve the healing process.
Bioactive glasses are the materials of the future. In addition, the performance and applicability of this material depends only on the imagination and ingenuity of researchers. The development of lower cost coating methods is an important step in order for the obtained bio-devices to be accessible to as many people as possible.
Author Contributions
Conceptualization, M.M., D.F. and A.F.; methodology, M.M., A.F., E.A.; validation, E.A., A.F.; writing—original draft preparation, M.M., O.-C.M., L.C., D.F.; writing—review and editing, A.F. and E.A.; visualization, A.F. and E.A.; supervision, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by University Politehnica of Bucharest.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mesquita-Guimaraes, J.; Henriques, B.; Silva, F.S. Bioactive glass coatings. In Bioactive Glasses. Materials, Properties and Applications; Ylanen, H., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Duxford, UK, 2018; pp. 103–118. [Google Scholar] [CrossRef]
- Sola, A.; Bellucci, D.; Cannillo, V.; Cattini, A. Bioactive glass coatings: A review. Surf. Eng. 2011, 27, 560–572. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass (R). J. Mater. Sci.-Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Greenspan, D.C. Glass and medicine: The larry hench story. Int. J. Appl. Glass Sci. 2016, 7, 134–138. [Google Scholar] [CrossRef]
- Moritz, N.; Vallittu, P.K. Bioactive silicate glass in implantable medical devices: From research to clinical applications. In Bioactive Glasses. Fundamentals, Technology and Applications; Aldo, R., Boccaccini, D.S.B., Hupa, L., Eds.; The Royal Society of Chemistry: Lomdon, UK, 2017; pp. 442–470. [Google Scholar]
- Bahrami, M.S.; Eshghinejad, P.; Bakhsheshi-Rad, H.R.; Karamian, E.; Chen, X.B. Electrophoretic deposition of bioglass/graphene oxide composite on Ti-alloy implants for improved antibacterial and cytocompatible properties. Mater. Technol. 2020, 35, 69–74. [Google Scholar] [CrossRef]
- Ranga, N.; Poonia, E.; Jakhar, S.; Sharma, A.K.; Kumar, A.; Devi, S.; Duhan, S. Enhanced antimicrobial properties of bioactive glass using strontium and silver oxide nanocomposites. J. Asian Ceram. Soc. 2019, 7, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Seuss, S.; Heinloth, M.; Boccaccini, A.R. Development of bioactive composite coatings based on combination of PEEK, bioactive glass and Ag nanoparticles with antibacterial properties. Surf. Coat. Technol. 2016, 301, 100–105. [Google Scholar] [CrossRef]
- Echezarreta-Lopez, M.M.; Landin, M. Using machine learning for improving knowledge on antibacterial effect of bioactive glass. Int. J. Pharm. 2013, 453, 641–647. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate Bioglass (R) against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]
- Krishnan, V. Future Applications of Bioglass. Adv. Struct. Mater. 2016, 53, 317–336. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Particulate Bioglass (R) reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin. Oral Implants Res. 2002, 13, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, D.L.; Montfort, M.J.; McLoughlin, S.W. Differential healing response of bone adjacent to porous implants coated with hydroxyapatite and 45S5 bioactive glass. J. Biomed. Mater. Res. 2001, 55, 603–612. [Google Scholar] [CrossRef]
- Migonney, V. History of biomaterials. In Biomaterials; Migonney, V., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–10. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive Glass: Chronology, Characterization, and Genetic Control of Tissue Regeneration. In Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B., Ed.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 51–70. [Google Scholar] [CrossRef]
- Amaral, M.; Abreu, C.S.; Oliveira, F.J.; Gomes, J.R.; Silva, R.F. Biotribological performance of NCD coated Si3N4-bioglass composites. Diam. Relat. Mater. 2007, 16, 790–795. [Google Scholar] [CrossRef]
- Dominguez-Trujillo, C.; Ternero, F.; Rodriguez-Ortiz, J.A.; Pavon, J.J.; Montealegre-Melendez, I.; Arevalo, C.; Garcia-Moreno, F.; Torres, Y. Improvement of the balance between a reduced stress shielding and bone rit ingrowth by bioactive coatings onto porous titanium substrates. Surf. Coat. Technol. 2018, 338, 32–37. [Google Scholar] [CrossRef]
- Ananth, K.P.; Suganya, S.; Mangalaraj, D.; Ferreira, J.M.F.; Balamurugan, A. Electrophoretic bilayer deposition of zirconia and reinforced bioglass system on Ti6Al4V for implant applications: An in vitro investigation. Mater. Sci. Eng. C 2013, 33, 4160–4166. [Google Scholar] [CrossRef]
- Li, Z.; Khun, N.W.; Tang, X.Z.; Liu, E.J.; Khor, K.A. Mechanical, tribological and biological properties of novel 45S5 Bioglass (R) composites reinforced with in situ reduced graphene oxide. J. Mech. Behav. Biomed. 2017, 65, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Keranen, P.; Moritz, N.; Alm, J.J.; Ylanen, H.; Kommonen, B.; Aro, H.T. Bioactive glass microspheres as osteopromotive inlays in macrotextured surfaces of Ti and CoCr alloy bone implants: Trapezoidal surface grooves without inlay most efficient in resisting torsional forces. J. Mech. Behav. Biomed. 2011, 4, 1483–1491. [Google Scholar] [CrossRef]
- Drnovsek, N.; Novak, S.; Dragin, U.; Ceh, M.; Gorensek, M.; Gradisar, M. Bioactive glass enhances bone ingrowth into the porous titanium coating on orthopaedic implants. Int. Orthop. 2012, 36, 1739–1745. [Google Scholar] [CrossRef] [Green Version]
- Babu, M.M.; Rao, P.V.; Veeraiah, N.; Prasad, P.S. Effect of Al3+ ions substitution in novel zinc phosphate glasses on formation of HAp layer for bone graft applications. Colloid Surf. B 2020, 185, 110591. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Husanu, M.A.; Mercioniu, I.; Santos, L.F.; Fernandes, H.R.; Ferreira, J.M.F. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Profeta, A.C.; Prucher, G.M. Bioactive-glass in periodontal surgery and implant dentistry. Dent. Mater. J. 2015, 34, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Jebahi, S.; Oudadesse, H.; Ben Saleh, G.; Saoudi, M.; Mesadhi, S.; Rebai, T.; Keskes, H.; el Feki, A.; el Feki, H. Chitosan-based bioglass composite for bone tissue healing: Oxidative stress status and antiosteoporotic performance in a ovariectomized rat model. Korean J. Chem. Eng. 2014, 31, 1616–1623. [Google Scholar] [CrossRef]
- Jebahi, S.; Oudadesse, H.; el Feki, H.; Rebai, T.; Keskes, H.; Pellen, P.; el Feki, A. Antioxidative/oxidative effects of strontium-doped bioactive glass as bone graft. In vivo assays in ovariectomised rats. J. Appl. Biomed. 2012, 10, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Price, N.; Bendall, S.P.; Frondoza, C.; Jinnah, R.H.; Hungerford, D.S. Human osteoblast-like cells (MG63) proliferate on a bioactive glass surface. J. Biomed. Mater. Res. 1997, 37, 394–400. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current Perspectives: Calcium Phosphate Nanocoatings and Nanocomposite Coatings in Dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef]
