Abstract
Exopolysaccharide (EPS) show remarkable properties in various food applications. In this review paper, EPS composition, structural characterization, biosynthesis pathways, and recent advancements in the context of application of EPS-producing Lactobacillus spp. in different food industries are discussed. Various chemical and physical properties of Lactobacillus EPS, such as the structural, rheological, and shelf-life enhancement of different food products, are mentioned. Moreover, EPSs play a characteristic role in starter culture techniques, yogurt production, immunomodulation, and potential prebiotics. It has been seen that the wastes of fermented and non-fermented products are used as biological food for EPS extraction. The main capabilities of probiotics are the use of EPS for technological properties such as texture and flavor enhancement, juiciness, and water holding capacities of specific food products. For these reasons, EPSs are used in functional and fermented food products to enhance the healthy activity of the human digestive system as well as for the benefit of the food industry to lower product damage and increase consumer demand. Additionally, some pseudocereals such as amaranth and quinoa that produce EPS also play an important role in improving the organoleptic properties of food-grade products. In conclusion, more attention should be given to sustainable extraction techniques of LAB EPS to enhance structural and functional use in the developmental process of food products to meet consumer preferences.
1. Introduction
The food industry highly appreciates new and innovative trends in food and nutrition for healthy food design with the aim of minimal usage of synthetic food ingredients. Additionally, a significant boom has been observed in the number of new studies exploring the potential of various functional and nutraceutical ingredients in the design of healthier food products [1]. Various prebiotics and probiotics combinations are being investigated to enhance the functional value of food products [2]. Currently, probiotics, prebiotics, para-probiotics, and post-biotics are the main research focuses for functional and nutraceutical food product development. Lactic acid bacteria (LAB) are highly significant in a food system and have potential to be used in all these categories of biotics [1]. Among LAB, by-products of Lactobacillus genus are the most common sources of probiotics. Moreover, they are extensively used in fermented and non-fermented food products, served as functional and nutraceutical dietary supplements [3].
Lactobacillus play an important role as probiotics by modulating the human gastrointestinal system to confer different health benefits [4]. Lactobacillus synthesize various metabolites such as organic acids (lactic and acetic acid), aromatic compounds, fatty acids, and bacteriocins (lantibiotics). All these ingredients have great importance in the food industry [5] due to the status of Generally Recognized as Safe (GRAS) [6]. Some of the important Lactobacillus that are used by the food industry are Lactobacillus helveticus, L. plantarum, L. casei, L. curvatus, L. sakei, L. delbrueckii subsp. Bulgaricus, L. paracasei, L. pentosus, L. reuteri, L. vaginalis, L. fermentum, L. kifer, L. acidophillus, L. reuteri, L. brevis, Wisella cibaria, Streptococcus thermophilus, etc.
During the growth of Lactobacillus, they excrete extracellular polysaccharides, named exopolysaccharides (EPS). EPS are natural, non-toxic, and bio-products with diverse chemical structures and biological activities. Around 165 Lactobacillus species were recognized as EPS-producers, including L. plantarum, L. delbrueckii subsp. bulgaricus, L. casei, L. brevis, L. curvatus, L. helveticus, L. rhamnosus, L. acidophilus, and L. johnsonii [7]. The Lactobacillus produce a low yield of EPS as compared to other bacteria. However, EPS from Lactobacillus strains have higher functionality potential and resistance to environmental stress [5].
EPS from Lactobacillus have diverse functions due to the presence of exceptional rheological and water-binding properties. They have an extensive range of emulsification applications in the food industry. Moreover, they play a dominant role as a thickener, gelling, encapsulation, and hygroscopic agents [8]. Table 1 summarizes the relationship of EPS structure with their bioactivities.

Table 1.
Lactobacillus-derived EPS and their relationship between structural activity.
2. Extraction of Lactobacillus EPS
EPS extraction methods include physical, chemical, or combinations from pure cultures or undefined mixed cultures [34]. The yield and composition of EPS was influenced by the extraction method [35]. Among physical methods, centrifugation, sonication, and heating were used to extract the EPS. Efficiency analysis indicated that the heating method exhibited a higher extraction yield (82 mg/g VSS) compared to sonication and centrifugation [35].
Furthermore, two physical extraction methods (centrifugation and ultra-sonication) were compared. The protein content was lower in EPS samples prepared by centrifugation compared to samples extracted by the ultra-sonication method [36].
The chemical-based extraction methods include cation exchange resin (CER), EDTA (Ethylene diamine tetra-acetic Acid), and NaOH methods [37,38,39]. Moreover, a study demonstrated that the physical method (sonication) was superior to the chemical ones (glutaraldehyde, formaldehyde, and NaOH). The protein content was higher (343–337 mg proteins/g EPS DW) with the physical method, compared to chemical extraction methods [40].
3. Lactobacillus EPS Composition
LAB can produce a wide variety of EPSs, with an infinite diversity of structures [41]. They differ in their side chains, in the linkages between repeating units, in their substitutions, and their charges [42]. They are composed of fructose, rhamnose, mannose, arabinose, galactose, glucose, and some sugar derivatives such as N-acetyl galactosamine and N-acetylglucosamine [43]. Their nature is dependent on the following factors: composition of the growth medium, cultural strain, and bacterial strain. Moreover, proteins and enzymes are involved in EPS production that can be controlled by the regulation of gene expression [43,44].
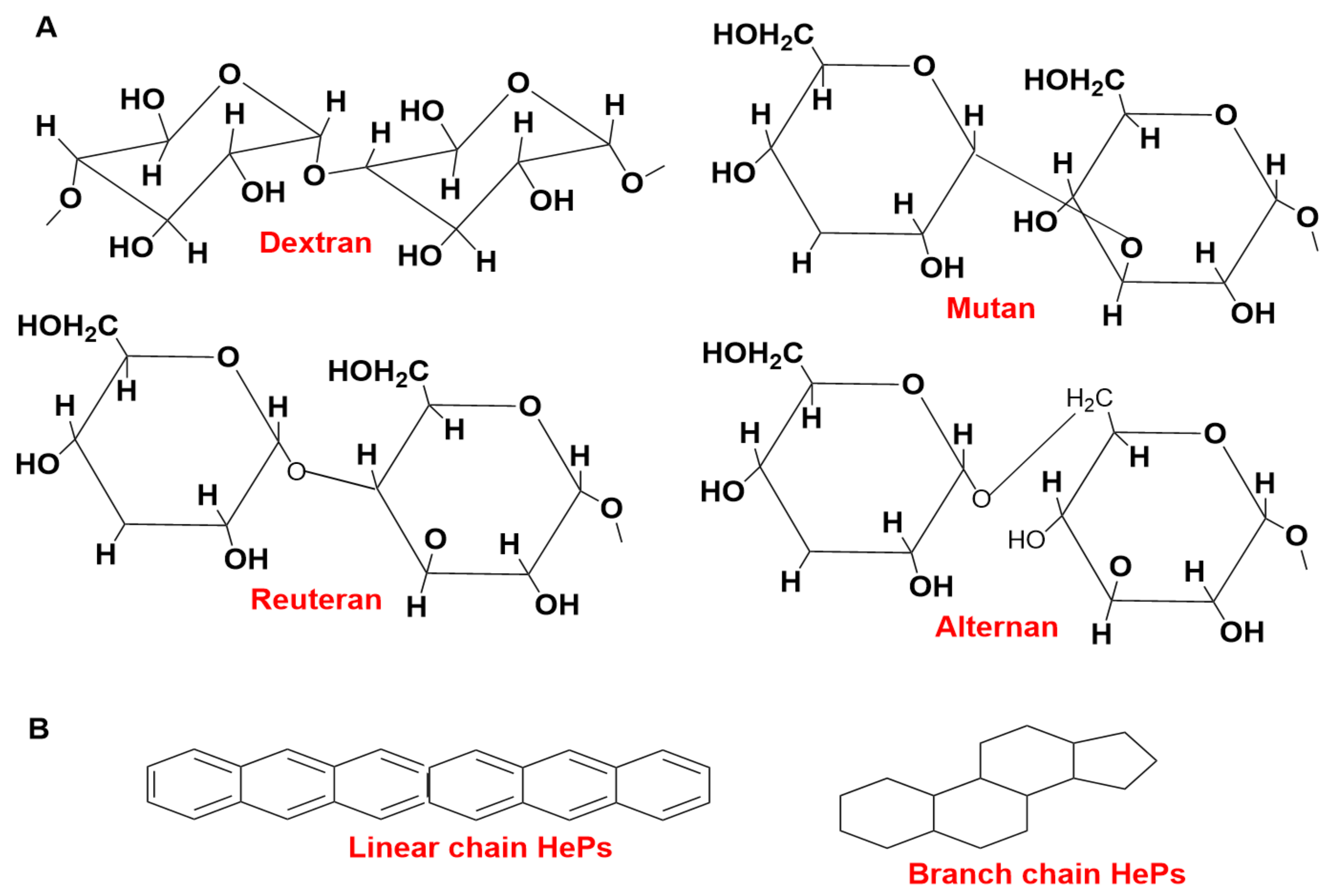
EPS are characterized into two groups: one is hetero-polysaccharides (HePS) and the other is homo-polysaccharides (HoPS) [45]. Lactobacillus homopolysaccharides (HoPSs) contain repeating units of the same type of sugar subunits with MW 105–106 Da [46]. α-glucans and β-fructans are synthesized from glucose and fructose by the activity of glycosyl hydrolase by utilizing the energy of glycosidic bonds present between them [47]. HoPSs are categorized into several different types: polyglucans, D-β-glucans, and D-α-glucans. The position of carbon bonds and glycosyl type depends on the linkage composition [46]. Lactobacillus produce some types of HoPSs, which are dextran, mutan, reuteran, alternan, β-D-glucans, levan, and insulin-type glucan [48]. The representative structures of HePSs and HoPSs are shown in Figure 1.
