Smell and Stress Response in the Brain: Review of the Connection between Chemistry and Neuropharmacology
Abstract
:1. Introduction
2. Stress Evaluation in Mammals
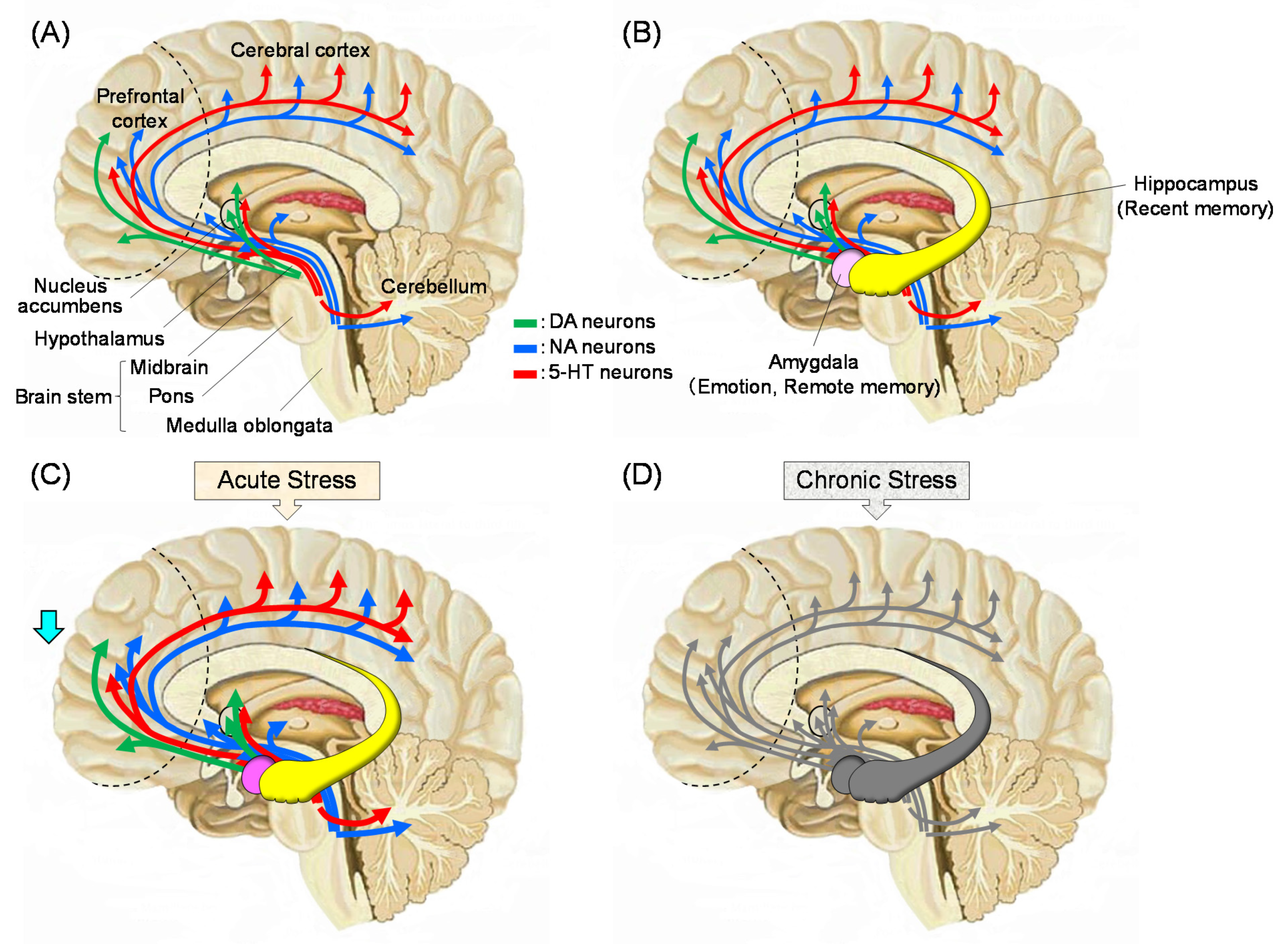
2.1. Stress
2.2. Stress-Responsive Biomarkers (Stress Markers)
2.3. Recent Exploratory Research on Novel Stress Marker Candidates
3. Olfaction and Stress Response in the Brain
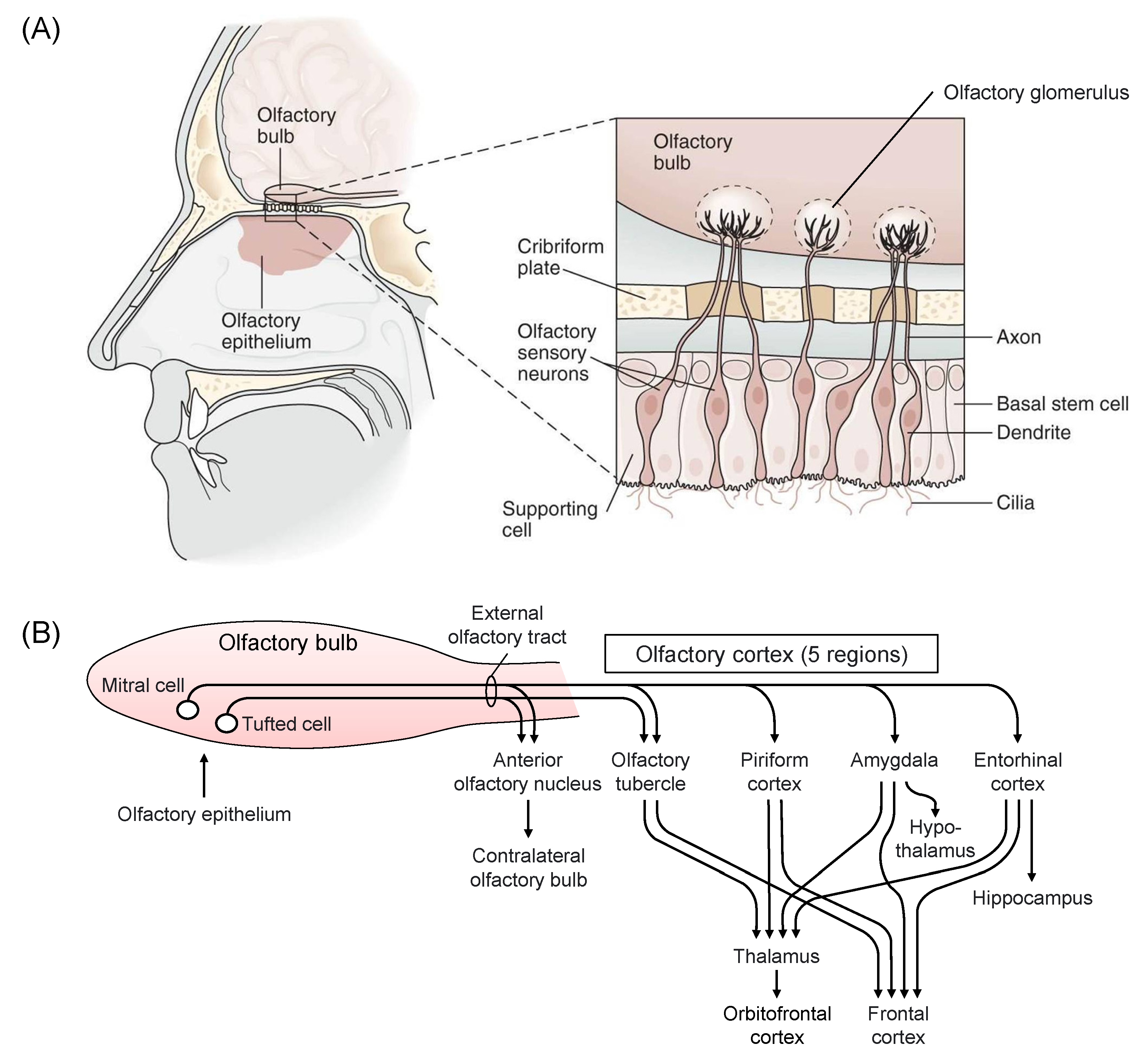
3.1. Olfactory System
3.2. Stress-Inducing Smell
3.3. Stress-Suppressing Smell
3.3.1. Effect on Humans
3.3.2. Effect on Rats
3.3.3. Effect on Mice
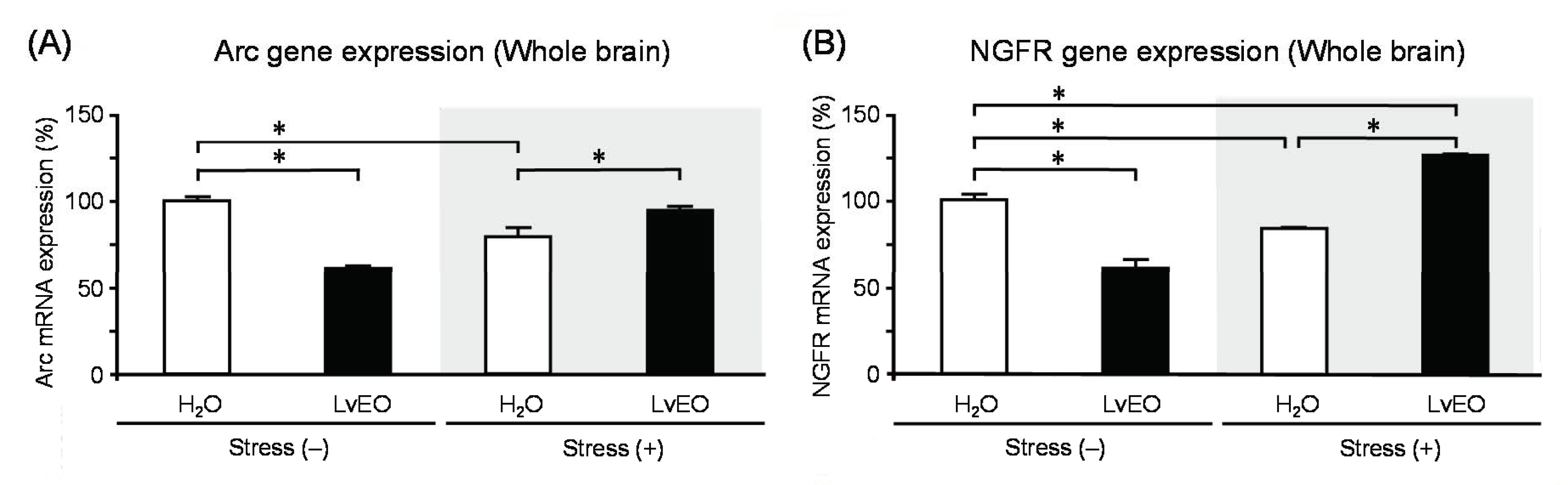
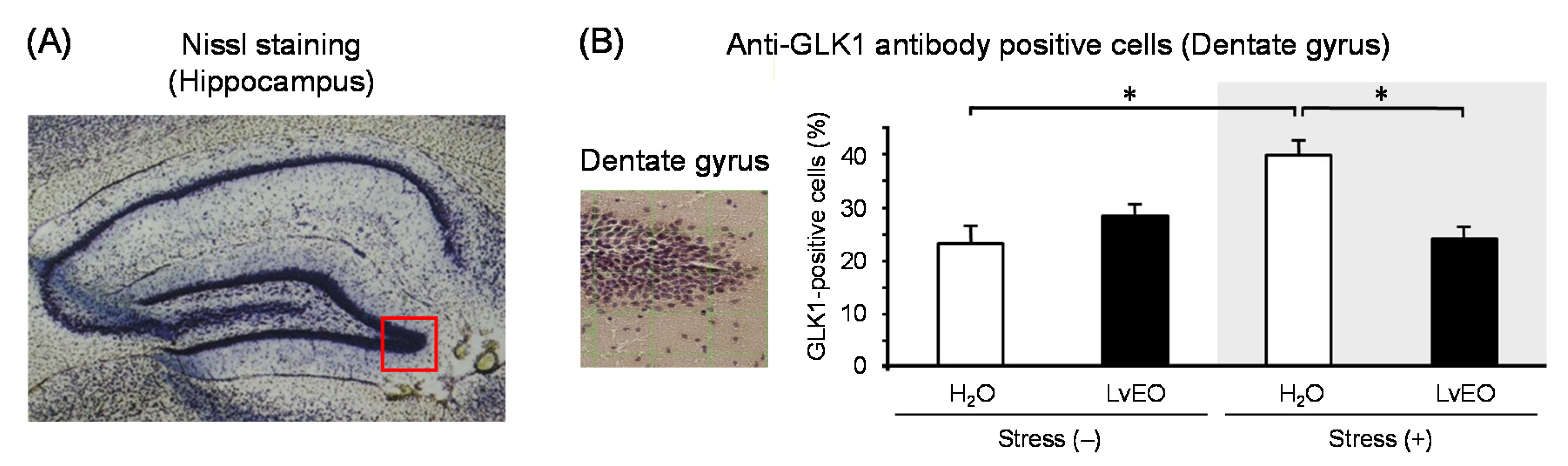
3.3.4. Biochemical Parameters in the Brain in Response to Olfaction
4. Recent Studies on the Effects of Aroma on Biochemical Parameters in the Brain
4.1. Coffee Beans
4.2. Lavender
4.3. Cypress
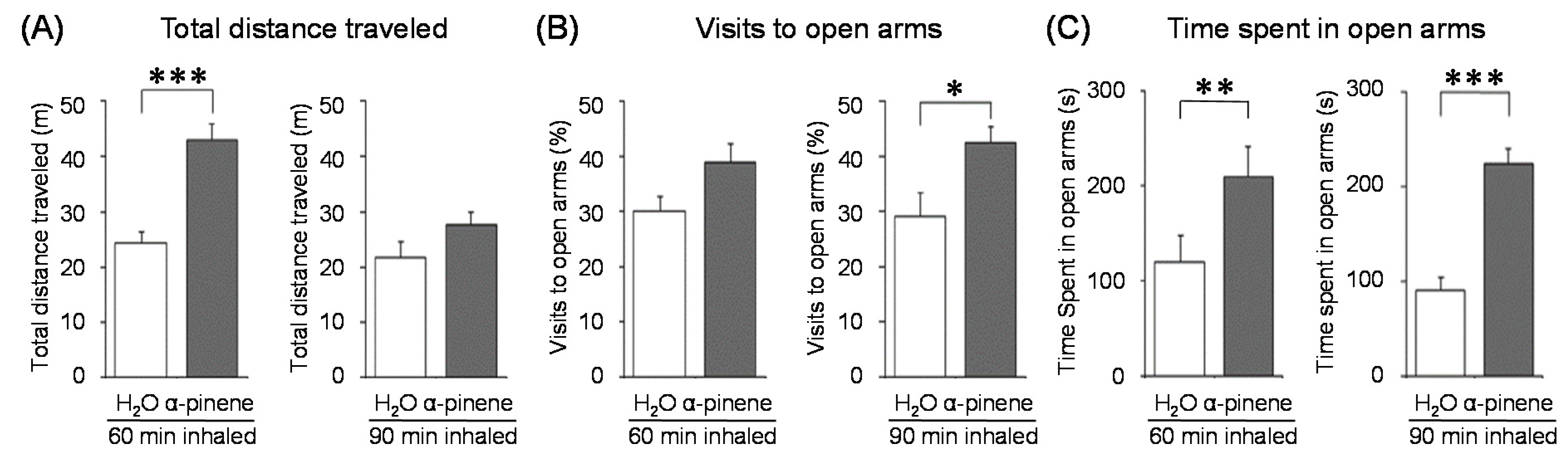
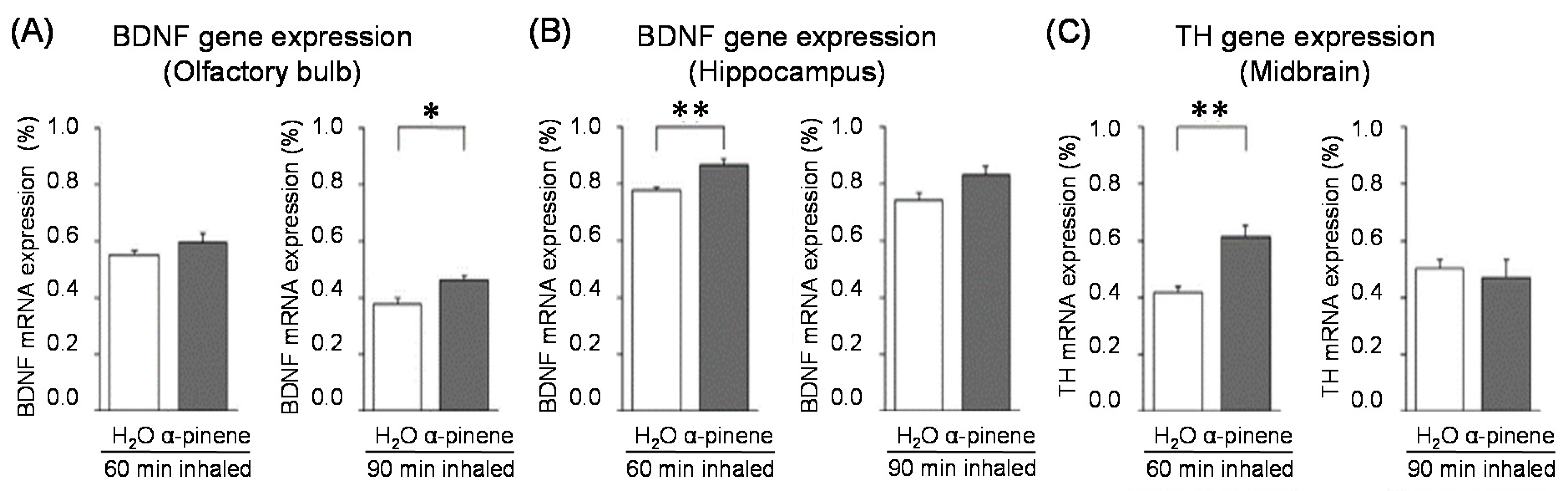
4.4. α-Pinene
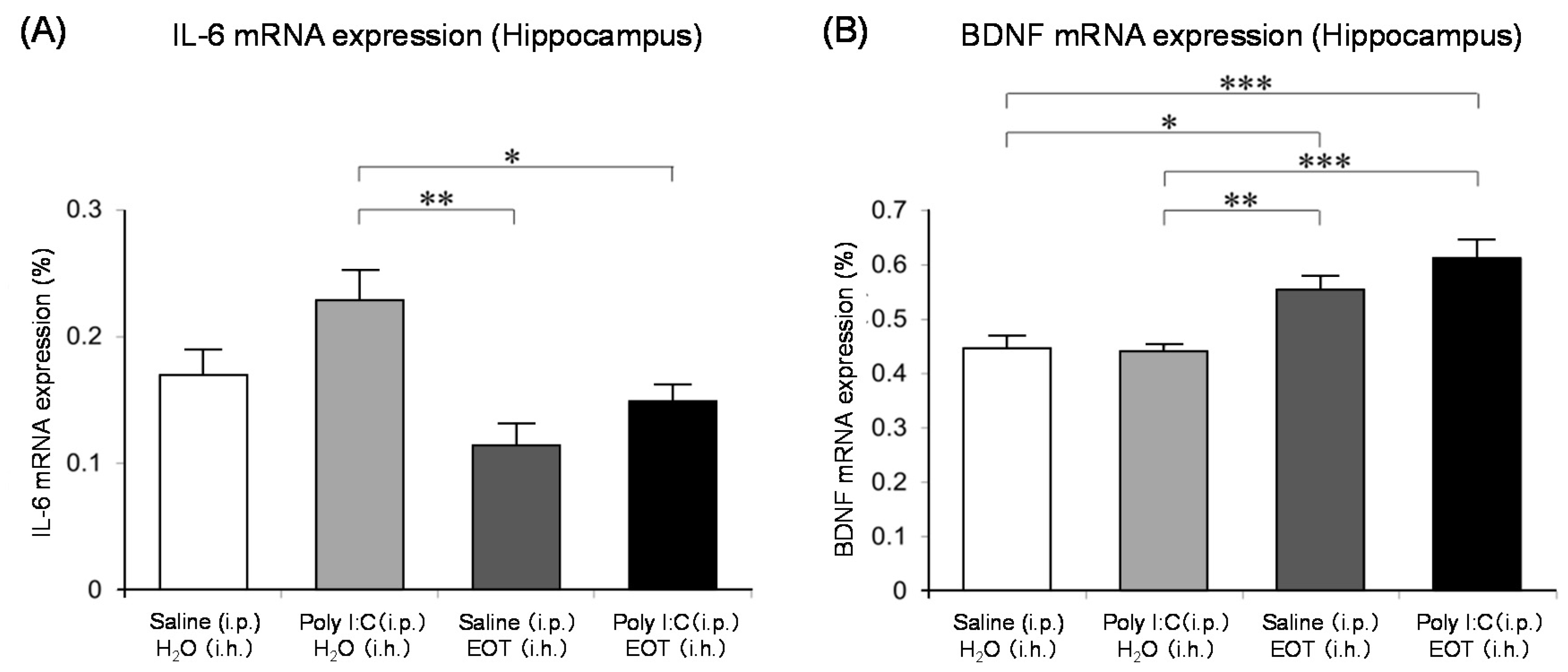
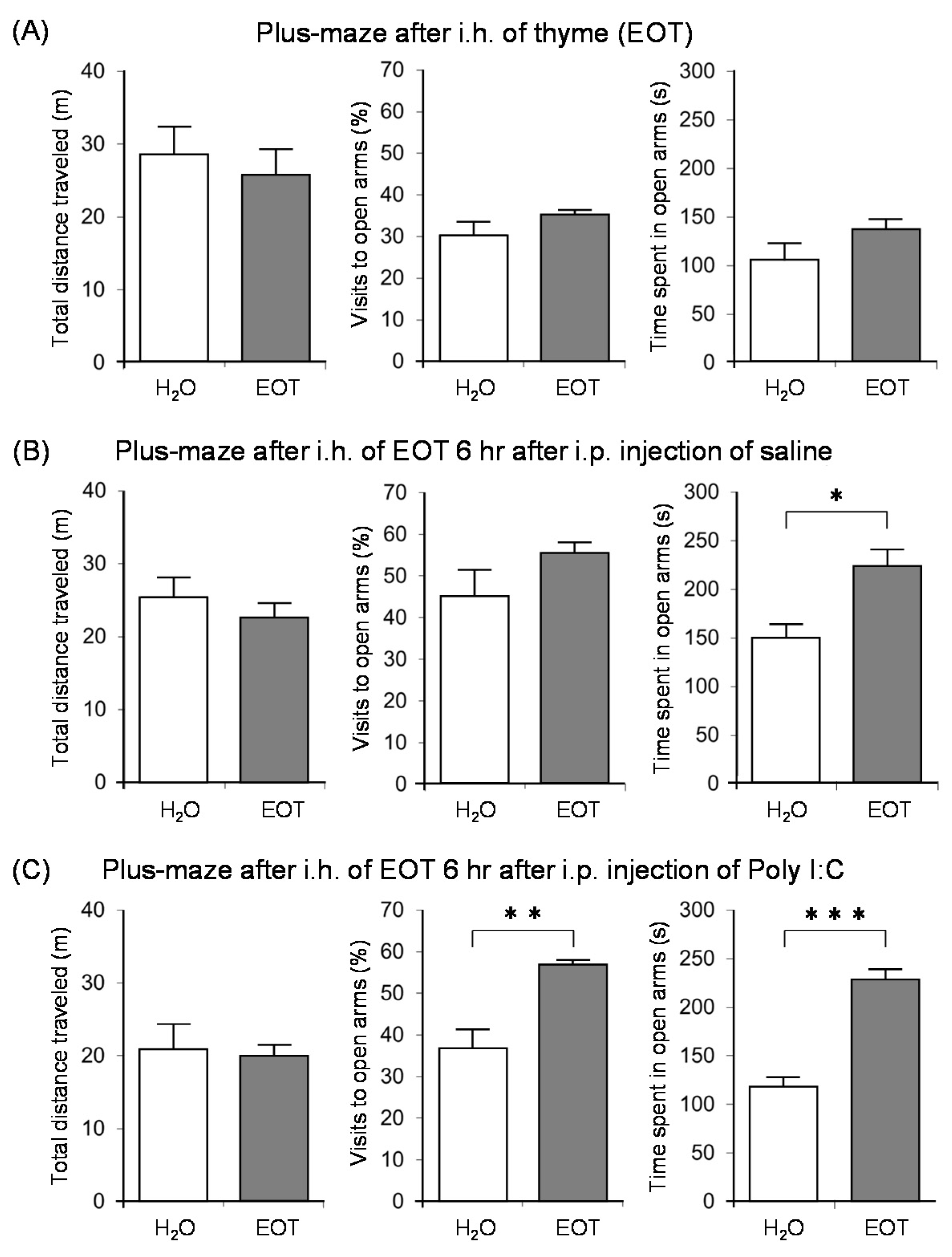
4.5. Thyme Linalool
4.6. Transfer of Fragrant Molecules to the Brain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Niimura, Y.; Matsui, A.; Touhara, K. Acceleration of olfactory receptor gene loss in primate evolution: Possible link to anatomical change in sensory systems and dietary transition. Mol. Biol. Evol. 2018, 35, 1437–1450. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F.L.; Silva, V.V.; Dal Pizzol, C.; Spir, L.G.; Praes, C.E.O.; Maibach, H. Physiological effect of olfactory stimuli inhalation in humans: An overview. Int. J. Cosmet. Sci. 2014, 36, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.M.; Schumann, A.Y.; Howell, A.N.; McConnell, P.A.; Yang, Q.X.; Uhde, T.W. Preliminary evidence for differential olfactory and trigeminal processing in combat veterans with and without PTSD. NeuroImage Clin. 2018, 17, 378–387. [Google Scholar] [CrossRef]
- Daniels, J.K.; Vermetten, E. Odor-induced recall of emotional memories in PTSD–Review and new paradigm for research. Exp. Neurol. 2016, 284, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Herten, N.; Pomrehn, D.; Wolf, O.T. Memory for objects and startle responsivity in the immediate aftermath of exposure to the Trier Social Stress Test. Behav. Brain Res. 2017, 326, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, Y.; Shirasu, M.; Okamoto, M.; Touhara, K. Subjective unpleasantness of malodors induces a stress response. Psychoneuroendocrinology 2019, 106, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, P.; Vestbrant, K.; Döllinger, L.; Liuzza, M.T.; Olsson, M.J.; Blomkvist, A.; Lundström, J.N. The scent of security: Odor of romantic partner alters subjective discomfort and autonomic stress responses in an adult attachment-dependent manner. Physiol. Behav. 2019, 198, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selye, H. A Syndrome produced by Diverse Nocuous Agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Homiack, D.; O’Cinneide, E.; Hajmurad, S.; Barrileaux, B.; Stanley, M.; Kreutz, M.R.; Schrader, L.A. Predator odor evokes sex-independent stress responses in male and female Wistar rats and reduces phosphorylation of cyclic-adenosine monophosphate response element binding protein in the male, but not the female hippocampus. Hippocampus 2017, 27, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, H.; Perski, A.; Berglund, H.; Savić, I. Chronic stress is linked to 5-HT1A receptor changes and functional disintegration of the limbic networks. NeuroImage 2011, 55, 1178–1188. [Google Scholar] [CrossRef]
- Kreinin, A.; Lisson, S.; Nesher, E.; Schneider, J.; Bergman, J.; Farhat, K.; Farah, J.; Lejbkowicz, F.; Yadid, G.; Raskin, L.; et al. Blood BDNF level is gender specific in severe depression. PLoS ONE 2015, 10, e0127643. [Google Scholar] [CrossRef] [Green Version]
- Butler, R.K.; Oliver, E.M.; Sharko, A.C.; Parilla-Carrero, J.; Kaigler, K.F.; Fadel, J.R.; Wilson, M.A. Activation of corticotropin releasing factor-containing neurons in the rat central amygdala and bed nucleus of the stria terminalis following exposure to two different anxiogenic stressors. Behav. Brain Res. 2016, 304, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Itoga, C.A.; Hellard, E.A.R.; Whitaker, A.M.; Lu, Y.-L.; Schreiber, A.L.; Baynes, B.B.; Baiamonte, B.A.; Richardson, H.N.; Gilpin, N.W. Traumatic stress promotes hyperalgesia via Corticotropin-Releasing Factor-1 Receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology 2016, 41, 2463–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janitzky, K.; Peine, A.; Kröber, A.; Yanagawa, Y.; Schwegler, H.; Roskoden, T. Increased CRF mRNA expression in the sexually dimorphic BNST of male but not female GAD67 mice and TMT predator odor stress effects upon spatial memory retrieval. Behav. Brain Res. 2014, 272, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Prusator, D.K.; Meerveld, B.G.-V. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: Importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain 2017, 158, 296–305. [Google Scholar] [CrossRef]