- Xuereb, M.; Camilleri, J.; Attard, N.J. Systematic Review of Current Dental Implant Coating Materials and Novel Coating Techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Potestio, I. Special Applications of Bioactive Glasses in Otology and Ophthalmology. Adv. Struct. Mater. 2016, 53, 227–248. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of Bioactive Glass Development and Clinical Applications. New J. Glass Ceram. 2013, 3, 30885. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.P.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Oonishi, H.; Hench, L.L.; Wilson, J.; Sugihara, F.; Tsuji, E.; Matsuura, M.; Kin, S.; Yamamoto, T.; Mizokawa, S. Quantitative comparison of bone growth behavior ingranules of Bioglasst, A-W glass-ceramic, and hydroxyapatite. J. Biomed. Mater. Res. 2000, 51, 37–46. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Coatings of titanium substrates with xCaO center dot (1−x)SiO2 sol-gel materials: Characterization, bioactivity and biocompatibility evaluation. Mater. Sci. Eng. C 2016, 58, 846–851. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Wang, Y.W.; Maitz, P.K.; Mirmohseni, F.; Cheng, T.L.; Peacock, L.; Little, D.G.; Schindeler, A.; Dehghani, F. Fabrication of a Biodegradable Implant with Tunable Characteristics for Bone Implant Applications. Biomacromolecules 2017, 18, 1736–1746. [Google Scholar] [CrossRef]
- Wang, L.P.; Long, N.J.; Li, L.H.; Lu, Y.; Li, M.; Cao, J.K.; Zhang, Y.; Zhang, Q.Y.; Xu, S.H.; Yang, Z.M.; et al. Multi-functional bismuth-doped bioglasses: Combining bioactivity and photothermal response for bone tumor treatment and tissue repair. Light Sci. Appl. 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granito, R.N.; Renno, A.C.; Ravagnani, C.; Bossini, P.S.; Mochiuti, D.; Jorgetti, V.; Driusso, P.; Peitl, O.; Zanotto, E.D.; Parizotto, N.A.; et al. In vivo biological performance of a novel highly bioactive glass-ceramic (Biosilicate (R)): A biomechanical and histomorphometric study in rat tibial defects. J. Biomed. Mater. Res. B 2011, 97b, 139–147. [Google Scholar] [CrossRef] [PubMed]
- El-Meliegy, E.; Hamzawy, E.M.A.; El-Kady, A.M.; Salama, A.; El-Rashedi, A. Development and bioactivity evaluation of bioglasses with low Na2O content based on the system Na2O-CaO-MgO-P2O5-SiO2. J. Mater. Sci. Mater. Med. 2012, 23, 2069–2080. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Gorustovich, A. Bioactive glasses as delivery systems for antimicrobial agents. J. Appl. Microbiol. 2017, 122, 1424–1437. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Nandi, S.K.; Dasgupta, S.; Datta, S.; Mukherjee, P.; Roy, S.; Singh, A.K.; Mandal, T.K.; Das, P.; Bhattacharya, R.; et al. Macro-to-micro porous special bioactive glass and ceftriaxone-sulbactam composite drug delivery system for treatment of chronic osteomyelitis: An investigation through in vitro and in vivo animal trial. J. Mater. Sci. Mater. Med. 2011, 22, 705–720. [Google Scholar] [CrossRef]
- Tan, Y.B.; Wang, X.H.; Wu, Q.H.; Yan, W.Q. Early peri-implant osteogenesis with functionally graded nanophase hydroxyapatite/bioglass coating on Ti alloys. Key Eng. Mater. 2007, 330, 553–556. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Chirica, I.M.; Stuart, B.W.; Galca, A.C.; Balescu, L.M.; Popescu-Pelin, G.; Grant, D.M.; Ferreira, J.M.F.; Stan, G.E. Phosphate bioglass thin-films: Cross-area uniformity, structure and biological performance tailored by the simple modification of magnetron sputtering gas pressure. Appl. Surf. Sci. 2021, 541, 148640. [Google Scholar] [CrossRef]
- Chakraborty, J.; Sengupta, S.; Ray, S.; Ghosh, S.; Kapoor, R.; Gouri, S.P.; Pande, G.; Datta, S. Multifunctional gradient coatings of phosphate-free bioactive glass on SS316L biomedical implant materials for improved fixation. Surf. Coat. Technol. 2014, 240, 437–443. [Google Scholar] [CrossRef]
- Lin, F.H.; Huang, Y.Y.; Hon, M.H.; Wu, S.C. Fabrication and Biocompatibility of a Porous Bioglass Ceramic in a Na2o-Cao-Sio2-P2o5 System. J. Biomed. Eng. 1991, 13, 328–334. [Google Scholar] [CrossRef]
- Koller, G.; Cook, R.J.; Thompson, I.D.; Watson, T.F.; Di Silvio, L. Surface modification of titanium implants using bioactive glasses with air abrasion technologies. J. Mater. Sci. Mater. Med. 2007, 18, 2291–2296. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [Green Version]
- De Aza, P.N.; De Aza, A.H.; Pena, P.; De Aza, S. Bioactive glasses and glass-ceramics. Bol.-Soc. Esp. Ceram. Vidr. 2007, 46, 45–55. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Bioactive and mechanically strong Bioglass (R)-poly(D,L-lactic acid) composite coatings on surgical sutures. J. Biomed. Mater. Res. B 2006, 76b, 354–363. [Google Scholar] [CrossRef]
- Han, I.; Lee, I.S.; Choi, J.H.; Baik, H.K. Thinfilm deposition and characteristics of calcium-silicates bioglass. Key Eng. Mater. 2007, 343, 649–652. [Google Scholar] [CrossRef]
- Singh, R.K.; Srinivasan, A.; Kothiyal, G.P. Evaluation of CaO-SiO2-P2O5-Na2O-Fe2O3 bioglass-ceramics for hyperthermia application. J. Mater. Sci. Mater. Med. 2009, 20, 147–151. [Google Scholar] [CrossRef]
- Bhakta, S.; Faira, P.E.; Salata, L.A.; Neto, P.J.D.; Miller, C.A.; van Noort, R.; Reaney, I.M.; Brook, I.M.; Hatton, P.V. Determination of relative in vivo osteoconductivity of modified potassium fluorrichterite glass-ceramics compared with 45S5 bioglass. J. Mater. Sci. Mater. Med. 2012, 23, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Haftbaradaran-Esfahani, M.; Ahmadian, M.; Nassajpour-Esfahani, A.H. Fabrication and characterization of porous biomedical Vitallium alloy with 58S bioglass coating prepared by sol-gel method. Appl. Surf. Sci. 2020, 506, 144959. [Google Scholar] [CrossRef]
- Anand, A.; Lalzawmliana, V.; Kumar, V.; Das, P.; Devi, K.B.; Maji, A.K.; Kundu, B.; Roy, M.; Nandi, S.K. Preparation and in vivo biocompatibility studies of different mesoporous bioactive glasses. J. Mech. Behav. Biomed. 2019, 89, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, K.; Zhou, Z.Y.; Zhu, X.R.; Li, W.C.; Cao, C.L.; Zhou, K.; Liao, L.; Ai, F.R. Customized Borosilicate Bioglass Scaffolds With Excellent Biodegradation and Osteogenesis for Mandible Reconstruction. Front. Bioeng. Biotech. 2020, 8, 610284. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour, I.; Naghib, S.M.; Yaghtin, A.H. Synthesis and characterisation of nanostructured hardystonite coating on stainless steel for biomedical application. IET Nanobiotechnol. 2018, 12, 895–902. [Google Scholar] [CrossRef]
- Wajda, A.; Sitarz, M. Structural and microstructural studies of zinc-doped glasses from NaCaPO4-SiO2 system. J. Non-Cryst. Solids 2016, 441, 66–73. [Google Scholar] [CrossRef]
- Mozafari, M.; Rabiee, M.; Azami, M.; Maleknia, S. Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds. Appl. Surf. Sci. 2010, 257, 1740–1749. [Google Scholar] [CrossRef]