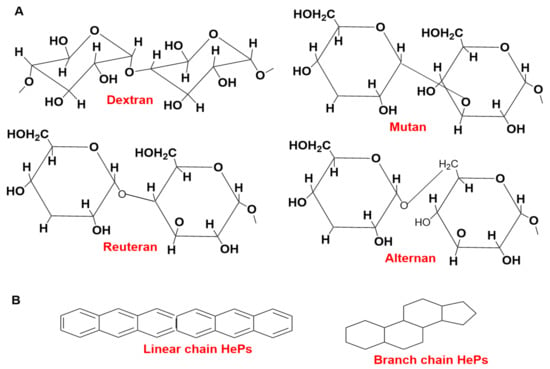
Figure 1.
The representative structures of HoPSs (A) and HePSs (B).
HePS are much more complex as compared to HoPSs; as HePS comprise of different monosaccharides and have three to eight repeating units of galactose, glucose, and rhamnose in different proportions. Some other components (uronic acid, N-acetyl-d-galactosamine, and N-acetyl-d glucosamine) play crucial roles in the technological and physiological functions [49,50]. Moreover, some growth conditions affect the secretion of HePSs, including pH, turbidity, composition, oxygen level, temperature, growth phase, amount, type, and glycosidic linkages [51].
These hetero-polysaccharides and hexo-polysaccharides require lipids such as isoprenoid glycosyl and have an intracellular scope containing repeating units; hence, they play a significant role in the developmental processes [52]. A polymerization process continues with the transposition of the repetitive units through a membrane that occurs outside the cell. As stated in previous studies, EPS having only the same type of sugar monomers in their composition are HoPS and HePS, which are defined as those that comprise two or more different types of sugar monomers [53]. Some factors actively respond to EPS production such as fermentation conditions, as these define and characterize which type and how much EPS is present, as well as the secretion and biosynthesis of microbial EPSs, which are much more dependent on the growth and developmental phases of microorganisms, as stated by Dertli et al. [52].
One of the main components, i.e., glucan-type EPS production, relies on the activity of a special enzyme-termed glucan sucrase activity of Lactobacillus; it has a greater effect on the catalysis reactions of transglycosylation and hydrolysis of sucrose (sugar) as a substrate, immediately after the breakdown of the sugar (sucrose); as a result, polymeric glucan is formed [32]. Glucose-containing homopolysaccharides are composed of a-D glucans; 1-dextran linked by a-1,6-glycosidic bonds; 2-mutan linked by a-1,3-glycosidic bonds; 3-reuteran containing both a-(1,4)- and a-(1,6)-glycosidic linkages, with a ratio of both 58% and 42%, respectively; and beta-glucans. Whereas homopolysaccharides containing fructose include fructans, the 1-inulin-type which is linked by b-2,1-glycosidic bonds and arranged at position b-2,6 and 2-levan contain b-2,6-glycosidic linkages, and some molecules also have a variation such as b-2,1-linked branches [5]. The biosynthesis and structures of HePS are much more complex as compared to HoPSs as HePS comprises different monosaccharides as they may have repeating units of galactose, glucose, and rhamnose in different proportions. Some other components play crucial roles in technological and physiological functions, such as uronic acid, N-acetyl-d-galactosamine, and N-acetyl-d glucosamine, and some other non-carbohydrate substituents such as phosphate, succinate, pyruvate, and acetate are mentioned [49,50].
A major role of LAB is its EPS characterization in regulating activities, such as Streptococcus thermophilus CH9 producing EPS that exhibits antitumor activity in in vitro characterization against HepG2 cells (human liver cancer) [54].
EPS produced by Leuconostoc citreum strain B-2 with D-glucopyranose units, which are α-(1→6)-linkages in the major branch chain, α-(1→3) linkages (with proportions of 75% and 19%, respectively), and a few α-(1→2) chains [55].
A recent study revealed that Lactobacillus reuteri E81 producing EPS contains an α-glucan homopolysaccharide with α-(1→3) and α-(1→6) glycosidic linkages. This EPS is a promising option for the food industry due to its special characteristics such as its hygroscopic nature and heat-resistant thermal properties [56]. The Leuconostoc mesenteroides strain NRRL B-512 F produces EPS, which comprises α-(1→6) linkages, α-(1→3) linkages in a proportion of 95% and 5%, respectively, and some α-(1→4) branch linkages [57]. All of these studies show that EPS extracted from Lactobacillus covers a wide range of properties and structures that have potential applications in several key areas. Some other studies on EPS’ role in other food products are discussed below.
4. EPS Synthesis Pathways
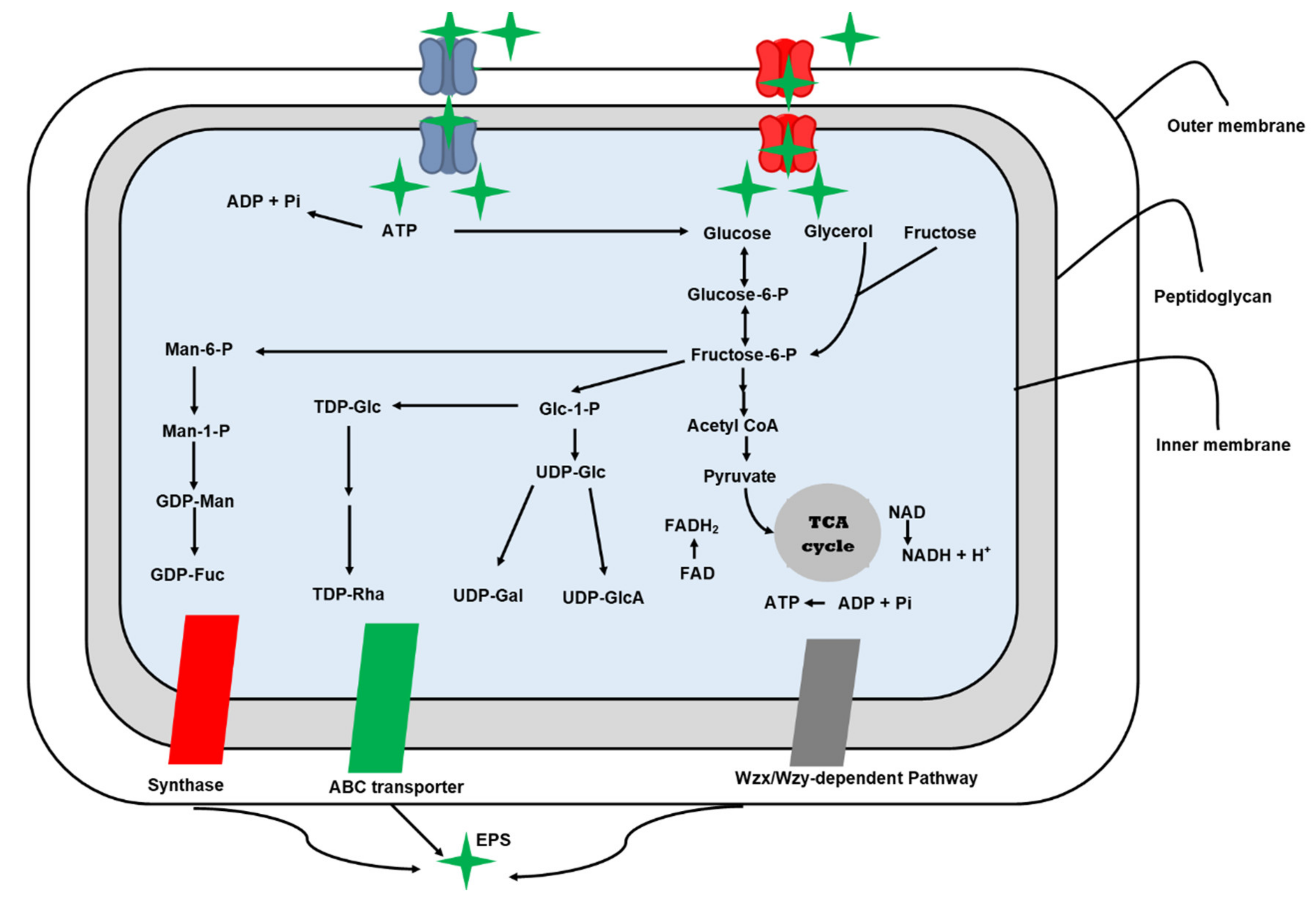
Microorganisms use four commons pathways for EPS biosynthesis i.e., extracellular, the synthase-dependent, the ATP-binding cassette transporter-dependent pathway, and the Wzx/Wzy-dependent pathway (Figure 2). The Wzx/Wzy-dependent and extracellular synthesis pathways are common mechanisms for Lactobacillus EPS production [58].
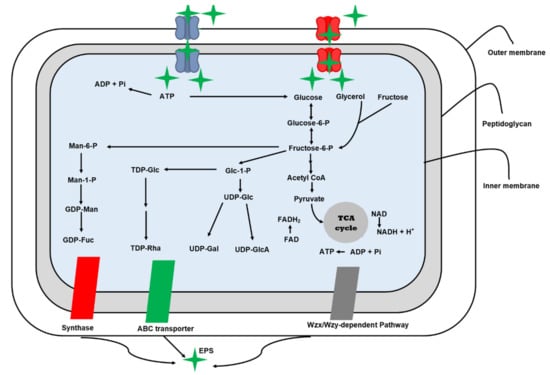
Figure 2.
