Changes in the Chemical, Technological, and Microbiological Properties of Kefir-Fermented Soymilk after Supplementation with Inulin and Acrocomia aculeata Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetable Material and Kefir Preparation
2.2. Kefir-Fermented Soymilk Characterization
2.3. Chemical Composition
2.4. Technological Characterization
2.5. Viability of Kefir Microorganism
2.6. Statistical Analysis
3. Results and Discussion
3.1. Bocaiúva Powder-Pulp Characterization
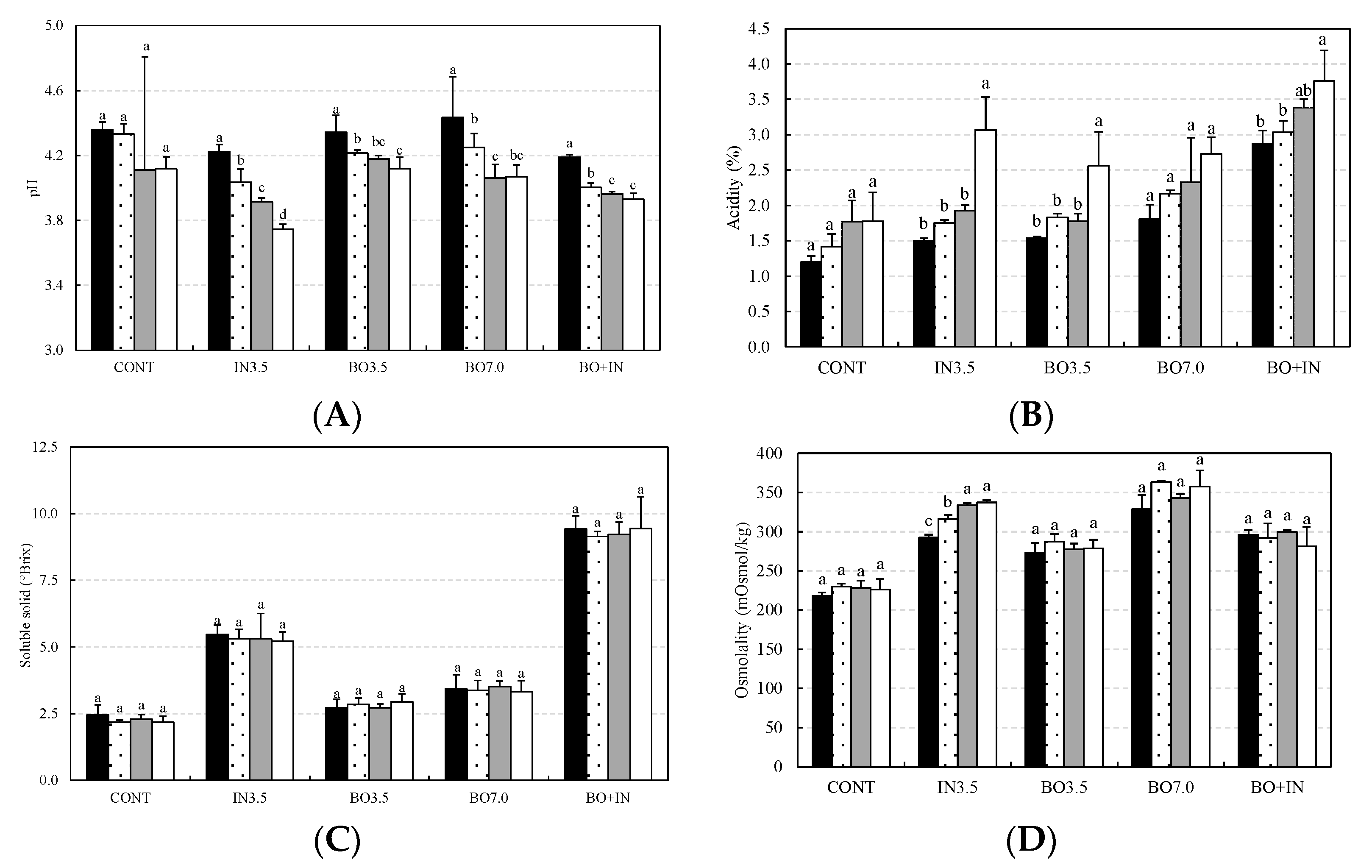
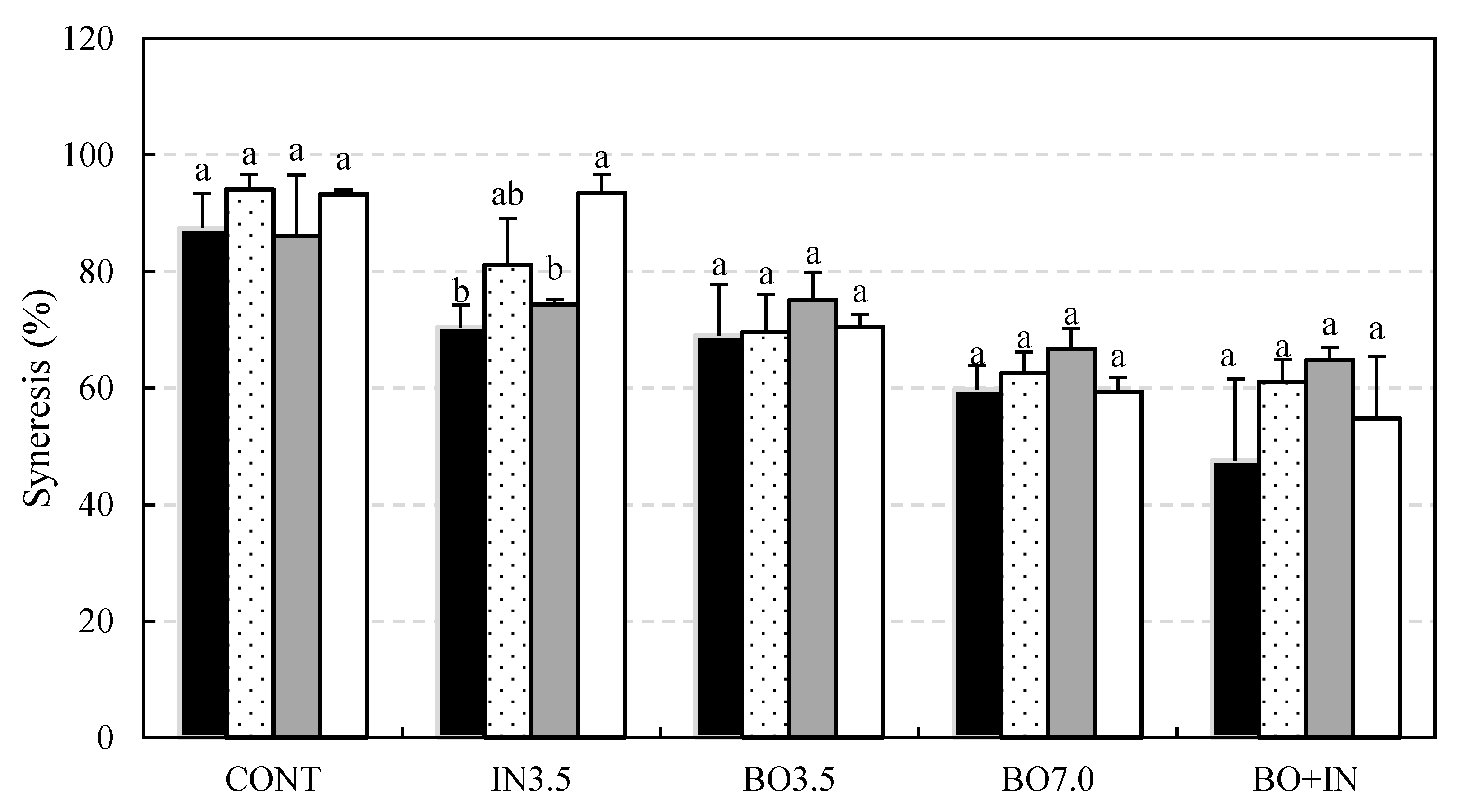
3.2. Chemical and Technological Characterization and Microbiological Viability of the Fermented Beverages during Storage
3.3. Bioactive Compounds in Kefir-Fermented Beverages Supplemented with Inulin and/or Bocaiúva Powder-Pulp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Damasco, G.; Fontes, C.; Françoso, R.; Haidar, R. The Cerrado biome: A forgotten biodiversity hotspot. Front. Young Minds 2018, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, G.d.C.; Schlindwein, M.M.; Padovan, M.P.; Vogel, E.; Ruviaro, C.F. Environmental performance of agroforestry systems in the Cerrado biome, Brazil. World Dev. 2019, 122, 339–348. [Google Scholar] [CrossRef]
- Bortolotto, I.M.; Hiane, P.A.; Ishii, I.H.; de Souza, P.R.; Campos, R.P.; Juraci Bastos Gomes, R.; Farias, C.d.S.; Leme, F.M.; de Oliveira Arruda, R.d.C.; de Lima Corrêa da Costa, L.B.; et al. A knowledge network to promote the use and valorization of wild food plants in the Pantanal and Cerrado, Brazil. Reg. Environ. Chang. 2017, 17, 1329–1341. [Google Scholar] [CrossRef]
- Bailão, E.F.L.C.; Devilla, I.A.; Da Conceição, E.C.; Borges, L.L. Bioactive compounds found in Brazilian Cerrado fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef]
- Coimbra, M.; Jorge, N. Proximate composition of guariroba (Syagrus oleracea), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata) palm fruits. Food Res. Int. 2011, 44, 2139–2142. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.; Laviola, B.G.; Santos, G.S.; de Sousa, H.U.; de Oliveira, H.B.; Veras, L.C.; Ciannella, R.; Favaro, S.P. Opportunities and challenges for sustainable production of A. aculeata through agroforestry systems. Ind. Crops Prod. 2017, 107, 573–580. [Google Scholar] [CrossRef]