- Dinaryand, P.; Seyedjafari, E.; Shafiee, A.; Jandaghi, A.B.; Doostmohammadi, A.; Fathi, M.H.; Farhadian, S.; Soleimani, M. New Approach to Bone Tissue Engineering: Simultaneous Application of Hydroxyapatite and Bioactive Glass Coated on a Poly(L-lactic acid) Scaffold. ACS Appl. Mater. Interfaces 2011, 3, 4518–4524. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gelderived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Biernat, M.; Ciolek, L.; Dzierzynska, M.; Ozieblo, A.; Sawicka, J.; Deptula, M.; Bauer, M.; Kamysz, W.; Pikula, M.; Jaegermann, Z.; et al. Porous chitosan/ZnO-doped bioglass composites as carriers of bioactive peptides. Int. J. Appl. Ceram. Technol. 2020, 17, 2807–2816. [Google Scholar] [CrossRef]
- Theodorou, G.; Goudouri, O.M.; Kontonasaki, E.; Chatzistavrou, X.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K.M. Comparative bioactivity study of 45S5 and 58S bioglasses in organic and inorganic environment. Bioceram. Dev. Appl. 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Ajay Rakkesh, R.; Aruna, P.; Ganesana, S.; Balakumar, S. Bioactivity and hemocompatibility of sol-gel bioactive glass synthesized under different catalytic conditions. New J. Chem. 2020, 44, 21026–21037. [Google Scholar] [CrossRef]
- Lombardi, M.; Gremillard, L.; Chevalier, J.; Lefebvre, L.; Cacciotti, I.; Bianco, A.; Montanaro, L. A comparative study between melt-derived and sol-gel synthesized 45S5 bioactive glasses. Key Eng. Mater. 2013, 541, 15–30. [Google Scholar] [CrossRef]
- Gouveia, P.F.; Mesquita-Guimaraes, J.; Galarraga-Vinueza, M.E.; Souza, J.C.M.; Silva, F.S.; Fredel, M.C.; Boccaccini, A.R.; Detsch, R.; Henriques, B. In-vitro mechanical and biological evaluation of novel zirconia reinforced bioglass scaffolds for bone repair. J. Mech. Behav. Biomed. 2021, 114, 104164. [Google Scholar] [CrossRef]
- Teghil, R.; Curcio, M.; De Bonis, A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings 2021, 11, 811. [Google Scholar] [CrossRef]
- Ducheyne, P. Bioglass Coatings and Bioglass Composites as Implant Materials. J. Biomed. Mater. Res. 1985, 19, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, J.W. Surface bio-modification of titanium implants by an enamel process. J. Ceram. Process. Res. 2005, 6, 338–344. [Google Scholar]
- Bharati, S.; Soundrapandian, C.; Basu, D.; Datta, S. Studies on a novel bioactive glass and composite coating with hydroxyapatite on titanium based alloys: Effect of gamma-sterilization on coating. J. Eur. Ceram. Soc. 2009, 29, 2527–2535. [Google Scholar] [CrossRef]
- Pazo, A.; Saiza, E.; Tomsiaa, P. Silicate glass coatings on Ti-based implants. Acta Mater. 1998, 46, 2551–2558. [Google Scholar] [CrossRef]
- Fuchs, G.A. The Biological and Biomechanical Properties of Metal Implants Coated with Bioglass Ceramic, as Exemplified in the Simple-Model of a Loaded, Cement-Free Total Hip-Prosthesis. Biomed. Tech. 1982, 27, 24–29. [Google Scholar] [CrossRef]
- Lacefield, W.R.; Hench, L.L. The Bonding of Bioglass to a Cobalt-Chromium Surgical Implant Alloy. Biomaterials 1986, 7, 104–108. [Google Scholar] [CrossRef]
- Kudo, K.; Miyasawa, M.; Fujioka, Y.; Kamegai, T.; Nakano, H.; Seino, Y.; Ishikawa, F.; Shioyama, T.; Ishibashi, K. Clinical-Application of Dental Implant with Root of Coated Bioglass-Short-Term Results. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 18–23. [Google Scholar] [CrossRef]
- Andersson, O.H.; Karlsson, K.H.; Hero, H.; Vedel, E.; Yliurpo, A.; Pajamaki, K.J.J.; Lindholm, T.S. Bioactive Double Glass Coatings for Co-Cr-Mo Alloy. J. Mater. Sci. Mater. Med. 1995, 6, 242–247. [Google Scholar] [CrossRef]
- Hench, L.L.; Greenspan, D.C. Bioglass Coated Al2O3 Ceramics. U.S. Patent 4103002, 25 July 1978. [Google Scholar]
- Greenspan, D.C.; Hench, L.L. Chemical and mechanical behavior of Bioglass coated alumina. Biomed. Mater. Res. 1976, 10, 503. [Google Scholar] [CrossRef]
- Griss, P.; Werner, E.; Heimke, G.; Rautekreinsen, U. Comparative Experimental Investigations with Bioglass LL Hench and Al2O3-Ceramic Coated with Mod Bioglass. II. Results of Experiments with Loaded Implants. Arch. Orthop. Traum. Surg. 1978, 92, 199–210. [Google Scholar] [CrossRef]
- Turley, P.K.; Shapiro, P.A.; Moffett, B.C. The Loading of Bioglass-Coated Aluminum-Oxide Implants to Produce Sutural Expansion of the Maxillary Complex in the Pigtail Monkey (Macaca-Nemestrina). Arch. Oral Biol. 1980, 25, 459–469. [Google Scholar] [CrossRef]
- Smith, J.R. Bone Dynamics Associated with the Controlled Loading of Bioglass-Coated Aluminum-Oxide Endosteal Implants. Am. J. Orthod. Dentofac. 1979, 76, 618–636. [Google Scholar] [CrossRef]
- Ignatius, A.; Peraus, M.; Schorlemmer, S.; Augat, P.; Burger, W.; Leyen, S.; Claes, L. Osseointegration of alumina with a bioactive coating under load-bearing and unloaded conditions. Biomaterials 2005, 26, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, A.; Hausmann, A.; Weber, M.; Fischer, J.; Fischer, H. Bioactive and Thermally Compatible Glass Coating on Zirconia Dental Implants. J. Dent. Res. 2015, 94, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Rohr, N.; Nebe, J.B.; Schmidli, F.; Muller, P.; Weber, M.; Fischer, H.; Fischer, J. Influence of bioactive glass-coating of zirconia implant surfaces on human osteoblast behavior in vitro. Dent. Mater. 2019, 35, 862–870. [Google Scholar] [CrossRef]
- Gomez-Vega, J.M.; Saiz, E.; Tomsia, A.P.; Marshall, G.W.; Marshall, S.J. Bioactive glass coatings with hydroxyapatite and Bioglass (R) particles on Ti-based implants. 1. Processing. Biomaterials 2000, 21, 105–111. [Google Scholar] [CrossRef]
- Peddi, L.; Brow, R.K.; Brown, R.F. Bioactive borate glass coatings for titanium alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3145–3152. [Google Scholar] [CrossRef]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011, 56, 68–75. [Google Scholar] [CrossRef]
- Bobzin, K.; Kopp, N.; Wiesner, S.; Puidokas, S.; Anavar, S.S.; Fischer, H.; Korsten, A.; Schickle, K. Investigation of a bioactive Ti-Co-based brazing coating on oxide high-performance ceramics in medical technology. Mater. Werkst 2014, 45, 504–511. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, R.; Kundu, B.; Datta, S.; Kumar, M.; Chanda, A.; Kundu, D. Clinical Outcome of Hydroxyapatite Coated, Bioactive Glass Coated, and Machined Ti6Al4V Threaded Dental Implant in Human Jaws: A Short-Term Comparative Study. Implant Dent. 2016, 25, 252–260. [Google Scholar] [CrossRef]
- Rodriguez, O.; Matinmanesh, A.; Phull, S.; Schemitsch, E.H.; Zalzal, P.; Clarkin, O.M.; Papini, M.; Towler, M.R. Silica-Based and Borate-Based, Titania-Containing Bioactive Coatings Characterization: Critical Strain Energy Release Rate, Residual Stresses, Hardness, and Thermal Expansion. J. Funct. Biomater. 2016, 7, 32. [Google Scholar] [CrossRef]
- Fujino, S.; Tokunaga, H.; Hata, S.; Esaiz, A.P.T. Graded glass coatings for Co-Cr implant alloys. J. Mater. Sci. 2005, 40, 2499–2503. [Google Scholar] [CrossRef]