Biosynthesis pathways of exopolysaccharides in microorganisms.
4.1. Extracellular Synthesis Pathway
The extracellular EPS biosynthesis pathway is typically used by the genera Pediococcus, Leuconostoc, and Weissella to generate HoPSs. This pathway consists of two major mechanisms [45]. The initial step is polymerization, which involves the transfer of a monosaccharide to an evolving polysaccharide chain. Enzymes such as glucansucrase and fructansucrase help speed up this process. The polymerized EPSs chain is released straight into the extracellular environment in the second stage [59].
4.2. Wzx/Wzy-Dependent EPSs Biosynthesis Pathway
The Wzx/Wzy-dependent EPSs biosynthesis pathway is used entirely by Streptococcus, Lactobacillus, and Leuconostoc. This pathway is more complex than extracellular EPSs production since it requires the employment of more enzymes and larger interaction sites [60]. It consists of five steps: (a) phosphorylation, (b) activation of monosaccharides, (c) synthesis of repeating units, (d) polymerization, (e) release of EPS.
For phosphorylation and transportation of monosaccharides and disaccharides, a phosphotransferase-assisted pathway and a permease-assisted pathway are required for the import of repeating subunits [19]. While, in activation of monosaccharides or the synthesis of sugar nucleotides, the phosphoglucomutase or galactose-1-phosphate uridylyltransferase starts the transformation from glucose-6-phosphate or galactose-1-phosphate to glucose-1-phosphate, which is then changed into UDP-galactose, dTDP-rhamnose, and UDP-glucose by using numerous enzymes such as UDP-galactose 4-epimerase, UDP-glucose pyrophosphorylase, and dTDP-glucose 4,6-dehydratase [19].
In the third step, the synthesis of repeating subunits occurs. Individual repeating subunits coupled with an undecaprenol diphosphate anchor on the surface membrane are accumulated in this stage by a series of glycosyltransferases. With the help of flippase, repeated subunits are transformed from the intracellular membrane to the extracellular membrane surface in the fourth phase (Wzx). The polymerization of repeated subunits by a polymerization protein (Wzy), followed by the release of long polymer chains into extracellular space [61,62].
5. Role of EPS in the Food Industry
5.1. Rheological and Technological Functions in Food Products
A recent study by Ayyash et al. [13] evaluated the rheological and bioactive potential of EPS synthesized by a novel probiotic, L. plantarum C70. In this study, camel milk was used as a potential source to isolate EPS-C70. The EPS-C70 had an average molecular weight (Mw) of 3.8 × 105 Da while having main monosaccharide elements such as glucose (74.6%), mannose (7.1%), galactose (5.0%), and arabinose (13.3%). EPS-C70 (10 mg/mL) showed 88.1% and 73.1% cytotoxic activity against breast cancer and colon cancer lines, respectively. Moreover, at the same concertation of EPS-C70 with 2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulphonic acid) (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH), the activity was 49.42% and 75.91%, respectively [13]. Additionally, EPSC70 has a strong bactericidal effect on some devastating foodborne pathogens such as Salmonella typhimurium, Listeria monocytogenes, E. coli O157:H7, and Staphylococcus aureus ATCC; this EPS shows a 2.0 to 3.0 log (CFU/mL) reduction in pathogenic foodborne microorganisms, which have initial growing populations of 9.0 log (CFU/mL). An overview of EPS technological and functional characteristics in the food industry are represented in Figure 3.

Figure 3.
Technological function of Lactobacillus EPS in the food industry.
Recently, Hashemi et al. [63] revealed that the use of ultra-sonication treatment considerably improved the fermentation process by increasing goat milk’s bioactive properties, i.e., EPS content, a-amylase, and a-glucosidase inhibition, antioxidant and anticancer activities, and peptide content. The obtained results show that ultra-sonication, initially at 30% and then at 60% amplitude, increased the growth of some inoculated strains, e.g., L plantarum LP3. However, a 90% amplitude not only reduced the bacterial population but also exerted antagonistic effects on EPS. Similarly, the EPS potential was evaluated by the production of low-fat akawi cheese isolated from camel milk. Angiotensin-converting enzyme (ACE) inhibition, anti-proliferative, a-amylase, antioxidant, and a-glucosidase inhibitory activities were compared between commercial EPS and camel milk-EPS cultures. The ACE inhibition activity of camel milk-EPS cultures was more than 70%, while the anti-proliferation activity improved from 38% to 48% during one week of storage. Overall, the camel milk-EPS cultures showed significant health-boosting properties compared to the commercial EPS cultures [64]. A study [65] isolated a novel bacterial strain, L. lactis, from milk with promising EPS-producing properties. The extracted EPS showed higher antioxidant properties and have the potential to be applied as a thickening agent in the food industry.
Likewise, Lactiplantibacillus plantarum has three distinct LBIO1, LBIO14, and LBIO28 strains, which are isolated from fermented dairy products and are used to investigate the impact of polymers that are seen in skim milk fermentations. These three strains are to be used based on the presence and absence of a ropy character, where LBIO1 and LBIO28 show the presence of a ropy character and LBIO14 exhibit its absence; this ropy character can be used to evaluate the synthesis of a type of EPS [66]. Due to the presence of a high molecular weight EPS, the two strains show a ropy character, which is beneficial in the food industry.
Among the three L. plantarum strains, LBIO1 can produce milk gels with a less permeable structure; therefore, this strain is used for the manufacture of ready-to-use dairy products to increase water retention or avoid syneresis and maintain the nutritional needs of consumers [67]. Another beneficial impact of non-ropy LAB spp. is that the milk fermented by L. plantarum LBIO28 strain showed the highest viscosity of the product, which indicates that the in-situ polymer production can mainly be used to modify the rheological properties of food products with low calories. This will ultimately serve the function of a natural fat replacer [68]. All of these characteristics show the technological aspects of the Lab spp. EPS for use in dairy products.
L. paracasei isolated from Kefir grains are used as a fermentation starter for the development of functional products. Milk fermented with L. paracasei is suitable for human consumption as it can produce immunomodulatory metabolites during the fermentation process, also playing a role in prebiotic development [69]. The three strains, CIDCA8339, CIDCA83123, and CIDCA83124, of L. paracasei are used to produce bioactive moieties during the fermentation process, and the milk supernatants downregulate the innate immune response induced in vitro by >75% [70]. Moreover, L. paracasei CIDCA 8339 maintains EPS production and probiotic development properties, which are affected by different temperature variations, with a technological function in the food industry [69]. These strains’ survival depends upon a suitable amount of viable microbe during the storage of a specific product. Similarly, the L. paracasei CIDCA 8339 survived in fermented milk stored at 4 °C, with no significant reduction in viable bacteria when stored for 225 days. This strain also showed variation, having the best protection against Salmonella attacks on intestinal cells [71]. Due to the absence of hemolytic activity, the EPS is used for the healthy activity of the gastrointestinal tract, which is why they are used in functional fermented foods [70].