- Curtis, A.L.; Leiser, S.C.; Snyder, K.; Valentino, R.J. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 2012, 62, 1737–1745. [Google Scholar] [CrossRef] [Green Version]
- Butler, R.K.; White, L.C.; Frederick-Duus, D.; Kaigler, K.F.; Fadel, J.R.; Wilson, M.A. Comparison of the activation of somatostatin- and neuropeptide Y-containing neuronal populations of the rat amygdala following two different anxiogenic stressors. Exp. Neurol. 2012, 238, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.; Zohar, J.; Kaplan, Z.; Arnt, J. Adjunctive treatment with brexpiprazole and escitalopram reduces behavioral stress responses and increase hypothalamic NPY immunoreactivity in a rat model of PTSD-like symptoms. Eur. Neuropsychopharmacol. 2018, 28, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Masuo, Y.; Ogura, A.; Kobayashi, M.; Masaki, T.; Furuta, Y.; Ono, T.; Takamatsu, K. Hippocalcin protects hippocampal neurons against excitotoxin damage by enhancing calcium extrusion. Neuroscience 2007, 145, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K. Salivary mental stress proteins. Clin. Chim. Acta 2013, 425, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.; Haraty, H.; Salame, Z.; Fares, Y.; Ojcius, D.M.; Sadier, N.S. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed. J. 2018, 41, 63–87. [Google Scholar] [CrossRef]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef] [PubMed]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary biomarkers of stress, anxiety and depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Rakwal, R.; Shibato, J.; Sawa, H.; Nagashima, K.; Ogawa, Y.; Yoshida, Y.; Iwahashi, H.; Niki, E.; Masuo, Y. Proteomics- and transcriptomics-based screening of differentially expressed proteins and genes in brain of Wig rat: A model for attention deficit hyperactivity disorder (ADHD) research. J. Proteome Res. 2008, 7, 2471–2489. [Google Scholar] [CrossRef]
- Masuo, Y.; Ishido, M.; Morita, M.; Oka, S.; Niki, E. Motor activity and gene expression in rats with neonatal 6-hydroxydopamine lesions. J. Neurochem. 2004, 91, 9–19. [Google Scholar] [CrossRef]
- Masuo, Y.; Ishido, M.; Morita, M.; Sawa, H.; Nagashima, K.; Niki, E. Behavioural characteristics and gene expression in the hyperactive wiggling (Wig) rat. Eur. J. Neurosci. 2007, 25, 3659–3666. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kulikova, S.P.; Shibato, J.; Rakwal, R.; Satoh, H.; Pinault, D.; Masuo, Y. DNA microarray unravels rapid changes in transcriptome of MK-801 treated rat brain. World J. Biol. Chem. 2015, 6, 389–408. [Google Scholar] [CrossRef]
- Masuo, Y.; Imai, T.; Shibato, J.; Hirano, M.; Jones, O.A.H.; Maguire, M.L.; Satoh, K.; Kikuchi, S.; Rakwal, R. Omic analyses unravels global molecular changes in the brain and liver of a rat model for chronic Sake (Japanese alcoholic beverage) intake. Electrophoresis 2009, 30, 1259–1275. [Google Scholar] [CrossRef]
- Ishido, M.; Masuo, Y.; Sayato-Suzuki, J.; Oka, S.; Niki, E.; Morita, M. Dicyclohexylphthalate causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurochem. 2004, 91, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Masuo, Y.; Ishido, M.; Morita, M.; Oka, S. Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plast. 2004, 11, 59–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuo, Y.; Morita, M.; Oka, S.; Ishido, M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: A study inspired by the physiological roles of PACAP in the brain. Regul. Pept. 2004, 123, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Rakwal, R.; Kouyama, N.; Katayama, Y.; Hayashi, M.; Shibato, J.; Ogawa, Y.; Yoshida, Y.; Iwahashi, H.; Masuo, Y. Gel-based proteomics of unilateral irradiated striatum after gamma knife surgery. J. Proteome Res. 2007, 6, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Shibato, J.; Rakwal, R.; Kouyama, N.; Katayama, Y.; Hayashi, M.; Masuo, Y. Transcriptomic analysis of rat brain tissue following gamma knife surgery: Early and distinct bilateral effects in the un-irradiated striatum. Mol. Cells 2009, 27, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. (Eds.) Principles of Neural Science, 4th ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Klein, B.; Bautze, V.; Maier, A.-M.; Deussing, J.; Breer, H.; Strotmann, J. Activation of the mouse odorant receptor 37 subsystem coincides with a reduction of novel environment-induced activity within the paraventricular nucleus of the hypothalamus. Eur. J. Neurosci. 2015, 41, 793–801. [Google Scholar] [CrossRef]