- Henao, J.; Poblano-Salas, C.; Monsalve, M.; Corona-Castuera, J.; Barceinas-Sanchez, O. Bio-active glass coatings manufactured by thermal spray: A status report. J. Mater. Res. Technol. 2019, 8, 4965–4984. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, M.; Vardelle, A.; Goutier, S. What Do We Know, What are the Current Limitations of Suspension Plasma Spraying? J. Therm. Spray Technol. 2015, 24, 1120–1129. [Google Scholar] [CrossRef] [Green Version]
- Jordan Eric, H.; Chen, J.; Maurice, G. The Solution Precursor Plasma Spray (SPPS) Process: A Review with Energy Considerations. J. Therm. Spray Technol. 2015, 24, 1153–1165. [Google Scholar] [CrossRef]
- Karthikeyan, J. The advantages and disadvantages of the cold spray coating process. In The Cold Spray Materials Deposition Process; Woodhead Publishing Series; Woodhead Publishing: Southston, UK, 2007; pp. 62–71. [Google Scholar]
- Fan, W.; Bai, Y. Review of Suspension and Solution Precursor Plasma Sprayed Thermal Barrier Coatings. Ceram. Int. 2016, 42, 14299–14312. [Google Scholar] [CrossRef]
- Killinger, A.; Müller, P.; Gadow, R. What Do We Know, What are the Current Limitations of Suspension HVOF Spraying? J. Therm. Spray Technol. 2015, 24, 1130–1142. [Google Scholar] [CrossRef]
- Monsalvea, M.; Ageorgesa, H.; Lopezb, E.; Vargasb, F.; Bolivar, F. Bioactivity and mechanical properties of plasma-sprayed coatings ofbioglass powder. Surf. Coat. Technol. 2013, 220, 60–66. [Google Scholar] [CrossRef]
- Lindgren, V.; Galea, V.P.; Nebergall, A.; Greene, M.E.; Rolfson, O.; Malchau, H. Radiographic and Clinical Outcomes of Porous Titanium-Coated and Plasma-Sprayed Acetabular Shells. J. Bone Jt. Surg. Am. 2018, 100, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Marghussian, V.K.; Mesgar, A.S.-M. Effects of composition on crystallization behaviour and mechanical properties of bioactive glass-ceramics in the MgO-CaO-SiO2-P2O5 system. Ceram. Int. 2000, 26, 415–420. [Google Scholar] [CrossRef]
- Goller, G. The effect of bond coat on mechanical properties ofplasma sprayed bioglass-titanium coatings. Ceram. Int. 2004, 30, 351–355. [Google Scholar] [CrossRef]
- Cannillo, V.; Sola, A. Different approaches to produce coatings with bioactive glasses:Enamelling vs. plasma spraying. J. Eur. Ceram. Soc. 2010, 30, 2031–2039. [Google Scholar] [CrossRef]
- Lopez-Sastre, A.; Gonzalo-Orden, J.M.; Altonaga, J.A.R.; Altonaga, J.R.; Orden, M.A. Coating titanium implants with bioglass and with hydroxyapatite—A comparative study in sheep. Int. Orthop. 1998, 22, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Verne, E.; Ferraris, M.; Ventrella, A.; Paracchini, L.; Krajewskic, A.; Ravaglioli, A. Sintering and Plasma Spray Deposition of Bioactive Glass-Matrix Composites for Medical Applications. J. Eur. Ceram. Soc. 1998, 18, 363–372. [Google Scholar] [CrossRef]
- Jallot, E.; Benhayoune, H.; Kilian, L.; Irigaray, J.L.; Barbotteau, Y.; Balossier, G.; Bonhomme, P. Dissolution Kinetics, Selective Leaching, and Interfacial Reactions of a Bioglass Coating Enriched in Alumina. J. Colloid Interface Sci. 2001, 233, 83–90. [Google Scholar] [CrossRef]
- Li, M.Q.; Zhang, R.; Wang, J.P.; Yang, S.Q. Study of different biocomposite coatings on Ti alloy by a subsonic thermal spraying technique. Biomed. Mater. 2007, 2, 1–5. [Google Scholar] [CrossRef]
- Newman, S.D.; Lotfibakhshaiesh, N.; O’Donnell, M.; Walboomers, F.; Horwood, N.; Jansen, J.A.; Amis, A.A.; Cobb, J.P.; Stevens, M.M. Enhanced Osseous Implant Fixation with Strontium-Substituted Bioactive Glass Coating. Tissue Eng. Part A 2014, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kitsugi, T.; Nakamura, T.; Oka, M.; Senaha, Y.; Goto, T.; Shibuya, T. Bone-bonding behavior of plasma-sprayed coatings of Bioglass(R), AW-glass ceramic, and tricalcium phosphate on titanium alloy. J. Biomed. Mater. Res. 1996, 30, 261–269. [Google Scholar] [CrossRef]
- Carvalho, F.L.S.; Borges, C.S.; Branco, J.R.T.; Pereira, M.M. Structural analysis of hydroxyapatite bioactive glass composite coatings obtained by plasma spray processing. J. Non-Cryst. Solids 1999, 247, 64–68. [Google Scholar] [CrossRef]
- Canas, E.; Vicent, M.; Bannier, E.; Carpio, P.; Orts, M.J.; Sánchez, E. Effect of particle size on processing of bioactive glass powder for atmospheric plasma spraying. J. Eur. Ceram. Soc. 2016, 36, 837–845. [Google Scholar] [CrossRef]
- Calvo, V.L.; Cabedo, M.V.; Bannier, E.; Recacha, E.C.; Boccaccini, A.R.; Arias, L.C.; Vilches, E.S. 45S5 bioactive glass coatings by atmospheric plasma spraying obtained from feedstocks prepared by different routes. J. Mater. Sci. 2014, 49, 7933–7942. [Google Scholar] [CrossRef] [Green Version]
- Poirier, T.; Planche, M.P.; Landemarre, O.; Coddet, C. Particles Spreading Phenomena in the Caseof Glass Thermal Spraying. J. Therm. Spray Technol. 2008, 17, 564–573. [Google Scholar] [CrossRef]
- Cai, F.; Miyata, C.; Huang, X.; Yang, Q. Microstructure, bioactivity and wear resistance of sintered composite Co-Cr-Mo/Bioglass((R)) for medical implant applications. Int. J. Surf. Sci. Eng. 2014, 8, 264–281. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Comprehensive Review of Bioactive Glass Coatings: State of the Art, Challenges and Future Perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Song, L.; Liu, X.G.; Huang, Y.; Huang, T.; Wu, Y.; Chen, J.Y.; Wu, F. Nanostructured bioactive glass-ceramic coatings deposited by the liquid precursor plasma spraying process. Appl. Surf. Sci. 2011, 257, 1898–1905. [Google Scholar] [CrossRef]
- Altomare, L.; Bellucci, D.; Bolelli, G.; Bonferroni, B.; Cannillo, V.; De Nardo, L.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A.; et al. Microstructure and in vitro behaviour of 45S5 bioglass coatings deposited by high velocity suspension flame spraying (HVSFS). J. Mater. Sci. Mater. Med. 2011, 22, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Ahmed, I.; Grant, D.M.; Nommeots-Nomm, A.; Hussain, T. Effect of processing on microstructure, mechanical properties and dissolution behaviour in SBF of Bioglass (45S5) coatings deposited by Suspension High Velocity Oxy Fuel (SHVOF) thermal spray. Surf. Coat. Technol. 2019, 372, 229–238. [Google Scholar] [CrossRef]
- Liu, X.R.; Li, Y.Q.; Li, S.J.; Lin, Y.C.; Li, V.L.; Chen, Y.H.; Lin, C.P.; Keerthi, M.; Shih, S.J.; Chung, R.J. Polyelectrolyte multilayer coatings for short/long-term release of antibacterial agents. Surf. Coat. Technol. 2020, 393, 125696. [Google Scholar] [CrossRef]
- Mesquita-Guimaraes, J.; Leite, M.A.; Souza, J.C.M.; Henriques, B.; Silva, F.S.; Hotza, D.; Boccaccini, A.R.; Fredel, M.C. Processing and strengthening of 58S bioactive glass-infiltrated titaniascaffolds. J. Biomed. Mater. Res. Part A 2016, 105, 590–600. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An Investigation of Bioactive Glass Powders by Sol-Gel Processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Chen, W.; Long, T.; Guo, Y.J.; Zhu, Z.A.; Guo, Y.P. Hydrothermal synthesis of hydroxyapatite coatings with oriented nanorod arrays. RSC Adv. 2014, 4, 185–191. [Google Scholar] [CrossRef]