5.2. Radical Scavenging and Anti-Tumor Properties
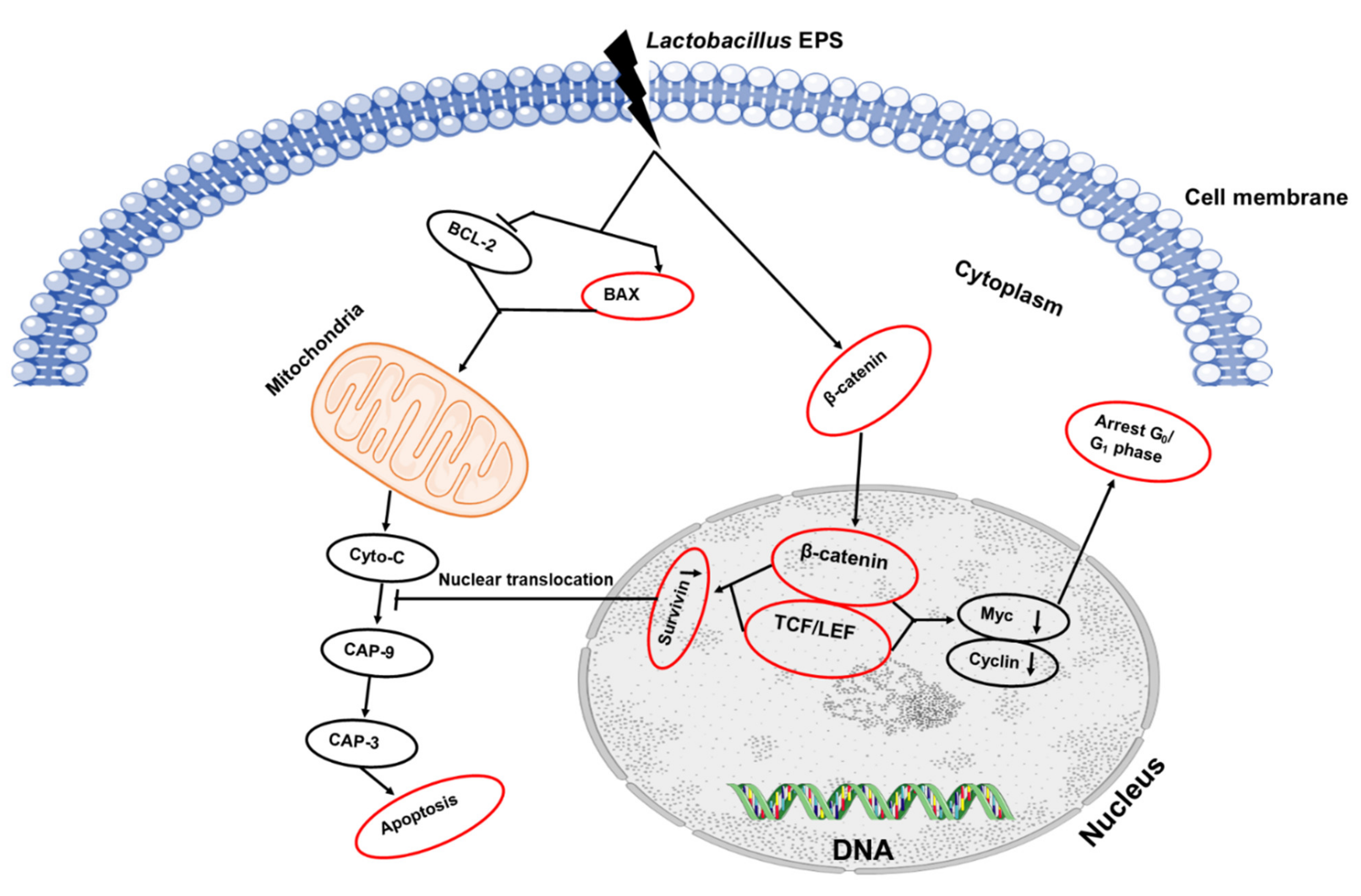
L. plantarum JLK0142, having robust probiotic properties and EPS-production capability, was isolated from traditionally fermented dairy tofu. The results showed that its EPS had strong hydroxyl radical-scavenging activity, and it effectively induced apoptosis in human colon cancer HT-29 cells, hence effectively improving immunomodulatory activity to stimulate the immune system [72]. In 2019, Wang and colleagues conducted an experiment in which non-EPS-producing starter cultures were used along with purified EPS (ingredient) and Lb. plantarum JLK0142 (adjunct culture) to produce three batches of cheese. The ripening period of 90 days did not induce any negative effects on L. plantarum JLK0142 count (7.99 log CFU/g) in cheese. All experimental cheeses (with adjunct culture or EPS ingredient) had proteolytic activity, higher moisture, and sensory evaluations, but lower hardness and cohesiveness as compared with the control cheese. Wang reported that water-soluble extracts from the experimental cheeses also outperformed the control in scavenging 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) and in inhibiting α-amylase, angiotensin-converting enzyme, hydroxyl radicals, and HT-29 cancerous cells growth in humans. Moreover, the improvement of low-fat cheese quality and bioactive moieties is directly proportional to the inoculation with the EPS-producing culture of the Lb. plantarum JLK0142 strain [73]. Schematic representation of the EPS anticancer mechanism is represented in Figure 4.
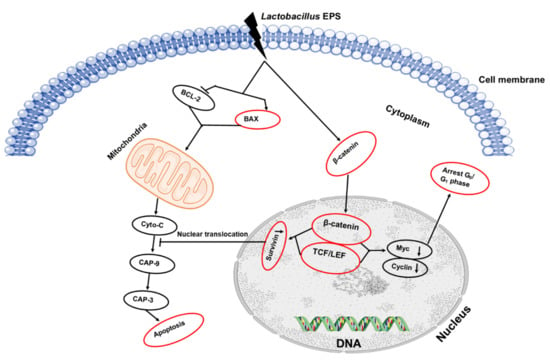
Figure 4.
Schematic representation of the EPS anticancer mechanism.
5.3. Flavor and Texture-Enhancing Properties
Whey, a lactose-rich byproduct, originated from cheese-making industries during the coagulation of a milk [74]. Some additional steps convert the lactose of whey using a β-galactosidase enzyme. This process also catalyzes the transgalactosylation and hydrolysis of lactose, which are further enhanced to be used as valuable by-products [75]. Many of the bacterial and fungal EPSs such as succinoglycan, curdlan, levan, pullunan, and EPS-605 are currently used as stabilizing and reducing agents for nanoparticle synthesis, which are metal in nature [76,77]. In food products, due to shear thinning properties (pseudoplastic non-Newtonian fluids), there may be a loss of EPS structural units, mainly caused by hydrodynamic forces [78], which plays a crucial role in forming the desired mouthfeel and flavor-enhancing properties [79]. Nowadays, this research is gaining attention due to the potential of converting cheese whey into valuable products.
Thus, EPS produced from L. plantarum is usually used as a textural agent, and potentially they can also be used as probiotics [80]. A specific strain (JNULCC001) of L. plantarum can convert cheese whey into a valuable EPS product as it shows a biosorption ability of methylene blue dye; it also plays a role in the thickening, gelling, stabilizing, and biosynthesis of selenium nanoparticles [12].
5.4. Lactobacillus EPS and Their Role as Bread Improvers
The EPS function in bread improvement mainly depends upon three factors, i.e., carbon source, incubation time, and sucrose concentration [81]. A liquid medium containing the Weisella cibaria C43-11 strain enriched with 10% sucrose indicated high EPS production. Liquid sourdoughs (LSs) with a dough yield (DY) of 500, based on wheat flour or pseudocereals (such as amaranth and quinoa) present in a 1:1 ratio with 3% sucrose as compared to the L. plantarum ITM21B strain, which does not produce EPS in mMRS, were tested [82]. The W. cibaria hydrolyzes protein fermentations, while the L. plantarum ITM21B strain causes protein degradation at a dough yield of 250. EPSs are responsible for technological and nutritional properties; similarly, phytases improve mineral bioavailability by reducing phytate components in bread [83]. Regarding the biotechnological aspects, the combination of EPS and pseudocereals is very helpful for enhancing the overall quality indicators of food products such as shelf-life, dough rheology, nutritional value, and flavor as well the aroma of the final products [84]. Hence, we can say that the use of pseudocereals enhanced the production of EPS, which are further used in bakery products as texture improvers.
All around the globe, the baking industry faces the major problem of how to reduce the salt levels in yeasted bread [85]. The industries have tried to solve this problem many times, but they have faced bad effects on organoleptic properties such as shelf-life reduction, crumb structure, and lack of salty taste. For this purpose, breads with a combination of sourdough levels (0%, 6%, 12%, 18%, 24%) and salt levels (0%, 0.3%, 1.2%) were prepared. These sourdough combinations have functional EPS of two spp., Weisella cibaria MG1 and Lactobacillus amylovorus DSM 19280, to reduce the quality problems in yeasted bread. The salt level was reduced to 0.3%, thus improving the sensory profile and increasing the volume and shelf life of bread, as well as the crumb texture [86]. Thus, functional sourdoughs containing EPS are the best way to compensate for the reduced level of salt in yeasted bread. In addition, we can naturally maintain a high quality of bread without the addition of synthetic additives.
Secondly, to find the functionality of the bread, the sourdoughs were fermented with EPS-producing (Weissella cibaria, Leuconostoc mesenteroides, Fructilactobacillus sanfranciscensis and Latilactobacillus sakei LS81) LAB spp. It is to be noted that EPS production by these lactobacilli spp. enhances the texture and specific volume but also reduces the stalling rate in bread and crumb hardness [18]. Similarly, the in situ production of EPS provides a natural replacer for hydrocolloids, enhancing the physicochemical properties of gluten and gluten-free bakery products [87].
5.5. Water-Holding Capacity and Juiciness of Meat Products
EPS-producing bacteria are currently used to improve the water-holding capacity and juiciness of meat products, but only a few experiments introducing EPS-producing LAB spp. to meat products are conducted to check the vulnerability and sustainability [88]. Applications of hydrocolloids improve the quality of meat products, including cooked hams. The in-situ usage of EPS-producing Lactobacillus spp. such as L. curvatus, L. sakei and L. plantarum in meat matrices improves the product characteristics, except that of isolated ones, and helps in the tumbling process during ham production [22].
EPS of the L. plantarum S2 strain was shown to be a potential candidate for use in the food industry, presenting the role of a natural preservative and also having the ability to be used as a starter culture as well as being an antimicrobial agent against four pathogenic strains: Staphylococcus aureus ATCC-6538, Listeria monocytogenes ATCC–7644, Enterococcus feacalis ATCC-29212, and Pseudomonas aeroginosa ATCC-27853 [89]. They also proposed that some factors that are essential for antimicrobial activity are pH, temperature, and several enzymes are α-chymotrypsin, α-amylase, trypsin, lysozyme, and pronase. This strain not only appeared to be used as a starter culture but also improved the food color, taste, smell, and appearance for prolonged use in the consumer market [90].
5.6. EPS as Emulsifying Agents
In recent years, many novel EPSs have emerged as promising emulsifying and stabilizing agents. These have enormous potential to develop and stabilize both oil-in-water as well as water-in-oil emulsions. This activity of EPS may provide the food industry with a suitable alternative to stabilizers and emulsifiers to enhance the organoleptic characteristics [91]. A recent study [92] studied EPS from Lactobacillus sakei L3 in terms of various technological attributes, including emulsifying activity. The emulsification indices of the Lactobacillus sakei L3 EPS varied from 2.61% to 62.30% with different vegetable oils, i.e., soybean oil, peanut oil, sunflower oil, and mustard oil. The most stable and active emulsion was with sunflower oil at 2.0 mg/mL L3 EPS concentration. More recently, EPS from Lactobacillus Plantarum S123 showed high emulsifying activity due to its spongy and highly porous structure. This EPS can be used as a potential stabilizer in food products [93]. Similarly, in another study, the Lactobacillus paracasei EPS emulsification index was quite high in toluene (79.20) and benzene (78.867) augmented medium, as compared to coconut oil [94].