- Gheusi, G.; Lledo, P.-M. Adult neurogenesis in the olfactory system shapes odor memory and perception. Prog. Brain Res. 2014, 208, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Kadohisa, M. Effects of odor on emotion, with implications. Front. Syst. Neurosci. 2013, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Krusemark, E.A.; Novak, L.R.; Gitelman, D.R.; Li, W. When the sense of smell meets emotion: Anxiety-state-dependent olfactory processing and neural circuitry adaptation. J. Neurosci. 2013, 33, 15324–15332. [Google Scholar] [CrossRef]
- Drobyshevsky, A.; Yu, L.; Yang, Y.; Khalid, S.; Luo, K.; Jiang, R.; Ji, H.; Derrick, M.; Kay, L.; Silverman, R.B.; et al. Antenatal insults modify newborn olfactory function by nitric oxide produced from neuronal nitric oxide synthase. Exp. Neurol. 2012, 237, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landers, M.S.; Sullivan, R.M. The development and neurobiology of infant attachment and fear. Dev. Neurosci. 2012, 34, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.A.; Molder, J.J.; Greba, Q.; Howland, J.G. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn. Mem. 2013, 20, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Albrechet-Souza, L.; Gilpin, N.W. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav. Pharmacol. 2019, 30, 105–114. [Google Scholar] [CrossRef]
- Kondoh, K.; Lu, Z.; Ye, X.; Olson, D.P.; Lowell, B.B.; Buck, L.B. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature 2016, 532, 103–106. [Google Scholar] [CrossRef]
- Vaz, R.P.; Cardoso, A.; Serrão, P.; Pereira, P.A.; Madeira, M.D. Chronic stress leads to long-lasting deficits in olfactory-guided behaviors, and to neuroplastic changes in the nucleus of the lateral olfactory tract. Horm. Behav. 2018, 98, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, I.D.; Monteiro, B.M.; Cornélio, G.O.; Fonseca, C.S.; Moraes, M.F.; Pereira, G.S. Odor-enriched environment rescues long-term social memory, but does not improve olfaction in social isolated adult mice. Behav. Brain Res. 2012, 228, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Nagai, K.; Nakashima, T. Order of exposure to pleasant and unpleasant odors affects autonomic nervous system response. Behav. Brain Res. 2013, 243, 109–117. [Google Scholar] [CrossRef]
- Wintermann, G.-B.; Donix, M.; Joraschky, P.; Gerber, J.; Petrowski, K. Altered olfactory processing of stress-related body odors and artificial odors in patients with panic disorder. PLoS ONE 2013, 8, e74655. [Google Scholar] [CrossRef]
- Belnoue, L.; Grosjean, N.; Ladevèze, E.; Abrous, D.N.; Koehl, M. Prenatal stress inhibits hippocampal neurogenesis but spares olfactory bulb neurogenesis. PLoS ONE 2013, 8, e72972. [Google Scholar] [CrossRef]
- Debiec, J.; Sullivan, R.M. The neurobiology of safety and threat learning in infancy. Neurobiol. Learn. Mem. 2017, 143, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Al Aïn, S.; Perry, R.E.; Nuñez, B.; Kayser, K.; Hochman, C.; Brehman, E.; LaComb, M.; Wilson, N.A.; Sullivan, R.M. Neurobehavioral assessment of maternal odor in developing rat pups: Implications for social buffering. Soc. Neurosci. 2016, 12, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, A.M.; Gilpin, N.W. Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity. Physiol. Behav. 2015, 147, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Wang, W.; Ding, X.; Yang, S.; Wang, A.; Yin, B.; Wei, W. Effects of maternal stress induced by predator odors during gestation on behavioral and physiological responses of offspring in Brandt’s vole (Lasiopodomys brandtii). Integr. Zool. 2018, 13, 723–734. [Google Scholar] [CrossRef]
- Kenny, S.L.; Wright, L.D.; Green, A.D.; Mashoodh, R.; Perrot, T.S. Expression of maternal behavior and activation of the bed nucleus of the stria terminalis during predatory threat exposure: Modulatory effects of transport stress. Physiol. Behav. 2014, 123, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kigar, S.L.; Chang, L.; Guerrero, C.R.; Sehring, J.R.; Cuarenta, A.; Parker, L.L.; Bakshi, V.P.; Auger, A.P. N6-methyladenine is an epigenetic marker of mammalian early life stress. Sci. Rep. 2017, 7, 18078. [Google Scholar] [CrossRef] [Green Version]