- Shankhwar, N.; Kothiyal, G.P.; Srinivasan, A. Influence of phosphate precursors on the structure, crystallization behaviour and bioactivity of sol-gel derived 45S5 bioglass. RSC Adv. 2015, 5, 100762–100768. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.-M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Z.; Meng, X.G.; Yu, H.J.; Yang, H.; He, T.; Wang, D.G.; Zhao, S.G. Preparation and Development of Bioglass by Sol-Gel Method. Key Eng. Mater. 2014, 591, 34–39. [Google Scholar] [CrossRef]
- Tian, B.; Chen, W.; Dong, Y.F.; Marymont, J.V.; Lei, Y.; Ke, Q.F.; Guo, Y.P.; Zhu, Z.N. Silver nanoparticle-loaded hydroxyapatite coating: Structure, antibacterial properties, and capacity for osteogenic induction in vitro. RSC Adv. 2016, 6, 8549–8562. [Google Scholar] [CrossRef]
- Snyder, K.L.; Holmes, H.R.; VanWagner, M.J.; Hartman, N.J.; Rajachar, R.M. Development of vapor deposited silica sol-gel particles for use as a bioactive materials system. J. Biomed. Mater. Res. A 2013, 101, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Vega, J.M.; Hozumi, A.; Sugimura, H.; Takai, O. Ordered mesoporous silica coatings that induce apatite formation in vitro. Adv. Mater. 2001, 13, 822–825. [Google Scholar] [CrossRef]
- Singh, B.N.; Pramanik, K. Development of novel silk fibroin/polyvinyl alcohol/sol-gel bioactive glass composite matrix by modified layer by layer electrospinning method for bone tissue construct generation. Biofabrication 2017, 9, 015028. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.M.; Collier, K.; Wulf, E.; Bormann, D.; Bach, F.W. Comparison of the Corrosion Behavior of Coated and Uncoated Magnesium Alloys in an In Vitro Corrosion Environment. Adv. Eng. Mater. 2011, 13, B313–B323. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Stamboulis, A.G.; Rashid, A.; Roether, J.A. Composite surgical sutures with bioactive glass coating. J. Biomed. Mater. Res. B 2003, 67b, 618–626. [Google Scholar] [CrossRef]
- Fathi, M.H.; Mohammadi, A.D. Preparation and characterization of sol-gel bioactive glass coating for improvement of biocompatibility of human body implant. Mater. Sci. Eng. A 2008, 474, 128–133. [Google Scholar] [CrossRef]
- Fathi, M.H.; Doostmohammadi, A. Bioactive glass nanopowder and bioglass coating for biocompatibility improvement of metallic implant. J. Mater. Process. Technol. 2009, 209, 1385–1391. [Google Scholar] [CrossRef]
- Sebdani, M.M.; Fathi, M.H. Novel hydroxyapatite-forsterite-bioglass nanocomposite coatings with improved mechanical properties. J. Alloy Compd. 2011, 509, 2273–2276. [Google Scholar] [CrossRef]
- Chen, Q.; Cordero-Arias, L.; Roether, J.A.; Cabanas-Polo, S.; Virtanen, S.; Boccaccini, A.R. Alginate/Bioglass (R) composite coatings on stainless steel deposited by direct current and alternating current electrophoretic deposition. Surf. Coat. Technol. 2013, 233, 49–56. [Google Scholar] [CrossRef]
- Pourhashem, S.; Afshar, A. Double layer bioglass-silica coatings on 316L stainless steel by sol-gel method. Ceram. Int. 2014, 40, 993–1000. [Google Scholar] [CrossRef]
- Omar, S.; Repp, F.; Desimone, P.M.; Weinkamer, R.; Wagermaier, W.; Cere, S.; Ballarre, J. Sol-gel hybrid coatings with strontium-doped 45S5 glass particles for enhancing the performance of stainless steel implants: Electrochemical, bioactive and in vivo response. J. Non-Cryst. Solids 2015, 425, 1–10. [Google Scholar] [CrossRef]
- Tabia, Z.; Bricha, M.; El Mabrouk, K.; Vaudreuil, S. Manufacturing of a metallic 3D framework coated with a bioglass matrix for implant applications. J. Mater. Sci. 2021, 56, 1658–1672. [Google Scholar] [CrossRef]
- Ye, X.Y.; Cai, S.; Dou, Y.; Xu, G.H.; Huang, K.; Ren, M.G.; Wang, X.X. Bioactive glass-ceramic coating for enhancing the in vitro corrosion resistance of biodegradable Mg alloy. Appl. Surf. Sci. 2012, 259, 799–805. [Google Scholar] [CrossRef]
- Shen, S.B.; Cai, S.; Xu, G.H.; Li, Y.; Zhang, T.; Zhang, M. Bond strength and corrosion resistance of bioglass coated magnesium alloy fabricated by uniaxial pressing and microwave hybrid heating. Mater. Des. 2015, 86, 610–615. [Google Scholar] [CrossRef]
- Lalk, M.; Reifenrath, J.; Rittershaus, D.; Bormann, D.; Meyer-Lindenberg, A. Biocompatibility and degradation behaviour of degradable magnesium sponges coated with bioglass-method establishment within the framework of a pilot study. Mater. Werkst 2010, 41, 1025–1034. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Michalczyk, C.; Singer, F.; Virtanen, S.; Boccaccini, A.R. In vitro study of polycaprolactone/bioactive glass composite coatings on corrosion and bioactivity of pure Mg. Appl. Surf. Sci. 2015, 355, 832–841. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.Y.; Wu, Y.; Jiang, J.; Ge, Y.S.; Gao, K.; Zhang, P.Y.; Wu, L.X. Enhancement of the osseointegration of a polyethylene terephthalate artificial ligament graft in a bone tunnel using 58S bioglass. Int. Orthop. 2012, 36, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Jugowiec, D.; Lukaszczyk, A.; Cieniek, L.; Kot, M.; Reczynska, K.; Cholewa-Kowalska, K.; Pamula, E.; Moskalewicz, T. Electrophoretic, deposition and characterization of composite chitosan-based coatings incorporating bioglass and sol-gel glass particles on the Ti-13Nb-13Zr alloy. Surf. Coat. Technol. 2017, 319, 33–46. [Google Scholar] [CrossRef]
- Scislowska-Czarnecka, A.; Menaszek, E.; Szaraniec, B.; Kolaczkowska, E. Ceramic modifications of porous titanium: Effects on macrophage activation. Tissue Cell 2012, 44, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 Containing Thin Films as Bioactive Coatings for Orthopaedic Implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- Gomez-Vega, J.M.; Hozumi, A.; Saiz, E.; Tomsia, A.P.; Sugimura, H.; Takai, O. Bioactive glass-mesoporous silica coatings on Ti6Al4V through enameling and triblock-copolymer-templated sol-gel processing. J. Biomed. Mater. Res. 2001, 56, 382–389. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Crouse, P.L.; Li, L.; Garrod, D. Combined laser/sol-gel synthesis of calcium silicate coating on Ti-6Al-4V substrates for improved cell integration. Appl. Surf. Sci. 2007, 253, 7998–8002. [Google Scholar] [CrossRef]
- Say, Y.; Aksakal, B. Enhanced corrosion properties of biological NiTi alloy by hydroxyapatite and bioglass based biocomposite coatings. J. Mater. Res. Technol. 2020, 9, 1742–1749. [Google Scholar] [CrossRef]
- Das, I.; Medda, S.K.; De, G.; Fagerlund, S.; Hupa, L.; Puska, M.A.; Vallittu, P.K. Hierarchically Designed Bioactive Glassy Nanocoatings for the Growth of Faster and Uniformly Dense Apatite. J. Am. Ceram. Soc. 2015, 98, 2428–2437. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloy Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Saino, E.; Maliardi, V.; Quartarone, E.; Fassina, L.; Benedetti, L.; De Angelis, M.G.C.; Mustarelli, P.; Facchini, A.; Visai, L. In Vitro Enhancement of SAOS-2 Cell Calcified Matrix Deposition onto Radio Frequency Magnetron Sputtered Bioglass-Coated Titanium Scaffolds. Tissue Eng. Part A 2010, 16, 995–1008. [Google Scholar] [CrossRef]
