Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer?
Abstract
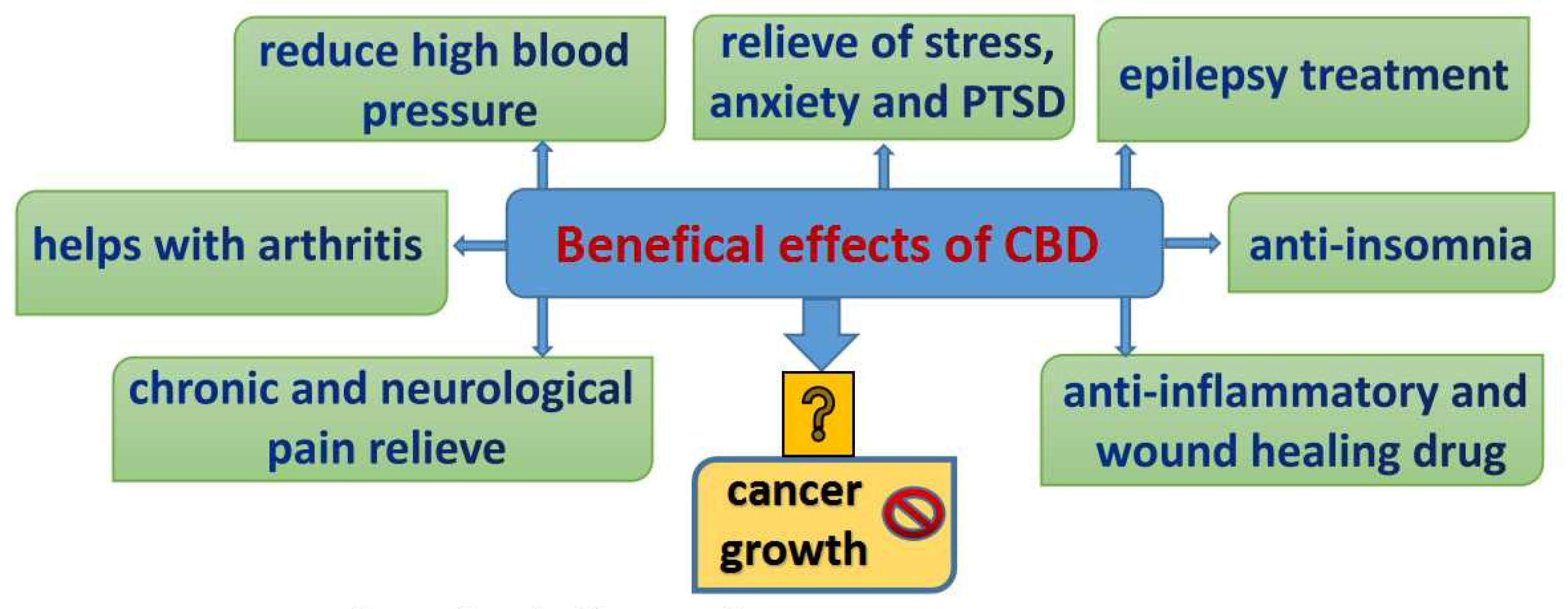
:1. Introduction
1.1. Cannabinoids and Their Working Mechanisms
1.2. Are CBD and Marijuana Usage the Same?
1.3. Antitumorigenic and Biphasic Properties
2. Role of CBD in Cancer Treatment and Its Potential Influence on Cancers’ Therapies Outcomes
2.1. CBD Success in Cancers
2.2. Does CBD Usage Affect the Potency of Classic Chemotherapy?
2.3. CBD and Targeted Therapies
2.4. Future of Epigenetic Treatments on Ovarian Cancer
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 3 May 2021).
- Kim, S.; Han, Y.; Kim, S.I.; Kim, H.-S.; Kim, S.J.; Song, Y.S. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis. Oncol. 2018, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilbao, M.; Aikins, J.K.; Ostrovsky, O. Is routine omentectomy of grossly normal omentum helpful in surgery for ovarian cancer? A look at the tumor microenvironment and its clinical implications. Gynecol. Oncol. 2021, 161, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Berchuck, A.; Dizon, D.S. Princ Pract Gynecol Oncol Seventh Ed Lippincott Williams Wilkins; Principles and Practice of Gynecologic Oncology, 7th edition, 2017. Classification: LCC RC280.G5|NLM WP 145|DDC 616.99/465–dc23 LC. Available online: https://lccn.loc.gov/2016048223 (accessed on 3 May 2021).