- Úbeda-Contreras, J.; Marín-Blasco, I.; Nadal, R.; Armario, A. Brain c-fos expression patterns induced by emotional stressors differing in nature and intensity. Brain Struct. Funct. 2018, 223, 2213–2227. [Google Scholar] [CrossRef]
- Cherng, C.-F.G.; Chang, C.P.; Su, C.-C.; Tzeng, W.-Y.; Chuang, J.-Y.; Chen, L.-H.; Lin, K.-Y.; Yu, L. Odors from proximal species reverse the stress-decreased neurogenesis via main olfactory processing. Behav. Brain Res. 2012, 229, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Stockman, S.L.; McCarthy, M.M. Predator odor exposure of rat pups has opposite effects on play by juvenile males and females. Pharmacol. Biochem. Behav. 2017, 152, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Dremencov, E.; Lapshin, M.; Komelkova, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Tseilikman, V. Chronic predator scent stress alters serotonin and dopamine levels in the rat thalamus and hypothalamus, respectively. Gen. Physiol. Biophys. 2019, 38, 187–190. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Tseilikman, V.E.; Karpenko, M.N.; Pestereva, N.S.; Tseilikman, O.B.; Komelkova, M.V.; Kondashevskaya, M.V.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; et al. Intermittent hypoxic conditioning alleviates post-traumatic stress disorder-induced damage and dysfunction of rat visceral organs and brain. Int. J. Mol. Sci. 2020, 21, 345. [Google Scholar] [CrossRef] [Green Version]
- Hegab, I.M.; Shang, G.; Ye, M.; Jin, Y.; Wang, A.; Yin, B.; Yang, S.; Wei, W. Defensive responses of Brandt’s voles (Lasiopodomys brandtii) to chronic predatory stress. Physiol. Behav. 2014, 126, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopfel, D.; Perez, P.D.; Verbitsky, A.; Bravo-Rivera, H.; Ma, Y.; Quirk, G.J.; Zhang, N. Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nat. Commun. 2019, 10, 2372. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.M.; Lodge, D.J. Adolescent stress contributes to aberrant dopamine signaling in a heritable rodent model of susceptibility. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109701. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Aou, S. Prenatal bisphenol A exposure is associated with medial amygdala neuron hyperresponsiveness to predator odor in rats. J. Toxicol. Sci. 2018, 43, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Galliot, E.; Levaillant, M.; Beard, E.; Millot, J.-L.; Pourié, G. Enhancement of spatial learning by predator odor in mice: Involvement of amygdala and hippocampus. Neurobiol. Learn. Mem. 2010, 93, 196–202. [Google Scholar] [CrossRef]
- Homiack, D.; O’Cinneide, E.; Hajmurad, S.; Dohanich, G.P.; Schrader, L.A. Effect of acute alarm odor exposure and biological sex on generalized avoidance and glutamatergic signaling in the hippocampus of Wistar rats. Stress 2018, 21, 292–303. [Google Scholar] [CrossRef]
- Janitzky, K.; D’hanis, W.; Kröber, A.; Schwegler, H. TMT predator odor activated neural circuit in C57BL/6J mice indicates TMT-stress as a suitable model for uncontrollable intense stress. Brain Res. 2015, 1599, 1–8. [Google Scholar] [CrossRef]
- Matsukawa, M.; Imada, M.; Murakami, T.; Aizawa, S.; Sato, T. Rose odor can innately counteract predator odor. Brain Res. 2011, 1381, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Nakashima, T. Different patterns of neuronal activities in the infralimbic and prelimbic cortices and behavioral expression in response to two affective odors, 2,5-dihydro-2,4,5-trimethylthiazoline and a mixture of cis-3-hexenol and trans-2-hexenal, in the freely moving rat. Behav. Brain Res. 2011, 218, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Lee, C.-Y.; Matsuo, T.; Wu, K.; Asher, G.; Tang, L.; Saitoh, T.; Russell, J.; Klewe-Nebenius, D.; et al. Large-scale forward genetics screening identifies Trpa1 as a chemosensor for predator odor-evoked innate fear behaviors. Nat. Commun. 2018, 9, 2041. [Google Scholar] [CrossRef] [Green Version]
- Miyazono, S.; Hasegawa, K.; Miyazaki, S.; Sakakima, H.; Konno, S.; Meguro, S.; Sasajima, H.; Noguchi, T.; Osada, K.; Kashiwayanagi, M. Etizolam attenuates the reduction in cutaneous temperature induced in mice by exposure to synthetic predator odor. Eur. J. Pharmacol. 2018, 824, 157–162. [Google Scholar] [CrossRef]