- Berbecaru, C.; Alexandru, H.V.; Stan, G.E.; Marcov, D.A.; Pasuk, I.; Ianculescu, A. First stages of bioactivity of glass-ceramics thin films prepared by magnetron sputtering technique. Mater. Sci. Eng. B 2010, 169, 101–105. [Google Scholar] [CrossRef]
- Stan, G.E.; Pasuk, I.; Husanu, M.A.; Enculescu, I.; Pina, S.; Lemos, A.F.; Tulyaganov, D.U.; El Mabrouk, K.; Ferreira, J.M.F. Highly adherent bioactive glass thin films synthetized by magnetron sputtering at low temperature. J. Mater. Sci. Mater. Med. 2011, 22, 2693–2710. [Google Scholar] [CrossRef]
- Stan, G.E.; Popa, A.C.; Galca, A.C.; Aldica, G.; Ferreira, J.M.F. Strong bonding between sputtered bioglass-ceramic films and Ti-substrate implants induced by atomic inter-diffusion post-deposition heat-treatments. Appl. Surf. Sci. 2013, 280, 530–538. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Enculescu, M.; Tanase, C.; Tulyaganov, D.U.; Ferreira, J.M.F. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. 2015, 51, 313–327. [Google Scholar] [CrossRef]
- Popa, A.C.; Marques, V.M.F.; Stan, G.E.; Husanu, M.A.; Galca, A.C.; Ghica, C.; Tulyaganov, D.U.; Lemos, A.F.; Ferreira, J.M.F. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Films 2014, 553, 166–172. [Google Scholar] [CrossRef]
- Stuart, B.; Gimeno-Fabra, M.; Joel Segal, I.A.; Grant, D.M. Preferential sputtering in phosphate glass systems for the processing of bioactive coatings. Thin Solid Films 2015, 589, 534–542. [Google Scholar] [CrossRef]
- van Oirschot, B.A.J.A.; Alghamdi, H.S.; Narhi, T.O.; Anil, S.; Aldosari, A.A.; van den Beucken, J.J.J.P.; Jansen, J.A. In vivo evaluation of bioactive glass-based coatings on dental implants in a dog implantation model. Clin. Oral Implants Res. 2014, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Perez-Tanoira, R.; Garcia-Pedrazuela, M.; Hyyrynen, T.; Soininen, A.; Aarnisalo, A.; Nieminen, M.T.; Tiainen, V.M.; Konttinen, Y.T.; Kinnari, T.J. Effect of S53P4 bone substitute on staphylococcal adhesion and biofilm formation on other implant materials in normal and hypoxic conditions. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef]
- Bibby, J.K.; Bubb, N.L.; Wood, D.J.; Mummery, P.M. Fluorapatite-mullite glass sputter coated Ti6Al4V for biomedical applications. J. Mater. Sci. Mater. Med. 2005, 16, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goh, B.T.; Wolke, J.; Tideman, H.; Stoelinga, P.; Jansen, J. Soft tissue adaptation to modified titanium surfaces. J. Biomed. Mater. Res. A 2010, 95a, 543–549. [Google Scholar] [CrossRef]
- Chanchareonsook, N.; Tideman, H.; Lee, S.; Hollister, S.J.; Flanagan, C.; Jansen, J.A. Mandibular reconstruction with a bioactive-coated cementless Ti6Al4V modular endoprosthesis in Macaca fascicularis. Int. J. Oral Max. Surg. 2014, 43, 758–768. [Google Scholar] [CrossRef]
- Pou, J.; Lusquinos, F.; Comesana, R.; Boutinguiza, M. Production of biomaterial coatings by laser-assisted processes. In Advances in Laser Materials Processing; Woodhead Publishing: Sawston, UK, 2010. [Google Scholar] [CrossRef]
- Kuo, P.H.; Joshi, S.S.; Lu, X.N.; Ho, Y.H.; Xiang, Y.; Dahotre, N.B.; Du, J.C. Laser coating of bioactive glasses on bioimplant titanium alloys. Int. J. Appl. Glass Sci. 2019, 10, 307–320. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, J.Y.; Mukaeyama, S.; Sun, S.H.; Nakano, T. Preparation of Titanium Alloy/Bioactive Glass Composite for Biomedical Applications via Selective Laser Melting. Mater. Trans. 2019, 60, 1779–1784. [Google Scholar] [CrossRef]
- Comesaña, R.; Quintero, F.; Lusquiños, F.; Pascual, M.J.; Boutinguiza, M.; Durán, A.; Pou, J. Laser cladding of bioactive glass coatings. Acta Biomater. 2010, 9, 953–961. [Google Scholar] [CrossRef]
- Baino, F.; Montealegre, M.A.; Orlygsson, G.; Novajra, G.; Vitale-Brovarone, C. Bioactive glass coatings fabricated by laser cladding on ceramic acetabular cups: A proof-of-concept study. J. Mater. Sci. 2017, 52, 9115–9128. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.Z.; Yao, L.; Bao, Q.H. Characterization of some methods of preparation for bioactive glass coating on implants. Surf. Rev. Lett. 2006, 13, 93–102. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Chen, C.Z.; Wang, D.G. The current techniques for preparing bioglass coatings. Surf. Rev. Lett. 2005, 12, 505–513. [Google Scholar] [CrossRef]
- Liste, S.; Serra, J.; González, P.; Borrajo, J.P.; Chiussi, S.; León, B.; Pérez-Amor, M. The role of the reactive atmosphere in pulsed laser deposition of bioactive glass films. Thin Solid Films 2004, 453, 224–228. [Google Scholar] [CrossRef]
- Sanz, C.K.; dos Santos, A.R.; da Silva, M.H.P.; Marcal, R.; Tute, E.M.; Meza, E.L.; Mello, A.; Borghi, F.F.; Camargo, S.A.D. Niobo-phosphate bioactive glass films produced by pulsed laser deposition on titanium surfaces for improved cell adhesion. Ceram. Int. 2019, 45, 18052–18058. [Google Scholar] [CrossRef]
- Berbecaru, C.; Alexandru, H.V.; Ianculescu, A.; Popescu, A.; Socol, G.; Sima, F.; Mihailescu, I. Bioglass thin films for biomimetic implants. Appl. Surf. Sci. 2009, 255, 5476–5479. [Google Scholar] [CrossRef]
- Floroian, L.; Sima, F.; Florescu, M.; Badea, M.; Popescu, A.C.; Serban, N.; Mihailescu, I.N. Double layered nanostructured composite coatings with bioactive silicate glass and polymethylmetacrylate for biomimetic implant applications. J. Electroanal. Chem. 2010, 648, 111–118. [Google Scholar] [CrossRef]
- D’Alessio, L.; Teghil, R.; Zaccagnino, M.; Zaccardo, I.; Ferro, D.; Marotta, V. Pulsed laser ablation and deposition of bioactive glass as coating material for biomedical applications. Appl. Surf. Sci. 1999, 138, 527–532. [Google Scholar] [CrossRef]
- Czel, G.; Teghil, R.; Janovszky, D. Pulsed laser deposition of bioglass coatings on dental implants. Mater. Sci. Forum 2003, 414–415, 9–14. [Google Scholar] [CrossRef]
- Tanaskovic, D.; Jokic, B.; Socol, G.; Popescu, A.; Mihailescu, I.N.; Petrovic, R.; Janackovic, D. Synthesis of functionally graded bioactive glass-apatite multistructures on Ti substrates by pulsed laser deposition. Appl. Surf. Sci. 2007, 254, 1279–1282. [Google Scholar] [CrossRef]
- Wang, D.G.; Chen, C.Z.; Yang, X.X.; Ming, X.C.; Zhang, W.L. Effect of bioglass addition on the properties of HA/BG composite films fabricated by pulsed laser deposition. Ceram. Int. 2018, 44, 14528–14533. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.C.; Urzica, I.; Dyia, H.M.; Bleotu, C.; et al. Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings. Molecules 2016, 21, 740. [Google Scholar] [CrossRef] [Green Version]
- Mihailescu, N.; Stan, G.E.; Ristoscu, C.; Sopronyi, M.; Mihailescu, I.N. Bioactive glass thin films synthesized by advanced pulsed laser techniques. J. Phys. Conf. Ser. 2016, 764, 012020. [Google Scholar] [CrossRef]