The emulsifying activity of EPS from Lactobacillus is less well studied and has a lower potential. However, there are some promising EPSs from another genus that have provided some interesting and highly productive results. Furthermore, a study [95] discovered a novel EPS from Pantoea sp. BCCS 001 GH with promising antioxidant and emulsifying abilities. This EPS is expressed as one of the most effective emulsifying agents against various oils/hydrocarbons combinations. The high degradation temperature (318 °C) of this EPS makes it safe to be used in the dairy industry. The genus Pandoraea is generally not known for EPS-producing bacterial strains. However, a study indicated that two bacterial strains, i.e., Pandorea pnomenusa MS5 Pandorea pnomenusa AS3, had effective emulsifying and anti-biofilm activity [91].
Galactan EPS is Generally Regarded as Safe (GRAS) and has a favorable potential to be used as an emulsifier in the food industry. Recently, a study evaluated the Galactan EPS oil-in-water emulsion capacity extracted from Weissella confusa KR 780676. This EPS at 1% of concentration exhibited substantial emulsifying activity (50–70%) and stability (100%) against olive and groundnut oil. This emulsion was stable from −20 °C to 60 °C as well as in a wide range of pH (3.0–8.0) [96]. Pseudomonas ID1, a bacterium isolated from marine sediments of Antarctica Islands, released EPS with a molecular mass higher than 2 × 106 Da. It showed very high stable emulsifying activity against different food and cosmetic oils. Furthermore, the emulsifying activity was much higher than commercial gums such as xanthan gum and Arabic gum [97]. BMS is the first high molecular weight EPS from Leuconostoc citreum-BMS strain with good emulsifying activity. The emulsifying activity of EPS-BMS was the highest at pH 7 and 25 °C after 24 h of incubation. Moreover, EPS-BMS was able to stabilize the emulsions for 15 days in both acidic and neutral conditions. This EPS stabilized oil-in-water emulsions due to its thickening properties and developed a macromolecular block in the aqueous medium between the dispersed droplets [98].
5.7. Starter Culture and Potential Prebiotics
A high EPS-producing L. pentosus SLC 13 strain isolated from mustard pickle presented probiotic features, antibiotic tolerance, bacteriocin production ability, acid tolerance, bile tolerance, and immune-potentiation impacts [99]. It is recommended that plant-based fermented foods such as soymilk should be fermented with LAB isolated from plants. Whereas animal-based fermented foods such as cow milk drinks can be better fermented with animal-source LABs. In yogurt production, prebiotics could be used as an adjunct along with L. pentosus SLC 13 to boost the dairy industry production [92]. Another study [100] reported that capsular EPS (S. thermophilus ASCC 285) and ropy EPS (S. thermophilus ASCC 1275)-producing starter cultures can improve the physical attributes of set and stirred types of yogurt. These EPS-producing starter cultures reduced the level of syneresis and improved firmness. A recent study [101] conducted an in situ study to evaluate the impact of two EPS-producing starter cultures, i.e., YF-L903 and YC-X11 on the various quality attributes of goat milk set yogurt. There was a significant improvement in the physicochemical, microbiological, and sensory properties of yogurt. A significant reduction in syneresis and improvement in apparent viscosity was observed in the yogurt. In another study, the effect of EPS-producing starter cultures on the physicochemical properties of yogurt was assessed using an EPS-producing culture (Streptococcus thermophilus LB-50 and Lactobacillus delbrueckii ssp. bulgaricus LB-42) and using a non-EPS producing cultures (Lactobacillus delbrueckii ssp. bulgaricus LB-42 and Streptococcus thermophilus LB-53). The EPS-producing cultures induced low structural degradation and high structural recovery. Additionally, a compact milk gel formation also substantially reduced the syneresis [102].
Bifidobacterium is generally not considered a suitable starter culture due to its inferior fermentation properties. In 2019, a research study indicated that ropy EPS-producing Bifidobacterium longum YS108R is an appropriate adjunct culture for fermentation of various novel dairy products. This B. longum YS108R strain enhanced the fermented milk’s physicochemical properties by reducing syneresis as well as increasing apparent viscosity and water-holding capacity. Additionally, it was observed that this ropy EPS-producing strain in combination with a commercial starter culture also reduced acetic acid production with improved sensory properties [103]. More research in this area is needed in the future to improve the acceptability, which can be achieved by properly regulating the ratio of seasoning or sugar to acid [104].
5.8. Texture Development in Yogurt
EPS produced by L. paracasei is used as starter cultures in yogurt preparations under controlled fermentation conditions [24]. The average molecular weight of EPS-S11 is 1.68 × 107 Da and is composed of glucose, mannose, galactose, and glucuronic acid with a molar ratio of 0.92:0.87:1:0.24 [105,106]. The zeta potential of casein solution dose-dependently increased concomitantly with the particle size by adding EPS-S11. However, the turbidity of the casein solution decreased in a dose-dependent manner, which indicates that this EPS inhibits casein aggregation that might enhance the stability. The average particle size and turbidity of the complex formed by sodium caseinate affected, as mentioned, the amount of EPS produced from probiotic bacterium, which increases with an increase in EPS content [107]. In this process, by comparing the particle size analysis and turbidity, there is a dose-dependent increase in the zeta potential of sodium caseinate solution by increasing the amount of EPS-S11 in the solution. These results showed that the sodium caseinate stability is enhanced when there is an interaction between EPS-S11 and sodium caseinate. It is known that when the pH value is lowered, the isoelectric point is held and there is an interaction exhibited by sodium caseinate that has a positive charge with a negative charge EPS-S11 [24]. Thus, we can conclude that EPS-S11 produced by L. paracasei H9 plays a crucial role in finalizing yogurt texture [24,108]
EPS from LAB are known as “natural bio-thickeners” in dairy product development [109]. EPS produced by yogurt starter cultures could affect the texture of yogurt and improve sensory characteristics such as mouthfeel, shininess, clean-cut, ropiness, and creaminess. Furthermore, the yogurt cultures producing EPS may decrease the extent of syneresis (lower whey separation) [110]. Another study [111] found that the Streptococcus thermophilus zlw TM11 strain showed the highest EPS content and viscosity out of 19 isolated strains from Chinese traditional dairy products. The combination of Streptococcus thermophilus zlw TM11 and Lactobacillus delbrueckii subsp. bulgaricus 3 4.5 was used as a starter culture for yogurt and compared with the commercial starter cultures. The results showed that this novel starter combination had the lowest syneresis (8.5%) as compared to the commercial starter cultures. Moreover, the yogurt had a better texture and sensory profile. Non-fat yogurts produced with different ropy EPS-producing co-cultures, i.e., Lactobacillus delbrueckii subsp. bulgaricus LTM, Streptococcus thermophilus S3.3, and Lactobacillus delbrueckii subsp. bulgaricus H+-ATPase-defective mutant L6 expressed different rheological properties under different fermentation conditions. Non-fat yogurt fermented with mutant L6 had a higher water-holding capacity and pH compared to those produced with the LTM strain; moreover, mutant strains improved the textural properties of non-fat yogurt [112].
6. Lactobacillus EPS as an Immunomodulatory Agent
A dairy-based viscous and carbonated drink “kefir” is becoming popular around the globe [113]. Some LAB spp. can generate EPS, which are extracted from Tibetan kefir grains. Among these, some strains produce the highest quality EPS, named: L. lactis subsp. lactis LZ-R-12 (having the highest EPS yield), L. helveticus LZ-R-5, and L pentosus LZ-R-17 [26]. You and colleagues reported that L. pentosus LZ-R-17 can isolate EPS (R-17-EPS) from fermented milk, after which it is refined by anion exchange chromatography.
Kefir is known for its remarkable properties, such as lowering cholesterol, which proved to be beneficial in immunomodulatory functions, its antitumor and antimicrobial roles, and positive effects on gut microbiota [36,114]. Another study [115] reported that immunomodulating EPS performed a dual role. It regulates macrophages’ immune functions without any side-effects [70].
Cytotoxicity of R-17-EPS was assessed using RAW264.7 cells that gave the potential of control as 100% by comparing with the blank sample. When the concentration of R-17-EPS was kept at 50–400 µg/mL, it increased the feasibility of RAW264.7 macrophage cells in a dose-dependent manner (Pb 0.05); hence, it was found that R-17-EPS exhibited no cytotoxicity [26]. Also, the compositional structure of R-17-EPS was researched, which was →2)-α-D-Galp-(1→ and →4)-β-D-Glcp-(1→ residue having a molar ratio of 1.00:3.12, respectively, which shows a powerful impact on immunomodulatory activity. Thus, R-17-EPS can be employed as an immunomodulator in functional foods, due to chain conformation and specific glycosidic bonds [116]. Moreover, future research should be carried out to maximize the industrial applications of Tibetan kefir, which comprise in vivo experiments, to highlight its therapeutic applications.
A grain-based fermented food, tarhana, contains yogurt as an ingredient and is rich in microflora formed by yeast and LAB spp, which have beneficial impacts on gut microbiota [117]. Furthermore, a recent study [118] evaluated the ropy EPSs present in tarhana produced from six L. plantarum strains. The presence of sucrose and maltose significantly enhanced the production of ropy exopolysaccharide. The acidic environment positively influenced the viscosity of ropy EPS. PFC311E showed viscosity at neutral pH. Overall, ropy EPS production was between 120 and 400 mg/L. However, the L. plantarum strain PFC311 produced the highest level of ropy EPS. Additionally, there was degradation of ropy EPS at >300 °C, except for PFC311E, which degenerated at 295.7 °C.