- Coimbra, M.; Jorge, N. Characterization of the Pulp and Kernel Oils from Syagrus oleracea, Syagrus romanzoffiana, and Acrocomia aculeata. J. Food Sci. 2011, 76, C1156–C1161. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Fatty acids and bioactive compounds of the pulps and kernels of Brazilian palm species, guariroba (Syagrus oleraces), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata). J. Sci. Food Agric. 2012, 92, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.; Nieminen, L.; Blasbalg, T.; Riggs, J.; Lands, W. Healthy intakes of n-3 and n–6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83, 1483S–1493S. [Google Scholar] [CrossRef] [PubMed]
- Temme, E.; Mensink, R.; Hornstra, G. Comparison of the effects of diets enriched in lauric, palmitic, or oleic acids on serum lipids and lipoproteins in healthy women and men. Am. J. Clin. Nutr. 1996, 63, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, C.A.L.; Machado, M.E.; Caramão, E.B. Characterization of bio-oils obtained from pyrolysis of bocaiuva residues. Renew. Energy 2016, 91, 21–31. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- Kumari, A.; Angmo, K.; Monika, S.; Bhalla, T. Functional and technological application of probiotic L. casei PLA5 in fermented soymilk. Int. Food Res. J. 2018, 25, 2164–2172. [Google Scholar]
- Albuquerque, M.; Bedani, R.; LeBlanc, J.; Saad, S. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int. J. Food Microbiol. 2017, 261, 35–41. [Google Scholar] [CrossRef]
- de Moraes Filho, M.; Busanello, M.; Prudencio, S.; Garcia, S. Soymilk with okara flour fermented by Lactobacillus acidophilus: Simplex-centroid mixture design applied in the elaboration of probiotic creamy sauce and storage stability. LWT 2018, 93, 339–345. [Google Scholar] [CrossRef]
- Karaçalı, R.; Özdemİr, N.; Çon, A. Aromatic and functional aspects of kefir produced using soya milk and Bifidobacterium species. Int. J. Dairy Technol. 2018, 71, 921–933. [Google Scholar] [CrossRef]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Wang, Z.; Li, X.; Zhou, J. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Egea, M.; Santos, D.d.; Oliveira Filho, J.d.; Ores, J.d.C.; Takeuchi, K.; Lemes, A. A review of nondairy kefir products: Their characteristics and potential human health benefits. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fiorda, F.A.; Pereira, G.V.d.M.