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.J.; Straughn, J.M.; Buchsbaum, D.J.; Arend, R.C. Epigenetic therapy for the treatment of epithelial ovarian cancer: A clinical review. Gynecol. Oncol. Rep. 2017, 20, 81–86. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.-K.; Kim, H.S.; Chung, H.H.; Song, Y.S. Epigenetic Therapies as a Promising Strategy for Overcoming Chemoresistance in Epithelial Ovarian Cancer. J. Cancer Prev. 2013, 18, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.; Nelson, K.A.; Mahmoud, F.A. Established and potential therapeutic applications of cannabinoids in oncology. Support. Care Cancer 2003, 11, 137–143. [Google Scholar] [CrossRef]
- Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda; Board on Population Health and Public Health Practice; Health and Medicine Division The Health Effects of Cannabis and Cannabinoids. Health Eff. Cannabis Cannabinoids 2017, 15, 1.
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol. Sci. 2020, 41, 3085–3098. [Google Scholar] [CrossRef] [PubMed]
- Wiese, B.; Wilson-Poe, A.R. Emerging Evidence for Cannabis’ Role in Opioid Use Disorder. Cannabis Cannabinoid Res. 2018, 3, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Boehnke, K.F.; Litinas, E.; Clauw, D.J. Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. J. Pain 2016, 17, 739–744. [Google Scholar] [CrossRef]
- Wilson, N.; Kariisa, M.; Seth, P.; Smith, H.; Davis, N.L. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, P. Rationale for cannabis-based interventions in the opioid overdose crisis. Harm Reduct. J. 2017, 14, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitcomb, B.; Lutman, C.; Pearl, M.; Medlin, E.; Prendergast, E.; Robison, K.; Burke, W. Use of cannabinoids in cancer patients: A Society of Gynecologic Oncology (SGO) clinical practice statement. Gynecol. Oncol. 2020, 157, 307–311. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2015, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, O.L.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Sallaberry, C.; Astern, L. The Endocannabinoid System, Our Universal Regulator. Endocannabinoid Syst. Our Univers. Regul. 2018, 34, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Espejo-Porras, F.; Fernández-Ruiz, J.; Pertwee, R.G.; Mechoulam, R.; García, C. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology 2013, 75, 155–163. [Google Scholar] [CrossRef]
- Rahn, E.J.; Zvonok, A.M.; Thakur, G.A.; Khanolkar, A.D.; Makriyannis, A.; Hohmann, A.G. Selective Activation of Cannabinoid CB2 Receptors Suppresses Neuropathic Nociception Induced by Treatment with the Chemotherapeutic Agent Paclitaxel in Rats. J. Pharmacol. Exp. Ther. 2008, 327, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Elikottil, M.J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid Manag. 2009, 5, 341–357. [Google Scholar] [CrossRef]
- Seeman, P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl. Psychiatry 2016, 6, e920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, H.P.; Bonawitz, S.C.; Ostrovsky, O. The effects of delta-9-tetrahydrocannabinol (THC) on inflammation: A review. Cell. Immunol. 2020, 352, 104111. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kaplan, B.L. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurier, R.B. Prospects for cannabinoids as anti-inflammatory agents. J. Cell. Biochem. 2003, 88, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [CrossRef]
- SanGiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativaL. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Prenderville, J.A.; Kelly, Á.M.; Downer, E.J. The role of cannabinoids in adult neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corroon, J.; Phillips, J.A. A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res. 2018, 3, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, D.; Smith, T.J.; Song, M.; Spirtos, N.M. Care After Chemotherapy: Peripheral Neuropathy, Cannabis for Symptom Control, and Mindfulness. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Mishima, K.; Fujiwara, M. Therapeutic Potential of Non-Psychotropic Cannabidiol in Ischemic Stroke. Pharmaceuticals 2010, 3, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Soboci, A.A.; Czarnecka, A.M.; Król, M.; Botta, B. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, A.; Tolón, M.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Anand, U.; Jones, B.; Korchev, Y.; Bloom, S.R.; Pacchetti, B.; Anand, P.; Sodergren, M.H. CBD Effects on TRPV1 Signaling Pathways in Cultured DRG Neurons. J. Pain Res. 2020, 13, 2269–2278. [Google Scholar] [CrossRef]
- A Pumroy, R.; Samanta, A.; Liu, Y.; Hughes, T.E.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular mechanism of TRPV2 channel modulation by cannabidiol. eLife 2019, 8. [Google Scholar] [CrossRef]