- Arakawa, H.; Arakawa, K.; Blandino, P., Jr.; Deak, T. The role of neuroinflammation in the release of aversive odor cues from footshock-stressed rats: Implications for the neural mechanism of alarm pheromone. Psychoneuroendocrinology 2011, 36, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Kiyokawa, Y.; Tamogami, S.; Watanabe, H.; Takeuchi, Y.; Mori, Y. Identification of a pheromone that increases anxiety in rats. Proc. Natl. Acad. Sci. USA 2014, 111, 18751–18756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konar, A.; Rastogi, M.; Bhambri, A. Brain region specific methylation and Sirt1 binding changes in MAOA promoter is associated with sexual dimorphism in early life stress induced aggressive behavior. Neurochem. Int. 2019, 129, 104510. [Google Scholar] [CrossRef]
- Tzeng, W.-Y.; Chen, L.-H.; Cherng, C.G.; Tsai, Y.-N.; Yu, L. Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrus. Psychoneuroendocrinology 2014, 42, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.Z.; Atsak, P.; Bretton, Z.H.; Holt, E.S.; Alam, R.; Morton, M.P.; Abbas, A.I.; Leonardo, E.D.; Bolkan, S.S.; Hen, R.; et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 2018, 43, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Yoto, A.; Fukui, N.; Kaneda, C.; Torita, S.; Goto, K.; Nanjo, F.; Yokogoshi, H. Black tea aroma inhibited increase of salivary chromogranin-A after arithmetic tasks. J. Physiol. Anthr. 2018, 37, 3. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.H.; Lee, M.-Y.; Hur, M.-H. The effects of aromatherapy on intensive care unit patients’ stress and sleep quality: A nonrandomised controlled trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 2856592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, H.; Mardani, A.; Basirinezhad, M.H.; Hamidzadeh, A.; Eskandari, F. The effects of Lavender and Chamomile essential oil inhalation aromatherapy on depression, anxiety and stress in older community-dwelling people: A randomized controlled trial. Explore 2021. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fukuda, S.; Maeda, K.; Kawasaki, T.; Kono, Y.; Jissho, S.; Taguchi, H.; Yoshiyama, M.; Yoshikawa, J. Aromatherapy alleviates endothelial dysfunction of medical staff after night-shift work: Preliminary observations. Hypertens. Res. 2010, 34, 264–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, M.; Morimoto, K. Effect of lavender aroma on salivary endocrinological stress markers. Arch. Oral Biol. 2008, 53, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Tonosaki, K. Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 2007, 150, 89–96. [Google Scholar] [CrossRef]
- Hur, M.-H.; Song, J.-A.; Lee, J.; Lee, M.S. Aromatherapy for stress reduction in healthy adults: A systematic review and meta-analysis of randomized clinical trials. Maturitas 2014, 79, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Satou, T.; Ohashi, M.; Hayashi, S.; Sadamoto, K.; Koike, K. Interspecies comparison of chemical composition and anxiolytic-like effects of lavender oils upon inhalation. Nat. Prod. Commun. 2011, 6, 1769–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Yoshino, A.; Yamanaka, A.; Asanuma, C.; Satou, T.; Hayashi, S.; Masuo, Y.; Sadamoto, K.; Koike, K. Effects of inhaled lavender essential oil on stress-loaded animals: Changes in anxiety-related behavior and expression levels of selected mRNAs and proteins. Nat. Prod. Commun. 2012, 7, 1539–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukada, M.; Kano, E.; Miyoshi, M.; Komaki, R.; Watanabe, T. Effect of "rose essential oil" inhalation on stress-induced skin-barrier disruption in rats and humans. Chem. Senses 2011, 37, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Orhan, I.E.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.-M.; Shen, C.-W. Aromatherapy benefits autonomic nervous system regulation for elementary school faculty in Taiwan. Evid.-Based Complement. Altern. Med. 2011, 2011, 946537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.-M.; Koo, M.; Yu, Z.-R. Effects of music and essential oil inhalation on cardiac autonomic balance in healthy individuals. J. Altern. Complement. Med. 2009, 15, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Kuchta, K.; Kimura, M.; Rauwald, H.W.; Kamei, T.; Imanishi, J. Effects of bergamot (Citrus bergamia (Risso) Wright & Arn.) essential oil aromatherapy on mood states, parasympathetic nervous system activity, and salivary cortisol levels in 41 healthy females. Forsch. Komplementmedizin 2015, 22, 43–49. [Google Scholar] [CrossRef]