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; Vandenberghe, L.P.S.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Inhibitory Power of Kefir: The Role of Organic Acids. J. Food Prot. 2000, 63, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Biotechnological innovations in kefir production: A review. Br. Food J. 2008, 110, 283–295. [Google Scholar] [CrossRef]
- Satir, G.; Guzel-Seydim, Z.B. How kefir fermentation can affect product composition? Small Rumin. Res. 2016, 134, 1–7. [Google Scholar] [CrossRef]
- Baú, T.; Garcia, S.; Ida, E. Changes in soymilk during fermentation with kefir culture: Oligosaccharides hydrolysis and isoflavone aglycone production. Int. J. Food Sci. Nutr. 2015, 66, 845–850. [Google Scholar] [CrossRef]
- Baú, T.R.; Garcia, S.; Ida, E.I. Evaluation of a functional soy product with addition of soy fiber and fermented with probiotic kefir culture. Braz. Arch. Biol. Technol. 2014, 57, 402–409. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, D.C.; de Oliveira Filho, J.G.; Santana, A.C.A.; de Freitas, B.S.M.; Silva, F.G.; Takeuchi, K.P.; Egea, M.B. Optimization of soymilk fermentation with kefir and the addition of inulin: Physicochemical, sensory and technological characteristics. LWT 2019, 104, 30–37. [Google Scholar] [CrossRef]
- Kundu, P.; Dhankhar, J.; Sharma, A. Development of non dairy milk alternative using soymilk and almond milk. Curr. Res. Nutr. Food Sci. J. 2018, 6, 203–210. [Google Scholar] [CrossRef]
- Silva, C.F.G.d.; Santos, F.L.; Santana, L.R.R.d.; Silva, M.V.L.; Conceição, T.d.A. Development and characterization of a soymilk Kefir-based functional beverage. Food Sci. Technol. 2018, 38, 543–550. [Google Scholar] [CrossRef] [Green Version]
- da Silva Fernandes, M.; Lima, F.; Rodrigues, D.; Handa, C.; Guelfi, M.; Garcia, S.; Ida, E. Evaluation of the isoflavone and total phenolic contents of kefir-fermented soymilk storage and after the in vitro digestive system simulation. Food Chem. 2017, 229, 373–380. [Google Scholar] [CrossRef]
- Yang, M.; Yang, X.; Chen, X.; Wang, J.; Liao, Z.; Wang, L.; Zhong, Q.; Fang, X. Effect of Kefir on Soybean Isoflavone Aglycone Content in Soymilk Kefir. Front. Nutr. 2020, 7, 587665. [Google Scholar] [CrossRef] [PubMed]