Soybean whey, as a byproduct produced of L. plantarum W1 named EPS-W1 marked by phenylalanyl-tRNA sequencing method using a set of primers for PCR amplification, shows remarkable properties in production aspects, such as in functional foods, food industries, and biomedical applications [119]. Some other LAB spp. is also indicated for use in food industries, e.g., L. fermentum Lf2 and L. gasseri FR4 [49,120]. Moreover, L. brevis E25 extracts EPS solutions in concentrations of 0%, 20%, and 40% to enhance its adhesiveness on food surfaces [21].
EPSs produced by L. vaginalis SHA110 (EPS-lvg) and L. reuteri SHA101 (EPS-lr) separated from the cecum of hens have a significant role in anticancer activity against Caco-2 cancer cells [43]. He investigated that the water solubility, emulsifying activity, and water-holding capacity of EPS-lvg and EPS-lr were used in food industries. The functional characteristics of both EPSs enable food scientists to consider it a good source in terms of bio-absorbents, food hydrocolloids, heavy metal sequestrating agents, and an effective drug delivery agent. The in vitro mechanism reveals the scavenging activity and reducing the power of EPS-lvg and EPS-Ir on hydroxyl, DPPH, and superoxide anion radicals, which shows promising returns in the food industry as an antioxidant [121].
7. Conclusions and Future Perspectives
There are many examples of the use of Lactobacillus EPS, including dairy, meat, non-dairy, and bakery industry applications. In dairy foods, Lactobacillus EPS are primarily used as thickeners and texturizers to increase the viscosity of products. They may also act as stabilizers by binding water, decreasing syneresis, and interacting with various milk constituents. As for plant-derived fermented foods, yogurt-like beverages are increasingly produced with EPS-synthesizing Lactobacillus strains to enhance the texture, organoleptic properties, and storage stability of these plant-based products. The positive effect of EPS on baked goods is based on their ability to bind water and form a network with different dough components. Thus, EPS may improve the rheology, structure, and volume of bread, resulting in decreased staling rates and an extended shelf life.
EPS plays a major role in maintaining technological aspects such as texture and flavor enhancements, improved stability, increased shelf life, and rheological properties. EPS-producing LAB spp. is superior because of the improved functional and technical quality of their products. Novel EPS shows immunomodulation, antioxidant activity, emulsifying activity, and radical-scavenging activity.
Although lactic acid bacteria can typically synthesize small amounts of EPS, having many benefits to using them, the main problem is that the biopolymer production capacity of strains varies greatly in quantity and quality. This problem can be eliminated by carefully selecting the voltage and optimizing the production parameters. The type and quantity of EPS and the optimal growth conditions of the microorganisms used should be in line with the specific production process and characteristics of the food. A better understanding of EPS’ structural capabilities in the food industry is a challenge to further improve their application.
Author Contributions
Conceptualization, A.B., Y.X. and M.S.R.R., draft preparation: A.B., Y.X., M.S.R.R. and H.M.M.; tables and figures preparation, E.R., M.U. and M.S.; writing—review and editing, Y.X., M.S.R.R., H.M.M., M.K.I.K., E.R. and R.M.A.; supervision, M.K.I.K., E.R. and R.M.A. All authors contributed equally to the original drafting, editing, and finalizing of the review paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the University of Agriculture, Faisalabad, Pakistan for their support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation List
| EPS | exopolysaccharide |
| LAB | lactic acid bacteria |
| GRAS | Generally Recognized as Safe |
| HePS | hetero-polysaccharides |
| HoPS | homo-polysaccharides |
| ABTS | 2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulphonic acid) |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ACE | Angiotensin-converting enzyme |
References
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- Shoukat, M.; Sorrentino, A. Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 2021, 56, 2088–2097. [Google Scholar] [CrossRef]
- Aponte, M.; Murru, N.; Shoukat, M. Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current Perspective. Front. Microbiol. 2020, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Jiménez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbés, T. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014, 05, 1765–1775. [Google Scholar] [CrossRef] [Green Version]
- Oleksy-Sobczak, M.; Klewicka, E. Optimization of Media Composition to Maximize the Yield of Exopolysaccharides Production by Lactobacillus rhamnosus Strains. Prob. Antimicrob. Proteins 2020, 12, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Widyastuti, Y.; Rohmatussolihat; Febrisiantosa, A. The Role of Lactic Acid Bacteria in Milk Fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Abedfar, A.; Hosseininezhad, M.; Sadeghi, A.; Raeisi, M.; Feizy, J. Investigation on “spontaneous fermentation” and the productivity of microbial exopolysaccharides by Lactobacillus plantarum and Pediococcus pentosaceus isolated from wheat bran sourdough. LWT 2018, 96, 686–693. [Google Scholar] [CrossRef]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Y.; Tian, J.; Zhou, J.; Xu, Q.; Feng, L.; Rui, X.; Fan, X.; Zhang, Q.; Chen, X.; et al. Influences of drying methods on the structural, physicochemical and antioxidant properties of exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 157, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, M.; Feng, L.; Liang, X.; Song, X.; Mu, G.; Tuo, Y.; Jiang, S.; Qian, F. Lactobacillus plantarum-12 Exopolysaccharides Have Anti-Biofilm Activity Against Shigella flexneri. Appl. Environ. Microbiol. 2020, 86, e000694-20. [Google Scholar] [CrossRef]
- Li, C.; Ding, J.; Chen, D.; Shi, Z.; Wang, L. Bioconversion of cheese whey into a hetero-exopolysaccharide via a one-step bi-oprocess and its applications. Biochem. Eng. J. 2020, 161, 107701. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef]
- Soumya, M.; Sasikumar, K.; Pandey, A.; Nampoothiri, K.M. Cassava starch hydrolysate as sustainable carbon source for exopolysaccharide production by Lactobacillus plantarum. Bioresour. Technol. Rep. 2019, 6, 85–88. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A. Functional and technological properties of exopolysaccharide producing autochthonous Lactobacillus plantarum strain AAS3 from dry fish based fermented food. LWT 2019, 114, 108387. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, T.; Zhao, X.; Hao, X.; Yang, Z. Interaction of exopolysaccharide produced by Lactobacillus plantarum YW11 with whey proteins and functionalities of the polymer complex. J. Food Sci. 2020, 85, 4141–4151. [Google Scholar] [CrossRef]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and antioxidant activity of an acidic exopoly-saccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.; Wu, F.; Jin, Y.; Xu, X.; Gänzle, M.G. Comparison of the Functionality of Exopolysaccharides Produced by Sourdough Lactic Acid Bacteria in Bread and Steamed Bread. J. Agric. Food Chem. 2020, 68, 8907–8914. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Zhang, H.; Ashraf, M.; Fang, H.; Zeng, X.; Wu, Y.; Khurshid, M.; Zhao, L.; He, Z. Antibacterial and antioxidant activity of exopolysaccharide mediated silver nanoparticle synthesized by Lactobacillus brevis isolated from Chinese koumiss. Colloids Surfaces B Biointerfaces 2020, 186, 110734. [Google Scholar] [CrossRef] [PubMed]
- Houssam, A.; Yahya, R.; Reda, B.; Nabil, G.; Salwa, K.; Milena, B.; Riadh, B.S.; Chihib, N.E.; Ennouamane, S.; Abdeslam, A. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Prob. Antimicrob. Proteins 2020, 12, 683–696. [Google Scholar]
- Karasu, E.N.; Ermis, E. Determination of the effect of exopolysaccharide (EPS) from Lactobacillus brevis E25 on adhesion of food powders on the surfaces, using the centrifuge technique. J. Food Eng. 2019, 242, 106–114. [Google Scholar] [CrossRef]
- Hilbig, J.; Loeffler, M.; Herrmann, K.; Weiss, J. Application of exopolysaccharide-forming lactic acid bacteria in cooked ham model systems. Food Res. Int. 2019, 119, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Malaka, R.; Maruddin, F.; Dwyana, Z.; Vargas, M.V. Assessment of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus ropy strain in different substrate media. Food Sci. Nutr. 2020, 8, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Lv, S.; Shi, T.-T.; Liu, K.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Luo, J.-P. Exopolysaccharides from yoghurt fermented by Lactobacillus paracasei: Production, purification and its binding to sodium caseinate. Food Hydrocoll. 2020, 102, 105635. [Google Scholar] [CrossRef]
- Saif, F.A.A.; Sakr, E.A. Characterization and bioactivities of exopolysaccharide produced from probiotic Lactobacillus plantarum 47FE and Lactobacillus pentosus 68FE. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100231. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhao, X.; Ma, K.; Chen, X.; Zhang, C.; Wang, G.; Dong, M.; Rui, X.; Zhang, Q.; et al. Isolation, purification, characterization and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan kefir. Int. J. Biol. Macromol. 2020, 158, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Prob. Antimicrob. Proteins 2019, 11, 1132–1142. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef]