- Saiyudthong, S.; Marsden, C.A. Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother. Res. 2010, 25, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Kawai, E.; Takeda, R.; Ota, A.; Morita, E.; Imai, D.; Suzuki, Y.; Yokoyama, H.; Ueda, S.-Y.; Nakahara, H.; Miyamoto, T.; et al. Increase in diastolic blood pressure induced by fragrance inhalation of grapefruit essential oil is positively correlated with muscle sympathetic nerve activity. J. Physiol. Sci. 2020, 70, 2. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Kang, P.; Lee, H.S.; Seol, G.H. Effects of inhalation of essential oil of Citrus aurantium L. var. amara on menopausal symptoms, stress, and estrogen in postmenopausal women: A randomized controlled trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 796518. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.K.; Kang, W.S.; Woo, J.-M.; Kim, J.W. Effects of essential oil from Chamaecyparis obtusa on cytokine genes in the hippocampus of maternal separation rats. Can. J. Physiol. Pharmacol. 2014, 92, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Kasuya, H.; Hata, E.; Satou, T.; Yoshikawa, M.; Hayashi, S.; Masuo, Y.; Koike, K. Effect on emotional behavior and stress by inhalation of the essential oil from Chamaecyparis obtusa. Nat. Prod. Commun. 2013, 8, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, E.; Ohira, T. Inhalation of Japanese cedar (Cryptomeria japonica) wood odor causes psychological relaxation after monotonous work among female participants. Biomed. Res. 2018, 39, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasuya, H.; Okada, N.; Kubohara, M.; Satou, T.; Masuo, Y.; Koike, K. Expression of BDNF and TH mRNA in the brain following inhaled administration of α-pinene. Phytother. Res. 2015, 29, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-H.; Kim, C.; Seong, K.; Hur, M.-H.; Lim, H.M.; Lee, M.S. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evid.-Based Complement. Altern. Med. 2012, 2012, 984203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.; Beserra, F.; Souza, M.; Totti, B.; Rozza, A. Limonene: Aroma of innovation in health and disease. Chem.-Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, N.; Yamano, E.; Ishii, A.; Tanaka, M.; Nakamura, J.; Watanabe, Y. Involvement of the olfactory system in the induction of anti-fatigue effects by odorants. PLoS ONE 2018, 13, e0195263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Fujihara, M.; Murakami, E.; Miyoshi, M.; Tanaka, Y.; Koba, S.; Tachibana, H. Green odor and depressive-like state in rats: Toward an evidence-based alternative medicine? Behav. Brain Res. 2011, 224, 290–296. [Google Scholar] [CrossRef]
- Kunihiro, K.; Myoda, T.; Tajima, N.; Gotoh, K.; Kaneshima, T.; Someya, T.; Toeda, K.; Fujimori, T.; Nishizawa, M. Volatile components of the essential oil of Artemisia montana and their sedative effects. J. Oleo Sci. 2017, 66, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulluni, N.; Re, T.; Loiacono, I.; Lanzo, G.; Gori, L.; Macchi, C.; Epifani, F.; Bragazzi, N.; Firenzuoli, F. Cannabis essential oil: A preliminary study for the evaluation of the brain effects. Evid.-Based Complement. Altern. Med. 2018, 2018, 1709182. [Google Scholar] [CrossRef] [Green Version]
- Aponso, M.; Patti, A.; Bennett, L.E. Dose-related effects of inhaled essential oils on behavioural measures of anxiety and depression and biomarkers of oxidative stress. J. Ethnopharmacol. 2020, 250, 112469. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Hirano, M.; Shibato, J.; Rakwal, R.; Hwang, I.K.; Masuo, Y. Effects of coffee bean aroma on the rat brain stressed by sleep deprivation: A selected transcript- and 2D gel-based proteome analysis. J. Agric. Food Chem. 2008, 56, 4665–4673. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; Mohamed, S.R.; Hathout, A.S.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon 2011, 57, 984–991. [Google Scholar] [CrossRef]
- Li, C.-C.; Yu, H.-F.; Chang, C.-H.; Liu, Y.-T.; Yao, H.-T. Effects of lemongrass oil and citral on hepatic drug-metabolizing enzymes, oxidative stress, and acetaminophen toxicity in rats. J. Food Drug Anal. 2018, 26, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L.; Postu, P.A. Tetraclinis articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer’s disease amyloidosis model. Phytomedicine 2019, 56, 57–63. [Google Scholar] [CrossRef]
- Hashikawa-Hobara, N.; Otsuka, A.; Ishikawa, R.; Hashikawa, N. Roman chamomile inhalation combined with clomipramine treatment improves treatment-resistant depression-like behavior in mice. Biomed. Pharmacother. 2019, 118, 109263. [Google Scholar] [CrossRef]
- Komiya, M.; Takeuchi, T.; Harada, E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006, 172, 240–249. [Google Scholar] [CrossRef]