- Wang, D.G.; Xiao, F.H.; Li, Y.; Ming, X.C.; Zhai, J.Q.; Chen, C.Z. Properties of HA-based composite films fabricated by pulsed laser deposition with an in-situ heat treatment. Surf. Coat. Technol. 2020, 394, 125863. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. A study of the electrophoretic deposition of Bioglass (R) suspensions using the Taguchi experimental design approach. J. Eur. Ceram. Soc. 2010, 30, 2963–2970. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Ojaghi-Ilkhchi, M.; Farrokhi-Rad, M. Development of bioglass coating reinforced with hydroxyapatite whiskers on TiO2 nanotubes via electrophoretic deposition. Ceram. Int. 2021, 47, 1333–1343. [Google Scholar] [CrossRef]
- You, C.K.; Meng, X.W.; Kwon, T.Y.; Yang, Y.Z.; Ong, J.L.; Kim, S.; Kim, K.H. Development of hydroxyapatite thin film on titanium substrate by electrophoretic deposition. Key Eng. Mater. 2005, 284–286, 901–904. [Google Scholar] [CrossRef]
- Radice, S.; Kern, P.; Burki, G.; Michler, J.; Textor, M. Electrophoretic deposition of zirconia-Bioglass (R) composite coatings for biomedical implants. J. Biomed. Mater. Res. A 2007, 82a, 436–444. [Google Scholar] [CrossRef]
- Cho, J.; Cannio, M.; Boccaccini, A.R. The Electrophoretic Deposition of Bioglasso (R)/Carbon Nanotube composite layers for bioactive coatings. Int. J. Mater. Prod. Technol. 2009, 35, 260–270. [Google Scholar] [CrossRef]
- Schausten, M.C.; Meng, D.C.; Telle, R.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes and bioactive glass particles for bioactive composite coatings. Ceram. Int. 2010, 36, 307–312. [Google Scholar] [CrossRef]
- Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic Deposition of Hyaluronic Acid and Composite Films for Biomedical Applications. JOM 2010, 62, 72–75. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition of chitosan/45S5 Bioglass (R) composite coatings for orthopaedic applications. Surf. Coat. Technol. 2011, 205, 5260–5268. [Google Scholar] [CrossRef]
- Pishbin, F.; Mourino, V.; Flor, S.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic Deposition of Gentamicin-Loaded Bioactive Glass/Chitosan Composite Coatings for Orthopaedic Implants. ACS Appl. Mater. Interfaces 2014, 6, 8796–8806. [Google Scholar] [CrossRef]
- Estrada-Cabrera, E.; Torres-Ferrer, L.R.; Aztatzi-Aguilar, O.G.; De Vizcaya-Ruiz, A.; Meraz-Rios, M.A.; Zarate-Trivino, D.G.; Arizmendi-Morquecho, A.; Bugallo, A.D.; Prokhorov, E.; Luna-Barcenas, G. Chitosan-bioglass coatings on partially nanostructured anodized Ti-6Al-4V alloy for biomedical applications. Surf. Coat. Technol. 2019, 375, 468–476. [Google Scholar] [CrossRef]
- Molaei, A.; Yousefpour, M. Preparation of Chitosan-based nanocomposites and biomedical investigations in bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 701–713. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Electrophoretic deposition of bioactive glass composite coating on biomaterials and electrochemical behavior study: A review. Mater. Today Proc. 2018, 5, 20160–20169. [Google Scholar] [CrossRef]
- Meyer, N.; Rivera, L.R.; Ellis, T.; Qi, J.H.; Ryan, M.P.; Boccaccini, A.R. Bioactive and Antibacterial Coatings Based on Zein/Bioactive Glass Composites by Electrophoretic Deposition. Coatings 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.A.U.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A. Electrophoretic deposition of PEEK/bioactive glass composite coatings on stainless steel for orthopedic applications: An optimization for in vitro bioactivity and adhesion strength. Int. J. Adv. Manuf. Technol. 2020, 108, 1849–1862. [Google Scholar] [CrossRef]
- Yadav, V.S.; Sankar, M.R.; Pandey, L.M. Coating of bioactive glass on magnesium alloys to improve its degradation behavior: Interfacial aspects. J. Magnes. Alloys 2020, 8, 999–1015. [Google Scholar] [CrossRef]
- Tan, F.; Naciri, M.; Al-Rubeai, M. Osteoconductivity and Growth Factor Production by MG63 Osteoblastic Cells on Bioglass-Coated Orthopedic Implants. Biotechnol. Bioeng. 2011, 108, 454–464. [Google Scholar] [CrossRef]
- Tan, F.; Nasiri, M.; Al-Rubeai, M. In vitro osteoblastic cell activity on Bioglass-derived bioceramic surfaces deposited by a novel coblast technique. J. Biotechnol. 2010, 150, S105. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Beltran, A.M.; Alcudia, A.; Begines, B.; Rodriguez-Ortiz, J.A.; Torres, Y. Porous titanium substrates coated with a bilayer of bioactive glasses. J. Non-Cryst. Solids 2020, 544, 120206. [Google Scholar] [CrossRef]
- Beltran, A.M.; Begines, B.; Alcudia, A.; Rodriguez-Ortiz, J.A.; Torres, Y. Biofunctional and Tribomechanical Behavior of Porous Titanium Substrates Coated with a Bioactive Glass Bilayer (4555-1393). ACS Appl. Mater. Interfaces 2020, 12, 30170–30180. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Souza, J.G.S.; Cordeiro, J.M.; Bertolini, M.; de Avila, E.D.; Landers, R.; Rangel, E.C.; Fortulan, C.A.; Retamal-Valdes, B.; da Cruz, N.C.; et al. Synthesis of bioactive glass-based coating by plasma electrolytic oxidation: Untangling a new deposition pathway toward titanium implant surfaces. J. Colloid Interface Sci. 2020, 579, 680–698. [Google Scholar] [CrossRef]
- Panahi, Z.; Tamjid, E.; Rezaei, M. Surface modification of biodegradable AZ91 magnesium alloy by electrospun polymer nanocomposite: Evaluation of in vitro degradation and cytocompatibility. Surf. Coat. Technol. 2020, 386, 125461. [Google Scholar] [CrossRef]
- Shafiee, B.M.; Torkaman, R.; Mahmoudi, M.; Emadi, R.; Derakhshan, M.; Karamian, E.; Tavangarian, F. Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings 2020, 10, 1220. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Vrouwenvelder, W.C.A.; Groot, C.G.; Degroot, K. Better Histology and Biochemistry for Osteoblasts Cultured on Titaniumdoped Bioactive Glass—Bioglass 45s5 Compared with Iron-Containing, Titanium-Containing, Fluorine-Containing and Boron-Containing Bioactive Glasses. Biomaterials 1994, 15, 97–106. [Google Scholar] [CrossRef]
- Jurczyk, K.; Kubicka, M.M.; Ratajczak, M.; Jurczyk, M.U.; Niespodziana, K.; Nowak, D.M.; Gajecka, M.; Jurczyk, M. Antibacterial activity of nanostructured Ti-45S5 bioglass-Ag composite against Streptococcus mutans and Staphylococcus aureus. Trans. Nonferrous Met. Soc. China 2016, 26, 118–125. [Google Scholar] [CrossRef]
- Jurczyk, K.; Jurczyk, M.U. Nanostructured surfaces in biomaterials. In Nanobiomaterials; Woodhead Publishing: Southston, UK, 2018; pp. 179–195. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Prezas, P.R.; Ramos, D.J.; Sa-Nogueira, I.; Borges, J.P.; Lanca, M.C.; Silva, J.C.; Henriques, C.M.R.; Pires, E.; Kumar, J.S.; et al. Nontoxic glasses: Preparation, structural, electrical and biological properties. Int. J. Appl. Ceram. Technol. 2019, 16, 1885–1894. [Google Scholar] [CrossRef]
- Galdos, M.V.G.; Pastore, J.I.; Ballarre, J.; Cere, S.M. Dual-surface modification of titanium alloy with anodizing treatment and bioceramic particles for enhancing prosthetic devices. J. Mater. Sci. 2017, 52, 9151–9165. [Google Scholar] [CrossRef]
- Fu, T.; Sun, J.M.; Zhao, Y.T.; Wang, L.J.; Zhou, Y.C.; Ma, X. Hydrothermally crystallized Sr-containing bioactive glass film and its cytocompatibility. Ceram. Int. 2017, 43, 13689–13695. [Google Scholar] [CrossRef]