- Tiss, M.; Souiy, Z.; ben Abdeljelil, N.; Njima, M.; Achour, L.; Hamden, K. Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and Liver-Kidney toxicities in HFFD-rats. J. Funct. Foods 2020, 67, 103869. [Google Scholar] [CrossRef]
- Alves, V.; Scapini, T.; Camargo, A.; Bonatto, C.; Stefanski, F.; de Jesus, E.; Diniz, L.; Bertan, L.; Maldonado, R.; Treichel, H. Development of fermented beverage with water kefir in water-soluble coconut extract (Cocos nucifera L.) with inulin addition. LWT 2021, 145, 111364. [Google Scholar] [CrossRef]
- Mitmesser, S.; Combs, M. Prebiotics: Inulin and other oligosaccharides. In The Microbiota in Gastrointestinal Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 201–208. [Google Scholar]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Masson, M.L.; Ribeiro, J.C.B. Sensory acceptability and physical stability evaluation of a prebiotic soy-based dessert developed with passion fruit juice. Food Sci. Technol. 2012, 32, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Resolução 2 de janeiro de 2002. Aprova o regulamento técnico de substâncias bioativas e probióticos isolados com reivindicação de propriedades funcionais e ou sanitárias (Approves the Technical Regulation of Bioactive Substances and Isolated Probiotics with Claim of Functional and or Health Properties); Resolution no 2, January 2, 2002; Nacional de Vigilância Sanitária, A., Ed.; DOU: Brasília, Brasil, 2002. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Benassi, M.; Antunes, A. A comparison of metaphosphoric and oxalic acids as extractans solutions for the determination of vitamin C in selected vegetables. Arquivos Biol. Tecnol. 1988, 31, 507–513. [Google Scholar]
- Rodriguez-Amaya, D. A Guide to Carotenoid Analysis Foods; ILSI press: Washington, DC, USA, 2001. [Google Scholar]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Aryana, K. Folic acid fortified fat-free plain set yoghurt. Int. J. Dairy Technol. 2003, 56, 219–222. [Google Scholar] [CrossRef]
- Musara, C.; Pote, W. Application of osmometry in quality analysis of milk. J. Food Sci. Technol. 2014, 51, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-H.; Chen, S.-J.; Wang, Y.; Han, J.-R. Fermentation conditions of walnut milk beverage inoculated with kefir grains. LWT Food Sci. Technol. 2013, 50, 349–352. [Google Scholar] [CrossRef]
- Tharmaraj, N.; Shah, N. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J. Dairy Sci. 2003, 86, 2288–2296. [Google Scholar] [CrossRef]