- Murakami, T.; Matsukawa, M.; Katsuyama, N.; Imada, M.; Aizawa, S.; Sato, T. Stress-related activities induced by predator odor may become indistinguishable by hinokitiol odor. NeuroReport 2012, 23, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Yang, Z.-Y.; Fan, G.; Ren, J.-N.; Yin, K.-J.; Pan, S.-Y. Antidepressant-like effect of Citrus sinensis (L.) Osbeck essential oil and its main component limonene on mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, E.; Ono, T.; Uwano, T.; Takashima, Y.; Kawasaki, M. Rat preference for food-related odors. Brain Res. Bull. 1991, 27, 387–391. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamamoto, N.; Fujiwara, S.; Kamei, A.; Abe, K.; Nakamura, A. Inhalation of a racemic mixture (R,S)-linalool by rats experiencing restraint stress alters neuropeptide and MHC class I gene expression in the hypothalamus. Neurosci. Lett. 2017, 653, 314–319. [Google Scholar] [CrossRef]
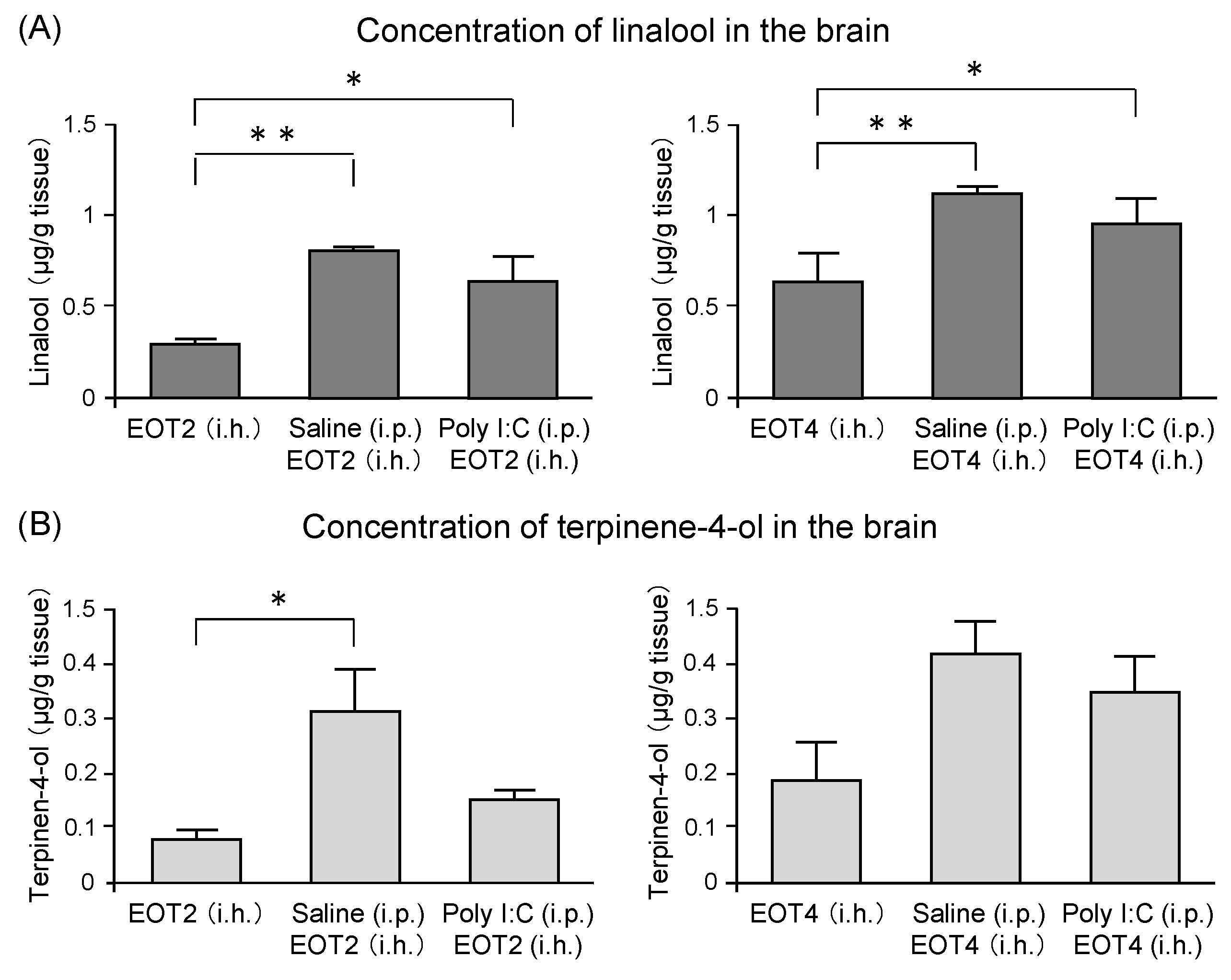
- Hayakawa, M.; Satou, T.; Koike, K.; Masuo, Y. Anti-fatigue activity of essential oil from thyme (linalool chemotype) in the polyriboinosinic:polyribocytidylic acid-induced brain fatigue mouse. Flavour Fragr. J. 2016, 31, 395–399. [Google Scholar] [CrossRef]
- Satou, T.; Hayakawa, M.; Goto, Y.; Masuo, Y.; Koike, K. Anxiolytic-like effects of essential oil from Thymus vulgaris was increased during stress. Flavour Fragr. J. 2018, 33, 191–195. [Google Scholar] [CrossRef]
- Takemoto, H.; Take, C.; Kojima, K.; Kuga, Y.; Hamada, T.; Yasugi, T.; Kato, N.; Koike, K.; Masuo, Y. Effects of sesame oil aroma on mice after exposure to water immersion stress: Analysis of behavior and gene expression in the brain. Molecules 2020, 25, 5915. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R. Essential oil inhaler (AromaStick®) improves heat tolerance in the Hot Immersion Test (HIT). Results from two randomized, controlled experiments. J. Therm. Biol. 2020, 87, 102478. [Google Scholar] [CrossRef] [PubMed]
- Saiyudthong, S.; Pongmayteegul, S.; Marsden, C.A.; Phansuwan-Pujito, P. Anxiety-like behaviour andc-fosexpression in rats that inhaled vetiver essential oil. Nat. Prod. Res. 2015, 29, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kiyokawa, Y.; Kodama, Y.; Arata, S.; Takeuchi, Y.; Mori, Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav. Brain Res. 2013, 240, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyokawa, Y.; Honda, A.; Takeuchi, Y.; Mori, Y. A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav. Brain Res. 2014, 267, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacother. 2019, 111, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily inhalation of α-pinene in mice: Effects on behavior and organ accumulation. Phytother. Res. 2014, 28, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Okuno, H. Regulation and function of immediate-early genes in the brain: Beyond neuronal activity markers. Neurosci. Res. 2011, 69, 175–186. [Google Scholar] [CrossRef]
- Morgan, J.; Cohen, D.; Hempstead, J.; Curran, T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 1987, 237, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.F.; McNaughton, B.L.; Barnes, C.A.; Worley, P.F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999, 2, 1120–1124. [Google Scholar] [CrossRef]
- Ardi, Z.; Albrecht, A.; Richter-Levin, A.; Saha, R.; Richter-Levin, G. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol. Dis. 2016, 88, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aykaç, A.; Aydın, B.; Cabadak, H.; Gören, M.Z. The change in muscarinic receptor subtypes in different brain regions of rats treated with fluoxetine or propranolol in a model of post-traumatic stress disorder. Behav. Brain Res. 2012, 232, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ozbeyli, D.; Aykac, A.; Alaca, N.; Hazar-Yavuz, A.N.; Ozkan, N.; Sener, G. Protective effects of vortioxetine in predator scent stress model of post-traumatic stress disorder in rats: Role on neuroplasticity and apoptosis. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Kawachi, I.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E. A prospective study of coffee drinking and suicide in women. Arch. Intern. Med. 1996, 156, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Yamato, T.; Yamasaki, S.; Misumi, Y.; Kino, M.; Obata, T.; Aomine, M. Modulation of the stress response by coffee: An in vivo microdialysis study of hippocampal serotonin and dopamine levels in rat. Neurosci. Lett. 2002, 332, 87–90. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Tomala, A.; Podolak, I.; Cielecka-Piontek, J. Cannabis sativa L. as a natural drug meeting the criteria of a multitarget approach to treatment. Int. J. Mol. Sci. 2021, 22, 778. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Hayakawa, M.; Kasuya, H.; Masuo, Y.; Koike, K. Mouse brain concentrations of α-pinene, limonene, linalool, and 1,8-cineole following inhalation. Flavour Fragr. J. 2017, 32, 36–39. [Google Scholar] [CrossRef]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef]