- Bargavi, P.; Chitra, S.; Durgalakshmi, D.; Radha, G.; Balakumar, S. Zirconia reinforced bio-active glass coating by spray pyrolysis: Structure, surface topography, in-vitro biological evaluation and antibacterial activities. Mater. Today Commun. 2020, 25, 101253. [Google Scholar] [CrossRef]
- Say, Y.; Aksakal, B. Effects of hydroxyapatite/Zr and bioglass/Zr coatings on morphology and corrosion behaviour of Rex-734 alloy. J. Mater. Sci. Mater. Med. 2016, 27, 105. [Google Scholar] [CrossRef]
- Araujo, M.S.; Bartolome, J.F.; Mello-Castanho, S. Tribological and mechanical behaviour of 45S5 Bioglass (R)-based compositions containing alumina and strontium. Ceram. Int. 2020, 46, 24347–24354. [Google Scholar] [CrossRef]
- Abushahba, F.; Soderling, E.; Aalto-Setala, L.; Sangder, J.; Hupa, L.; Narhi, T.O. Antibacterial properties of bioactive glass particle abraded titanium against Streptococcus mutans. Biomed. Phys. Eng. Express 2018, 4, 045002. [Google Scholar] [CrossRef]
- Burtscher, S.; Krieg, P.; Killinger, A.; Al-Ahmad, A.; Seidenstucker, M.; Latorre, S.H.; Bernstein, A. Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis. Materials 2019, 12, 3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, M.M.; Prasad, P.S.; Bindu, S.H.; Prasad, A.; Rao, P.V.; Govindan, N.P.; Veeraiah, N.; Ozcan, M. Investigations on Physico-Mechanical and Spectral Studies of Zn2+ Doped P2O5-Based Bioglass System. J. Compos. Sci. 2020, 4, 129. [Google Scholar] [CrossRef]
- Soundrapandian, C.; Bharati, S.; Basu, D.; Datta, S. Studies on novel bioactive glasses and bioactive glass-nano-HAp composites suitable for coating on metallic implants. Ceram. Int. 2011, 37, 759–769. [Google Scholar] [CrossRef]
- Khodaei, M.; Nejatidanesh, F.; Shirani, M.J.; Valanezhad, A.; Watanabe, I.; Savabi, O. The effect of the nano-bioglass reinforcement on magnesium based composite. J. Mech. Behav. Biomed. 2019, 100, 103396. [Google Scholar] [CrossRef]
- Yang, Y.W.; Lu, C.F.; Peng, S.P.; Shen, L.D.; Wang, D.; Qi, F.W.; Shuai, C.J. Laser additive manufacturing of Mg-based composite with improved degradation behaviour. Virtual Phys. Prototyp. 2020, 15, 278–293. [Google Scholar] [CrossRef]
- Daguano, J.K.M.E.; Strecker, K.; Ziemath, E.C.; Rogero, S.O.; Fernandes, M.H.V.; Santos, C. Effect of partial crystallization on the mechanical properties and cytotoxicity of bioactive glass from the 3CaO center dot P2O5-SiO2-MgO system. J. Mech. Behav. Biomed. 2012, 14, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Samudrala, R.K.; Azeem, P.A. Preliminary biological evaluation of tantalum containing soda lime borosilicate bioactive glasses. J. Alloys Compd. 2019, 810, 151853. [Google Scholar] [CrossRef]
- Yu, C.X.; Zhuang, J.J.; Dong, L.Q.; Cheng, K.; Weng, W.J. Effect of hierarchical pore structure on ALP expression of MC3T3-E1 cells on bioglass films. Colloid Surf. B 2017, 156, 213–220. [Google Scholar] [CrossRef]
- Adamek, G. Tantalum-45S5Bioglass composite foams prepared in thermal dealloying process. Int. J. Refract. Met. Hard Mater. 2019, 81, 58–62. [Google Scholar] [CrossRef]
- Alhalawani, A.M.F.; Mehrvar, C.; Stone, W.; Waldman, S.D.; Towler, M.R. A novel tantalum-containing bioglass. Part II. Development of a bioadhesive for sternal fixation and repair. Mater. Sci. Eng. C 2017, 71, 401–411. [Google Scholar] [CrossRef]
- Ning, C.Y.; Wang, Y.J.; Lu, W.W.; Chen, X.F.; Wu, G.; Zhao, N.R. Microstructure and mechanical performances of plasma-sprayed functionally gradient HA-ZrO2-Bioglass coatings. Key Eng. Mater. 2005, 280–283, 1893–1898. [Google Scholar]
- Ning, C.Y.; Wang, Y.J.; Lu, W.W.; Qiu, Q.X.; Lam, R.W.M.; Chen, X.F.; Chiu, K.Y.; Ye, J.D.; Wu, G.; Wu, Z.H.; et al. Nano-structural bioactive gradient coating fabricated by computer controlled plasma-spraying technology. J. Mater. Sci. Mater. Med. 2006, 17, 875–884. [Google Scholar] [CrossRef]
- Babu, M.M.; Prasad, P.S.; Rao, P.V.; Bindu, S.H.; Prasad, A.; Veeraiah, N.; Ozcan, M. Influence of ZrO2 Addition on Structural and Biological Activity of Phosphate Glasses for Bone Regeneration. Materials 2020, 13, 4058. [Google Scholar] [CrossRef]
- Aldini, N.N.; Fini, M.; Giavaresi, G.; Torricelli, P.; Martin, L.; Giardino, R.; Ravaglioli, A.; Krajewski, A.; Mazzocchi, M.; Dubini, B.; et al. Improvement in zirconia osseointegration by means of a biological glass coating: An in vitro and in vivo investigation. J. Biomed. Mater. Res. 2002, 61, 282–289. [Google Scholar] [CrossRef]
- Yang, D.W.; Zhu, J.W.; Wu, W.G. Improvement of biocompatibility and osteointegration of zirconia implants by means of bioactive glass coating: An experimental study in animal. Key Eng. Mater. 2007, 336–338, 1679–1682. [Google Scholar] [CrossRef]
- Nogueira, L.B.; Campos, T.P.R. Synthesis, chemical characterization and radiological response of Ho and HoZr bioglass seeds. J. Sol.-Gel. Sci. Technol. 2016, 77, 688–698. [Google Scholar] [CrossRef]
- Souza, L.; Lopes, J.H.; Encarnacao, D.; Mazali, I.O.; Martin, R.A.; Camilli, J.A.; Bertran, C.A. Comprehensive in vitro and in vivo studies of novel melt-derived Nb-substituted 45S5 bioglass reveal its enhanced bioactive properties for bone healing. Sci. Rep. 2018, 8, 12808. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.H.; Souza, L.P.; Domingues, J.A.; Ferreira, F.V.; Hausen, M.D.; Camilli, J.A.; Martin, R.A.; Duek, E.A.D.; Mazali, I.O.; Bertran, C.A. In vitro and in vivo osteogenic potential of niobium-doped 45S5 bioactive glass: A comparative study. J. Biomed. Mater. Res. B 2020, 108, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Vyas, V.K.; Harijan, P.K.; Ershad, M.; Pyare, R. Investigating in vitro bioactivity, magnetic and mechanical properties of iron and cobalt oxide reinforced (45S5-HA) biocomposite. J. Aust. Ceram. Soc. 2018, 54, 411–421. [Google Scholar] [CrossRef]
- Li, X.L.; Zhitomirsky, I. Deposition of poly(methyl methacrylate) and composites containing bioceramics and bioglass by dip coating using isopropanol-water co-solvent. Prog. Org. Coat. 2020, 148, 105883. [Google Scholar] [CrossRef]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci. Mater. Med. 2015, 26, 195. [Google Scholar] [CrossRef] [PubMed]
- Floroian, L.; Florescu, M.; Sima, F.; Popescu-Pelin, G.; Ristoscu, C.; Mihailescu, I.N. Synthesis of biomaterial thin films by pulsed laser technologies: Electrochemical evaluation of bioactive glass-based nanocomposite coatings for biomedical applications. Mater. Sci. Eng. C 2012, 32, 1152–1157. [Google Scholar] [CrossRef]
- Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Rosca, J.C.M.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional Bioglass-Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials 2020, 10, 385. [Google Scholar] [CrossRef] [Green Version]
- Boulila, S.; Oudadesse, H.; Badraoui, R.; Lefeuvre, B.; Mabrouk, M.; Chaabouni, K.; Mostafa, A.; Makni-Ayedi, F.; Barroug, A.; Rebai, T.; et al. Antioxidative/oxidative effects and retarding osteoconductivity of ciprofloxacin-loaded porous polyvinyl alcohol/bioactive glass hybrid. Med. Biol. Eng. Comput. 2017, 55, 17–32. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).