- Munhoz, C.; Guimarães, R.; Nozaki, V.; Sanjinez-Argandoña, E.; Macedo, M. Chemical composition and factors antinutritional of bocaiuva fruit. Rev. Ambiência 2018, 14, 212–224. [Google Scholar]
- Mooz, E.; Castelucci, A.; Spoto, M. Technological and nutritional potential of macaúba fruit Acrocomia aculeata (Jacq.) Lodd. Rev. Bras. Pesqui. Aliment. 2012, 3, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Egea, M.; Borsato, D.; Silva, R.; Yamashita, F. Osmo-dehydrated functional product containing fructo-oligosaccharides: Physical, chemical and sensorial characteristics. Braz. Arch. Biol. Technol. 2012, 55, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Aquino, A.; Móes, R.; Leão, K.; Figueiredo, A.; Castro, A. Physical-chemical and sensory characteristics of cookies formulated with acerola (Malpighia emarginata D.C.) residues flour. Rev. do Inst. Adolfo Lutz 2010, 69, 379–386. [Google Scholar]
- de Almeida, A.B.; Silva, A.K.C.; Lodete, A.R.; Egea, M.B.; Lima, M.C.P.M.; Silva, F.G. Assessment of chemical and bioactive properties of native fruits from the Brazilian Cerrado. Nutr. Food Sci. 2019, 49, 381–392. [Google Scholar] [CrossRef]
- Pertuzatti, P.; Barcia, M.; Rodrigues, D.; da Cruz, P.; Hermosín-Gutiérrez, I.; Smith, R.; Godoy, H. Antioxidant activity of hydrophilic and lipophilic extracts of Brazilian blueberries. Food Chem. 2014, 164, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Nout, M. Fermented foods and food safety. Food Res. Int. 1994, 27, 291–298. [Google Scholar] [CrossRef]
- EFSA. Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to carbohydrate-electrolyte solutions and reduction in rated perceived exertion/effort during exercise (ID 460, 466, 467, 468), enhancement of water absorption during exercise (ID 314, 315, 316, 317, 319, 322, 325, 332, 408, 465, 473, 1168, 1574, 1593, 1618, 4302, 4309), and maintenance of endurance performance (ID 466, 469) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2211. [Google Scholar]
- Sadowska, B.; Swiderski, F.; Rakowska, R.; Waszkiewicz-Robak, B.; Zebrowska-Krasuska, M.; Dybkowska, E. Beverage osmolality as a marker for maintaining appropriate body hydration. Rocz. Państwowego Zakładu Hig. 2017, 68, 167–173. [Google Scholar]