- Zárybnický, T.; Boušová, I.; Ambrož, M.; Skálová, L. Hepatotoxicity of monoterpenes and sesquiterpenes. Arch. Toxicol. 2018, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]


| Smell | Scientific Name | Main Component(s) | References | ||
|---|---|---|---|---|---|
| Human | Rat | Mouse | |||
| Many kinds of aroma, such as lavender, rosemary, bitter orange, peppermint, linalool, limonene | [4] (Review) | ||||
| Partner smell | [9] | ||||
| Black tea | 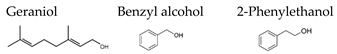 | [81] | |||
| Lavender | Lavandula | 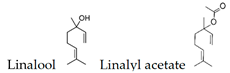 | [82,83,84,85,86,87] | [88] | [89,90] |
| Chamomile | Matricaria recutita | Chamizulene 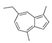 | [83] | ||
| Rosemary | Rosmarinus officinalis | 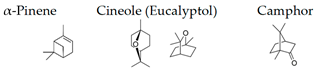 | [86] | ||
| Rose | Rosa | 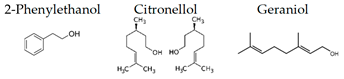 | [91] | [91] | [72] |
| 2-Phenylethanol | 2-Phenylethanol 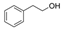 | [92] | |||
| Bergamot | Citrus bergamia | 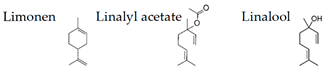 | [93,94,95] | [96] | |
| Grapefruit | Citrus paradisi | 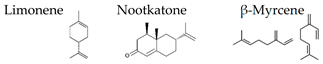 | [97] | ||
| Neroli | Citrus aurantium L. var. amara | Linalool 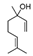 | [98] | ||
| Cypress | Chamaecyparis obtusa | α-Pinene 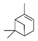 | [99] | [100] | [101] |
| Cedar | Cryptomeria japonica | α-Pinene  | [102] | ||
| α-Pinene | α-Pinene  | [103] | |||
| Mixture of essential oils | [104] | ||||
| Lavender | Lavandula officinalis | Linalool 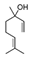 Linalyl acetate Linalyl acetate 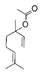 | |||
| Ylang-ylang | Cananga odorata | Germacrene D 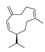 | |||
| Majoram | Origanum majorana | Terpinen-4-ol 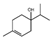 γ-Terpinene γ-Terpinene  | |||
| Neroli | Citrus aurantium | Linalool 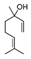 | |||
| Smell | Scientific name | Main component(s) | References | ||
| Human | Rat | Mouse | |||
| Limonene | Limonene  | [105] | |||
| Green |  | [106] | [73,107] | ||
| Yomogi | Artemisia montana | Cineol (Eucalyptol) 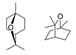 | [108] | ||
| Cannabis | Cannabis sativa | 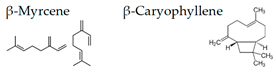 | [109] | ||
| Many kinds of volitile components | [110] | ||||
| Coffee bean | Coffea | Furfuryl alcohol 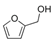 | [111] | ||
| Thyme | Tymus vulgaris | Carvacrol 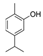 Thymol Thymol 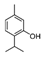 | [112] | ||
| Lemongrass | Cymbopogon flexuosus | Citral 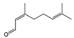 | [113] | ||
| Tetraclinis | Tetraclinis articulata | Hinokitiol 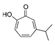 α-Pinene α-Pinene 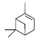 | [114] | ||
| Mixture of essential oil components | 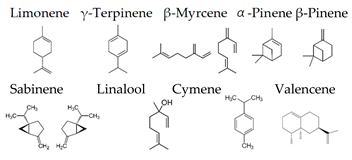 | [92] | |||
| Roman chamomile | Chamaemelum nobile | Angeric acid 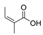 | [115] | ||
| Lemon | Citrus limon | Limonene  | [116] | ||
| Hinokitiol | Hinokitiol  | [117] | |||
| Navel orange | Citrus sinensis (L.) Osbeck | 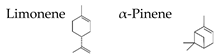 | [118] | ||
| Thyme linalool | Thymus vulgaris ct. linalool |  | [119,120] | [121,122] | |
| Sesame oil | 2-Methylpyrazine 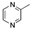 | [123] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuo, Y.; Satou, T.; Takemoto, H.; Koike, K. Smell and Stress Response in the Brain: Review of the Connection between Chemistry and Neuropharmacology. Molecules 2021, 26, 2571. https://doi.org/10.3390/molecules26092571
Masuo Y, Satou T, Takemoto H, Koike K. Smell and Stress Response in the Brain: Review of the Connection between Chemistry and Neuropharmacology. Molecules. 2021; 26(9):2571. https://doi.org/10.3390/molecules26092571
Chicago/Turabian StyleMasuo, Yoshinori, Tadaaki Satou, Hiroaki Takemoto, and Kazuo Koike. 2021. "Smell and Stress Response in the Brain: Review of the Connection between Chemistry and Neuropharmacology" Molecules 26, no. 9: 2571. https://doi.org/10.3390/molecules26092571