- Do, T.B.T.; Tran, T.A.L.; Tran, T.V.T.; Le, T.H.; Jayasena, V.; Nguyen, T.H.C.; Nguyen, C.C.; Kim, S.Y.; Le, Q.V. Novel Exopolysaccharide Produced from Fermented Bamboo Shoot-Isolated Lactobacillus Fermentum. Polymers 2020, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Sheng, S.; Hu, Z.; Liu, Y.; Li, J.; Zhang, H.; Liang, Y.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus kiferi as adjuvant enhanced the immuno-protective against Staphylococcus aureus infection. Int. J. Biol. Macromol. 2020, 161, 10–23. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A novel purified Lactobacillus acidophilus 20079 exopolysac-charide, LA-EPS-20079, molecularly regulates both apoptotic and NF-ΚB inflammatory pathways in human colon cancer. Microb. Cell Factories 2018, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Gangoiti, J.; Bai, Y.; Pijning, T.; Van Leeuwen, S.S.; Dijkhuizen, L. Structure-function relationships of family GH70 glucansucrase and 4,6-α-glucanotransferase enzymes, and their evolutionary relationships with family GH13 enzymes. Cell. Mol. Life Sci. 2016, 73, 2681–2706. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Guo, Q.; Zhang, H.; Wu, Y.; Hang, X.; Ai, L. Exopolysaccharide produced by Streptococcus thermophiles S-3: Molecular, partial structural and rheological properties. Carbohydr. Polym. 2018, 194, 132–138. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Reyes-Gavilán, C.D.L. Invited Review: Methods for the Screening, Isolation, and Characterization of Exopolysaccharides Produced by Lactic Acid Bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Tapia, J.M.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Malki, M.; Ballester, A. Extraction of extracellular polymeric substances from the acidophilic bacterium Acidiphilium 3.2Sup(5). Water Sci. Technol. 2009, 59, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Liu, J.; Zhang, D.; Chen, X.; Song, W.; Wu, F. Binding of dicamba to soluble and bound extracellular polymeric substances (EPS) from aerobic activated sludge: A fluorescence quenching study. J. Colloid Interface Sci. 2010, 345, 442–447. [Google Scholar] [CrossRef]
- Li, D.; Xi, H. Layered Extraction and Adsorption Performance of Extracellular Polymeric Substances from Activated Sludge in the Enhanced Biological Phosphorus Removal Process. Molecules 2019, 24, 3358. [Google Scholar] [CrossRef] [Green Version]
- Sheng, G.-P.; Yu, H.-Q.; Yu, Z. Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Appl. Microbiol. Biotechnol. 2005, 67, 125–130. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and complexation properties of Pb and Cd with EPS: Part II. Consequences of EPS extraction methods on Pb2+ and Cd2+ complexation. Enzym. Microb. Technol. 2006, 38, 246–252. [Google Scholar] [CrossRef]
- Bernal, P.; Llamas, M.A. Promising biotechnological applications of antibiofilm exopolysaccharides. Microb. Biotechnol. 2012, 5, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Rajoka, M.S.R.; Jin, M.; Haobin, Z.; Li, Q.; Shao, D.; Jiang, C.; Huang, Q.; Yang, H.; Shi, J.; Hussain, N. Functional characterization and biotechnological potential of exopolysaccharide produced by Lactobacillus rhamnosus strains isolated from human breast milk. LWT 2018, 89, 638–647. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.; Zhao, L. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar] [CrossRef]
- Ciszek-Lenda, M.; Nowak, B.; Sróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopoly-saccharide from Lactobacillus rhamnosus KL37: Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bac-teria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Vahed, S.Z.; Barzegari, A.; Saadat, Y.R.; Goreyshi, A.; Omidi, Y. Leuconostoc mesenteroides-derived anticancer pharmaceu-ticals hinder inflammation and cell survival in colon cancer cells by modulating NF-κB/AKT/PTEN/MAPK pathways. Biomed. Pharmacother. 2017, 94, 1094–1100. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Ale, E.C.; Perezlindo, M.J.; Pavón, Y.; Peralta, G.H.; Costa, S.; Sabbag, N.; Bergamini, C.; Reinheimer, J.A.; Binetti, A.G. Technological, rheological and sensory characterizations of a yogurt containing an exopolysaccharide extract from Lactobacillus fermentum Lf2, a new food additive. Food Res. Int. 2016, 90, 259–267. [Google Scholar] [CrossRef]
- Hussain, A.; Zia, K.M.; Tabasum, S.; Noreen, A.; Ali, M.; Iqbal, R.; Zuber, M. Blends and composites of exopolysaccharides; properties and applications: A review. Int. J. Biol. Macromol. 2017, 94, 10–27. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dertli, E.; Colquhoun, I.J.; Côté, G.L.; Le Gall, G.; Narbad, A. Structural analysis of the α-d-glucan produced by the sourdough isolate Lactobacillus brevis E25. Food Chem. 2018, 242, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Dertli, E.; Colquhoun, I.J.; Gunning, A.P.; Bongaerts, R.J.; Le Gall, G.; Bonev, B.B.; Mayer, M.J.; Narbad, A. Structure and bio-synthesis of two exopolysaccharides produced by Lactobacillus johnsonii FI9785. J. Biol. Chem. 2013, 288, 31938–31951. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Liu, H.; Liu, S.; Zhang, X.; Chen, P.; Li, W.; Xu, X.; Tian, W. Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9. Molecules 2018, 23, 2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, purification and characterization of exopolysaccharide produced by Leuconostoc pseudomesenteroides YF32 from soybean paste. Int. J. Biol. Macromol. 2018, 114, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ispirli, H.; Sagdic, O.; Yılmaz, M.T.; Dertli, E. Physicochemical characterisation of an α-glucan from Lactobacillus reuteri E81 as a potential exopolysaccharide suitable for food applications. Process. Biochem. 2019, 79, 91–96. [Google Scholar] [CrossRef]
- Siddiqui, N.N.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A. Structural analysis and characterization of dextran produced by wild and mutant strains of Leuconostoc mesenteroides. Carbohydr. Polym. 2014, 99, 331–338. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Kralj, S.; Piqué, A.V.; Leemhuis, H.; Van Der Maarel, M.J.E.C.; Dijkhuizen, L. Inulin and levan synthesis by probiotic Lactobacillus gasseri strains: Characterization of three novel fructansucrase enzymes and their fructan products. Microbiology 2010, 156, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [Green Version]
- Cuthbertson, L.; Mainprize, I.L.; Naismith, J.H.; Whitfield, C. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Gholamhosseinpour, A. Effect of ultrasonication treatment and fermentation by probiotic Lactobacillus plantarum strains on goat milk bioactivities. Int. J. Food Sci. Technol. 2020, 55, 2642–2649. [Google Scholar] [CrossRef]
- AL Dhaheri, A.; Al-Hemeiri, R.; Kizhakkayil, J.; Al-Nabulsi, A.; Abushelaibi, A.; Shah, N.P.; Ayyash, M. Health-promoting benefits of low-fat akawi cheese made by exopolysaccharide-producing probiotic Lactobacillus plantarum isolated from camel milk. J. Dairy Sci. 2017, 100, 7771–7779. [Google Scholar] [CrossRef]
- Almalki, M.A. Exopolysaccharide production by a new Lactobacillus lactis isolated from the fermented milk and its antiox-idant properties. J. King Saud Univ.-Sci. 2020, 32, 1272–1277. [Google Scholar] [CrossRef]
- Dohnalkova, A.C.; Marshall, M.J.; Arey, B.W.; Williams, K.H.; Buck, E.C.; Fredrickson, J.K. Imaging hydrated microbial ex-tracellular polymers: Comparative analysis by electron microscopy. Appl. Environ. Microbiol. 2011, 77, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachtarzi, N.; Speciale, I.; Kharroub, K.; De Castro, C.; Ruiz, L.; Ruas-Madiedo, P. Selection of Exopolysaccharide-Producing Lactobacillus Plantarum (Lactiplantibacillus Plantarum) Isolated from Algerian Fermented Foods for the Manufacture of Skim-Milk Fermented Products. Microorganisms 2020, 8, 1101. [Google Scholar] [CrossRef]
- Surber, G.; Mende, S.; Jaros, D.; Rohm, H. Clustering of Streptococcus thermophilus Strains to Establish a Relation between Exopolysaccharide Characteristics and Gel Properties of Acidified Milk. Foods 2019, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on ex-opolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Iraporda, C.; Acurcio, L.B.; de Cicco Sandes, S.H.; Costa, K.; Guimarães, G.M.; Arantes, R.M.E.; Neumann, E.; Nunes, Á.C.; Nicoli, J.R. Physicochemical, immunomodulatory and safety aspects of milks fermented with Lactobacillus paracasei isolated from kefir. Food Res. Int. 2019, 123, 48–55. [Google Scholar] [CrossRef]