- Wang, R.; Jin, X.; Su, S.; Lu, Y.; Guo, S. Soymilk gelation: The determinant roles of incubation time and gelation rate. Food Hydrocoll. 2019, 97, 105230. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, K.L.; Araújo, T.H.; Schneedorf, J.M.; de Souza Ferreira, C.; Moraes, G.d.O.I.; Coimbra, R.S.; Rodrigues, M.R. A novel beer fermented by kefir enhances anti-inflammatory and anti-ulcerogenic activities found isolated in its constituents. J. Funct. Foods 2016, 21, 58–69. [Google Scholar] [CrossRef]
- Diniz, R.O.; Perazzo, F.F.; Carvalho, J.C.T.; Schneenedorf, J.M. Atividade antiinflamatória de quefir, um probiótico da medicina popular. Rev. Bras. Farmacogn. 2003, 13, 19–21. [Google Scholar] [CrossRef]
- Alsayadi, M.; Al Jawfi, Y.; Belarbi, M.; Soualem-Mami, Z.; Merzouk, H.; Sari, D.C.; Sabri, F.; Ghalim, M. Evaluation of anti-Hyperglycemic and anti-hyperlipidemic activities of water kefir as probiotic on Streptozotocin-induced diabetic Wistar rats. J. Diabetes Mellit. 2014, 2014, 45054. [Google Scholar] [CrossRef] [Green Version]
- Azizi, N.; Kumar, M.; Yeap, S.; Abdullah, J.; Khalid, M.; Omar, A.; Osman, M.; Mortadza, S.; Alitheen, N. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant properties and phenolic compounds of vitamin C-rich juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.; Hovda, M.; Rode, T. Effect of high pressure and thermal processing on shelf life and quality of strawberry purée and juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, T.; Filbido, G.; de Oliveira Pinheiro, A.; Piereti, P.; Dalla Villa, R.; de Oliveira, A. In vitro bioaccessibility of the Cu, Fe, Mn and Zn in the baru almond and bocaiúva pulp and, macronutrients characterization. J. Food Compos. Anal. 2020, 86, 103356. [Google Scholar] [CrossRef]
- Debelo, H.; Novotny, J.; Ferruzzi, M. Vitamin A. Adv. Nutr. 2017, 8, 992–994. [Google Scholar] [CrossRef] [Green Version]
- Abreu, C.; Pinheiro, A.; Maia, G.; Carvalho, J.d.; Sousa, P.d. Chemical and physico-chemical evaluation of soybean beverages added tropical fruits. Aliment. Nutr. Araraquara 2008, 18, 291–296. [Google Scholar]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name | Inulin (%) | Bocaiúva Powder-Pulp (%) |
|---|---|---|
| CONT | - | - |
| IN3.5 | 3.5 | - |
| BO3.5 | - | 3.5 |
| BO7.0 | - | 7.0 |
| BO + IN | 3.5 | 3.5 |
| Analysis | Content |
|---|---|
| Moisture (g/100 g) | 6.22 ± 0.30 |