- Abrams, D. Integrating Cannabis into Clinical Cancer Care. Curr. Oncol. 2016, 23, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; A Walker, E. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5- HT 1A receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Bifulco, M.; Di Marzo, V. Targeting the endocannabinoid system in cancer therapy: A call for further research. Nat. Med. 2002, 8, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and cancer: Therapeutic implication. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Ramer, R. Anti-tumour actions of cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids2. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the endocannabinoid system as an anticancer target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sánchez, C.; Guzman, M. Endocannabinoids and Cancer; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; Volume 231, pp. 449–472. [Google Scholar] [CrossRef]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef]
- LaPrairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovanwright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; E Ponde, D.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Katsidoni, V.; Kastellakis, A.; Panagis, G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol. 2013, 16, 2273–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzavara, E.T.; Wade, M.; Nomikos, G.G. Biphasic Effects of Cannabinoids on Acetylcholine Release in the Hippocampus: Site and Mechanism of Action. J. Neurosci. 2003, 23, 9374–9384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarne, Y. Beneficial and deleterious effects of cannabinoids in the brain: The case of ultra-low dose THC. Am. J. Drug Alcohol Abus. 2019, 45, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Miyato, H.; Kitayama, J.; Yamashita, H.; Souma, D.; Asakage, M.; Yamada, J.; Nagawa, H. Pharmacological Synergism Between Cannabinoids and Paclitaxel in Gastric Cancer Cell Lines. J. Surg. Res. 2009, 155, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dariš, B.; Verboten, M.T.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef]
- Millar, S.; Stone, N.; Bellman, Z.; Yates, A.; England, T.; O’Sullivan, S. A systematic review of cannabidiol dosing in clinical populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.-Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E.; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [Green Version]
- Rey, A.A.; Purrio, M.; Viveros, M.-P.; Lutz, B. Biphasic Effects of Cannabinoids in Anxiety Responses: CB1 and GABAB Receptors in the Balance of GABAergic and Glutamatergic Neurotransmission. Neuropsychopharmacology 2012, 37, 2624–2634. [Google Scholar] [CrossRef] [Green Version]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; A Barrett, D.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Giacoppo, S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. DARU J. Pharm. Sci. 2015, 23, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Barrie, A.M.; Gushue, A.C.; Eskander, R.N. Dramatic response to Laetrile and cannabidiol (CBD) oil in a patient with metastatic low grade serous ovarian carcinoma. Gynecol. Oncol. Rep. 2019, 29, 10–12. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Delie, F.; Cohen, M.; Martin-Sabroso, C.; Mezzanzanica, D.; Figini, M.; Satta, A.; Torres-Suárez, A. Enhancing ovarian cancer conventional chemotherapy through the combination with cannabidiol loaded microparticles. Eur. J. Pharm. Biopharm. 2020, 154, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’Argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J. Pharmacol. Exp. Ther. 2003, 308, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Ossa, D.H.P.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; García-Taboada, E.; Aberturas, M.D.R.; Molpeceres, J.; Velasco, G.; Torres-Suárez, A.I. Local Delivery of Cannabinoid-Loaded Microparticles Inhibits Tumor Growth in a Murine Xenograft Model of Glioblastoma Multiforme. PLoS ONE 2013, 8, e54795. [Google Scholar] [CrossRef] [Green Version]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [Green Version]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2010, 129, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.H.; Tortolani, D.; Ayakannu, T.; Konje, J.C.; Maccarrone, M. (Endo)Cannabinoids and Gynaecological Cancers. Cancers 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Contassot, E.; Tenan, M.; Schnüriger, V.; Pelte, M.-F.; Dietrich, P.-Y. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol. Oncol. 2004, 93, 182–188. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. No Title. Surveillance, Epidemiol End Results Program Endometrial Cancer. Published online 2020.
- Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. [Google Scholar] [CrossRef]
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Ng, L.; Ozawa, T.; Stella, N. Quantitative Analyses of Synergistic Responses between Cannabidiol and DNA-Damaging Agents on the Proliferation and Viability of Glioblastoma and Neural Progenitor Cells in Culture. J. Pharmacol. Exp. Ther. 2017, 360, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Twelves, C.; Short, S.; Wright, S. Cannabinoid in Recurrent Glioma Study Group A two-part safety and exploratory efficacy randomized double-blind, placebo-controlled study of a 1:1 ratio of the cannabinoids cannabidiol and delta-9-tetrahydrocannabinol (CBD:THC) plus dose-intense temozolomide in patients with recurrent glioblastoma multiforme (GBM). J. Clin. Oncol. 2017, 35, 2046. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The Combination of Cannabidiol and Δ9-Tetrahydrocannabinol Enhances the Anticancer Effects of Radiation in an Orthotopic Murine Glioma Model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomko, A.; O’Leary, L.; Trask, H.; Achenbach, J.C.; Hall, S.R.; Goralski, K.B.; Ellis, L.D.; Dupré, D.J. Antitumor Activity of Abnormal Cannabidiol and Its Analog O-1602 in Taxol-Resistant Preclinical Models of Breast Cancer. Front. Pharmacol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Hu, C.-M.; Huang, C.-H.; Wey, S.-P.; Jan, T.-R. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol. Sin. 2010, 31, 1611–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef] [Green Version]
- Watzl, B.; Scuderi, P.; Watson, R.R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int. J. Immunopharmacol. 1991, 13, 1091–1097. [Google Scholar] [CrossRef]
- A Jones, P.; Laird, P.W. Cancer-epigenetics comes of age. Nat. Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinog. 2009, 31, 27–36. [Google Scholar] [CrossRef]
- Moufarrij, S.; Dandapani, M.; Arthofer, E.; Gomez, S.; Srivastava, A.; Lopez-Acevedo, M.; Villagra, A.; Chiappinelli, K.B. Epigenetic therapy for ovarian cancer: Promise and progress. Clin. Epigenet. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Kelly, T.K.; De Carvalho, D.D.; A Jones, P. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 2010, 28, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Reece, A.S.; Hulse, G.K. Chromothripsis and epigenomics complete causality criteria for cannabis- and addiction-connected carcinogenicity, congenital toxicity and heritable genotoxicity. Mutat. Res. Mol. Mech. Mutagen. 2016, 789, 15–25. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Receptor Activity |
|---|---|
| CB1 | Negative allo modulator |
| CB2 | Weak antagonist |
| FAAH | Weak inhibitor |
| FABP | Inhibitor |
| 5-HT1A | Full agonist |
| 5-HT2A | Weak partial agonist |
| 5-HT3A | Negative allo modulator |
| D2High (Dopamine) | Partial agonist |
| uOR (Opioid) | Negative allo modulator |
| δOR (Opioid) | Negative allo modulator |
| GPR55 | Negative allo modulator |
| PPAR-gamma | Full agonist |
| Adenosine A2A receptors | Negative allo modulator |
| TRPV1 | Negative allo modulator |
| TRPV2 | Full agonist |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffiths, C.; Aikins, J.; Warshal, D.; Ostrovsky, O. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules 2021, 11, 766. https://doi.org/10.3390/biom11050766
Griffiths C, Aikins J, Warshal D, Ostrovsky O. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules. 2021; 11(5):766. https://doi.org/10.3390/biom11050766
Chicago/Turabian StyleGriffiths, Courtney, James Aikins, David Warshal, and Olga Ostrovsky. 2021. "Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer?" Biomolecules 11, no. 5: 766. https://doi.org/10.3390/biom11050766