- Zavala, L.; Golowczyc, M.; Van Hoorde, K.; Medrano, M.; Huys, G.; Vandamme, P.; Abraham, A. Selected Lactobacillus strains isolated from sugary and milk kefir reduce Salmonella infection of epithelial cells in vitro. Benef. Microbes 2016, 7, 585–595. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Yang, Z. Manufacture of low-fat Cheddar cheese by exopolysaccharide-producing Lactobacillus plantarum JLK0142 and its functional properties. J. Dairy Sci. 2019, 102, 3825–3838. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Geiger, B.; Nguyen, H.-M.; Wenig, S.; Lorenz, C.; Kittl, R.; Mathiesen, G.; Eijsink, V.G.; Haltrich, D.; Nguyen, T.-H. From by-product to valuable components: Efficient enzymatic conversion of lactose in whey using β-galactosidase from Streptococcus thermophilus. Biochem. Eng. J. 2016, 116, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhou, L.; Yang, H.; Lv, R.; Tian, P.; Li, X.; Zhang, Y.; Chen, Z.; Lin, F. Self-Assembled Exopolysaccharide Nanoparticles for Bioremediation and Green Synthesis of Noble Metal Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 22808–22818. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Dineshkumar, K.; Yang, Y.-H. Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit. Rev. Microbiol. 2017, 43, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, Z.; Chen, C.; Han, J.; Ai, L.; Guo, B. Exopolysaccharides produced by Rhizobium radiobacter S10 in whey and their rheological properties. Food Hydrocoll. 2014, 36, 362–368. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-J.; Chen, Z.; Chen, P.T.; Ng, I.-S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef]
- Valerio, F.; Bavaro, A.R.; Di Biase, M.; Lonigro, S.L.; Logrieco, A.F.; Lavermicocca, P. Effect of Amaranth and Quinoa Flours on Exopolysaccharide Production and Protein Profile of Liquid Sourdough Fermented by Weissella cibaria and Lactobacillus plantarum. Front. Microbiol. 2020, 11, 967. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Garofalo, C.; Belleggia, L.; Maoloni, A.; Cardinali, F.; Mozzon, M.; Foligni, R.; Aquilanti, L.; Clementi, F. Selection of cereal-sourced lactic acid bacteria as candidate starters for the baking industry. PLoS ONE 2020, 15, e0236190. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Galle, S.; Gänzle, M.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide pro-ducing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Yusuf, S. Sodium Intake and Cardiovascular Health. Circ. Res. 2015, 116, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Belz, M.C.; Axel, C.; Arendt, E.K.; Lynch, K.M.; Brosnan, B.; Sheehan, E.M.; Coffey, A.; Zannini, E. Improvement of taste and shelf life of yeasted low-salt bread containing functional sourdoughs using Lactobacillus amylovorus DSM 19280 and Weisella cibaria MG1. Int. J. Food Microbiol. 2019, 302, 69–79. [Google Scholar] [CrossRef]
- Lynch, K.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Dincer, E.; Kivanc, M. Characterization of Lactobacillus plantarum strains isolated from Turkish pastırma and possibility to use of food industry. Food Sci. Technol. 2020, 40, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Rzepkowska, A.; Zielińska, D.; Ołdak, A.; Kolozyn-Krajewska, D. Organic whey as a source of Lactobacillus strains with selected technological and antimicrobial properties. Int. J. Food Sci. Technol. 2017, 52, 1983–1994. [Google Scholar] [CrossRef]
- Sacco, L.P.; Castellane, T.C.L.; Polachini, T.C.; de Macedo Lemos, E.G.; Alves, L.M.C. Exopolysaccharides produced by Pan-doraea shows emulsifying and anti-biofilm activities. J. Polym. Res. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Han, Y.; Zhou, Z. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int. J. Biol. Macromol. 2019, 141, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Malik, S.; Mehwish, H.M.; Ali, M.W.; Hussain, N.; Khurshid, M.; Rajoka, M.S.R.; Chen, Y. Isolation and functional characterization of exopolysaccharide produced by Lactobacillus plantarum S123 isolated from traditional Chinese cheese. Arch. Microbiol. 2021, 203, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Shankar, T.; Palpperumal, S.; Kathiresan, D.; Sankaralingam, S.; Balachandran, C.; Baskar, K.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Biomedical and therapeutic potential of exopolysaccharides by Lactobacillus paracasei isolated from sau-erkraut: Screening and characterization. Saudi J. Biol. Sci. 2021, 28, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide production of Pantoea sp. BCCS 001 GH: Physical characterizations, emulsification, and antioxidant activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Balyan, S.; Devi, P.B.; Shetty, P.H. Evaluation of oil-in-water (O/W) emulsifying properties of galactan exopolysaccharide from Weissella confusa KR780676. J. Food Sci. Technol. 2020, 57, 1579–1585. [Google Scholar] [CrossRef]
- Carrión, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Kao, C.-Y.; Liu, W.-S.; Fang, T.J. Characterization of high exopolysaccharide-producing Lactobacillus strains isolated from mustard pickles for potential probiotic applications. Int. Microbiol. 2017, 20, 75–84. [Google Scholar] [PubMed]
- Amatayakul, T.; Halmos, A.; Sherkat, F.; Shah, N. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 2006, 16, 40–51. [Google Scholar] [CrossRef]
- Madhubasani, G.; Prasanna, P.; Chandrasekara, A.; Gunasekara, D.; Senadeera, P.; Chandramali, D.; Vidanarachchi, J. Exopolysaccharide producing starter cultures positively influence on microbiological, physicochemical, and sensory properties of probiotic goats’ milk set-yoghurt. J. Food Proc. Preserv. 2020, 44, e14361. [Google Scholar] [CrossRef]
- Cartashev, A.; Rudic, V. The effect of starter culture producing exopolysaccharide on physicochemical properties of yoghurt. Chem. J. Mold. 2017, 12, 7–12. [Google Scholar] [CrossRef]
- Yan, S.; Yang, B.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Ropy exopolysaccharide-producing Bifidobacterium longum YS108R as a starter culture for fermented milk. Int. J. Food Sci. Technol. 2018, 54, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.L.; Huang, J.Y.; Kao, C.Y.; Fang, T.J. Fermented soymilk and soy and cow milk mixture, supplemented with orange peel fiber or Tremella flava fermented powder as prebiotics for high exopolysaccharide-producing Lactobacillus pentosus SLC 13. J. Sci. Food Agric. 2019, 99, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xia, Y.; Wang, G.; Zhang, H.; Zhu, S.; Ai, L. Bioactive exopolysaccharides from a S. thermophilus strain: Screening, purification and characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, W.; Guo, Q.; Xiong, Z.-Q.; Wang, G.; Xia, Y.; Lai, P.; Yin, B.; Ai, L. Characterization of a yogurt-quality improving exopolysaccharide from Streptococcus thermophilus AR333. Food Hydrocoll. 2018, 81, 220–228. [Google Scholar] [CrossRef]
- Abid, Y.; Joulak, I.; Ben Amara, C.; Casillo, A.; Attia, H.; Gharsallaoui, A.; Azabou, S. Study of interactions between anionic exopolysaccharides produced by newly isolated probiotic bacteria and sodium caseinate. Colloids Surfaces B Biointerfaces 2018, 167, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Ipsen, R.; Janzen, T.; Qvist, K. Microstructure and Rheology of Yogurt Made with Cultures Differing Only in Their Ability to Produce Exopolysaccharides. J. Dairy Sci. 2003, 86, 1632–1638. [Google Scholar] [CrossRef]
- Jaiswal, P.; Sharma, R.; Sanodiya, B.S.; Bisen, P.S. Microbial Exopolysaccharides: Natural Modulators of Dairy Products. J. Appl. Pharm. Sci. 2014, 4, 105–109. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Sezgin, E.; Seydim, A.C. Influences of exopolysaccharide producing cultures on the quality of plain set type yogurt. Food Control. 2005, 16, 205–209. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Improvement of the Texture of Yogurt by Use of Exopolysaccharide Producing Lactic Acid Bacteria. BioMed Res. Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Liu, J. Rheological and physical characteristics of non-fat set yogurt prepared with EPS-producing Streptococcus thermophilus and an H+-ATPase-defective mutant Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Food Prop. 2017, 20, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Shi, J.; Yang, X.; Liu, Y.; Nan, B.; Wang, Z. Isolation of exopolysaccharide-producing bacteria and yeasts from Tibetan kefir and characterisation of the exopolysaccharides. Int. J. Dairy Technol. 2016, 69, 410–417. [Google Scholar] [CrossRef]
- Abid, Y.; Casillo, A.; Gharsallah, H.; Joulak, I.; Lanzetta, R.; Corsaro, M.M.; Attia, H.; Azabou, S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int. J. Biol. Macromol. 2018, 108, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Abid, Y.; Azabou, S.; Joulak, I.; Casillo, A.; Lanzetta, R.; Corsaro, M.M.; Gharsallaoui, A.; Attia, H. Potential biotechnological properties of an exopolysaccharide produced by newly isolated Bacillus tequilensis-GM from spontaneously fermented goat milk. LWT 2019, 105, 135–141. [Google Scholar] [CrossRef]
- Lee, J.; Li, C.; Surayot, U.; Yelithao, K.; Lee, S.; Park, W.; Tabarsa, M.; You, S. Molecular structures, chemical properties and biological activities of polysaccharide from Smilax glabra rhizome. Int. J. Biol. Macromol. 2018, 120, 1726–1733. [Google Scholar] [CrossRef]
- Özel, S.; Sabanoğlu, S.; Çon, A.H.; Şimşek, Ö. Diversity and Stability of Yeast Species during the Fermentation of Tarhana. Food Biotechnol. 2015, 29, 117–129. [Google Scholar] [CrossRef]
- Şentürk, D.Z.; Dertli, E.; Erten, H.; Şimşek, Ö. Structural and technological characterization of ropy exopolysaccharides produced by Lactobacillus plantarum strains isolated from Tarhana. Food Sci. Biotechnol. 2019, 29, 121–129. [Google Scholar] [CrossRef]
- Do, T.B.T.; Tran, B.K.; Tran, T.V.T.; Le, T.H.; Cnockaert, M.; Vandamme, P.; Nguyen, T.H.C.; Nguyen, C.C.; Hong, S.H.; Kim, S.Y.; et al. Decoding the Capability of Lactobacillus plantarum W1 Isolated from Soybean Whey in Producing an Exopolysaccharide. ACS Omega 2020, 5, 33387–33394. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Ravindran, A.D. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).