| Ash (g/100 g) | 3.15 ± 0.06 |
| Protein (g/100 g) | 2.94 ± 0.30 |
| Lipids (g/100 g) | 9.15 ± 0.35 |
| Carbohydrates | 78.54 ± 0.25 |
| Energy | 408.27 ± 0.20 |
| pH | 5.57 ± 0.03 |
| Soluble solids (°Brix) | 3.10 ± 0.15 |
| Acidity (mg/mL) | 0.24 ± 0.01 |
| L* parameter | 53.24 ± 2.48 |
| a* parameter | 14.09 ± 1.14 |
| b* parameter | 39.20± 2.03 |
| Chroma | 37.97 ± 2.32 |
| Hue | 71.31 ± 0.89 |
| Vitamin C content (mg/100 g) | 13.12 ± 8.66 |
| Total carotenoids (µg/g) | 6.34 ± 1.50 |
| Total phenolic compounds (g/100 g) | 216.58 ± 7.12 |
| Antioxidant activity using DPPH method (µM TEAC/100 g) | 10.69 ± 0.003 |
| Antioxidant activity using ABTS method (µM TEAC/100 g) | 557.00 ± 36.56 |
| Time (d) | CONT | IN3.5 | BO3.5 | BO7.0 | BO + IN |
|---|---|---|---|---|---|
| L* | |||||
| 0 | 69.48 ± 2.84 a | 66.22 ± 4.27 a | 65.36 ± 2.24 b | 67.22 ± 0.89 b | 63.00 ± 1.06 b |
| 6 | 68.36 ± 1.79 a | 67.95 ± 2.58 a | 68.50 ± 1.31 a | 66.13 ± 0.58 a | 64.85 ± 1.79 a |
| 11 | 66.00 ± 4.58 ab | 60.30 ± 2.71 bc | 66.62 ± 1.42 c | 67.64 ± 1.33 d | 64.75 ± 0.48 bc |
| 16 | 60.9 ± 5.14 a | 59.93 ± 3.48 a | 65.17 ± 1.36 a | 67.66 ± 0.91 c | 63.87 ± 1.31 a |
| Chroma | |||||
| 0 | 17.27 ± 1.22 a | 8.62 ± 3.26 b | 14.60 ± 3.16 cab | 18.96 ± 1.69 d | 25.15 ± 1.05 e |
| 6 | 9.03 ± 1.84 a | 8.46 ± 3.30 b | 14.07 ± 1.99 c | 20.23 ± 0.74 d | 24.55 ± 1.06 e |
| 11 | 10.68 ± 4.48 a | 6.75 ± 2.33 ac | 12.95 ± 2.16 ab | 16.07 ± 1.59 c | 26.00 ± 0.90 d |
| 16 | 6.52 ± 3.35 a | 6.26 ± 2.25 b | 15.05 ± 2.22 c | 18.22 ± 1.97 ad | 24.49 ± 1.66 e |
| hue | |||||
| 0 | 77.55 ± 3.82 a | 73.15 ± 6.05 b | 83.00 ± 3.14 c | 88.32 ± 1.46 d | 85.64 ± 1.08 e |
| 6 | 74.32 ± 2.68 a | 73.32 ± 3.30 b | 83.56 ± 2.45 c | 89.07 ± 0.58 d | 86.62 ± 1.06 e |
| 11 | 75.85 ± 7.01 a | 68.49 ± 4.15 b | 81.28 ± 2.45 c | 84.87 ± 1.59 d | 85.93 ± 0.90 e |
| 16 | 63.49 ± 11.62 a | 64.42 ± 8.02 b | 83.43 ± 3.82 c | 87.20 ± 1.97 d | 87.11 ± 1.87 e |
| Time (days) | Lactobacillus (UFC/mL) | ||||
|---|---|---|---|---|---|
| CONT | IN3.5 | BO3.5 | BO7.0 | BO + IN | |
| 0 | 2.70 × 107 | 1.70 × 107 | 2.45 × 107 | 3.65 × 107 | 1.00 × 107 |
| 6 | 3.00 × 107 | 1.00 × 108 | 1.60 × 107 | 3.63 × 107 | 1.20 × 107 |
| 11 | 1.70 × 108 | 1.20 × 108 | 1.10 × 108 | 2.66 × 107 | 8.00 × 107 |
| 16 | 3.78 × 107 | 1.40 × 107 | 2.73 × 107 | 1.50 × 107 | 1.20 × 107 |
| Lactococcus (UFC/mL) | |||||
| CONT | IN3.5 | BO3.5 | BO7.0 | BO + IN | |
| 0 | 4.80 × 107 | 4.60 × 107 | 4.25 × 107 | 5.02 × 107 | 1.60 × 107 |
| 6 | 2.73 × 107 | 5.00 × 107 | 3.05 × 107 | 1.00 × 107 | 5.00 × 107 |
| 11 | 3.00 × 107 | 8.83 × 107 | 4.95 × 107 | 2.00 × 107 | 4.23 × 107 |
| 16 | 1.36 × 107 | 2.80 × 107 | 2.56 × 107 | 4.50 × 107 | 4.43 × 107 |
| Yeast (UFC/mL) | |||||
| CONT | IN3.5 | BO3.5 | BO7.0 | BO + IN | |
| 0 | 5.70 × 107 | 1.90 × 108 | 3.50 × 108 | 4.12 × 107 | 1.60 × 107 |
| 6 | 5.28 × 107 | 1.50 × 108 | 1.30 × 108 | 2.70 × 108 | 1.00 × 108 |
| 11 | 3.00 × 107 | 1.60 × 107 | 1.10 × 107 | 2.00 × 107 | 4.23 × 107 |
| 16 | 2.26 × 107 | 5.65 × 107 | 3.46 × 107 | 2.50 × 107 | 1.20 × 107 |
| Analysis | CONT | IN3.5 | BO3.5 | BO7.0 | BO + IN |
|---|---|---|---|---|---|
| Vitamin C (mg/100 g) | 28.56 ± 5.96 a | 20.64 ± 4.20 a | 12.18 ± 1.49 a | 17.94 ± 7.99 a | 13.62 ± 2.47 a |
| Total carotenoids (µg/g) | 0.115 ± 0.02 b | 0.206 ± 0.03 b | 0.146 ± 0.11 b | 0.563 ± 0.09 a | 0.231 ± 0.05 b |
| Total phenolic compounds (mg/100 g) | 37.17 ± 1.17 c | 39.40 ± 0.50 bc | 39.79 ± 1.64 bc | 45.80 ± 4.36 ab | 49.52 ± 3.08 a |
| Antioxidant activity using ABTS method (µM TEAC/g) | 335.00 ± 5.27 b | 376.75 ± 2.93 ab | 406.59 ± 20.38 a | 378.75 ± 12.69 ab | 388.61 ± 10.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.C.d.M.; Santana, R.V.; Almeida, A.B.d.; Takeuchi, K.P.; Egea, M.B. Changes in the Chemical, Technological, and Microbiological Properties of Kefir-Fermented Soymilk after Supplementation with Inulin and Acrocomia aculeata Pulp. Appl. Sci. 2021, 11, 5575. https://doi.org/10.3390/app11125575
Silva JCdM, Santana RV, Almeida ABd, Takeuchi KP, Egea MB. Changes in the Chemical, Technological, and Microbiological Properties of Kefir-Fermented Soymilk after Supplementation with Inulin and Acrocomia aculeata Pulp. Applied Sciences. 2021; 11(12):5575. https://doi.org/10.3390/app11125575
Chicago/Turabian StyleSilva, Juliane Cristina de Melo, Railany Vieira Santana, Adrielle Borges de Almeida, Katiuchia Pereira Takeuchi, and Mariana Buranelo Egea. 2021. "Changes in the Chemical, Technological, and Microbiological Properties of Kefir-Fermented Soymilk after Supplementation with Inulin and Acrocomia aculeata Pulp" Applied Sciences 11, no. 12: 5575. https://doi.org/10.3390/app11125575






