Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
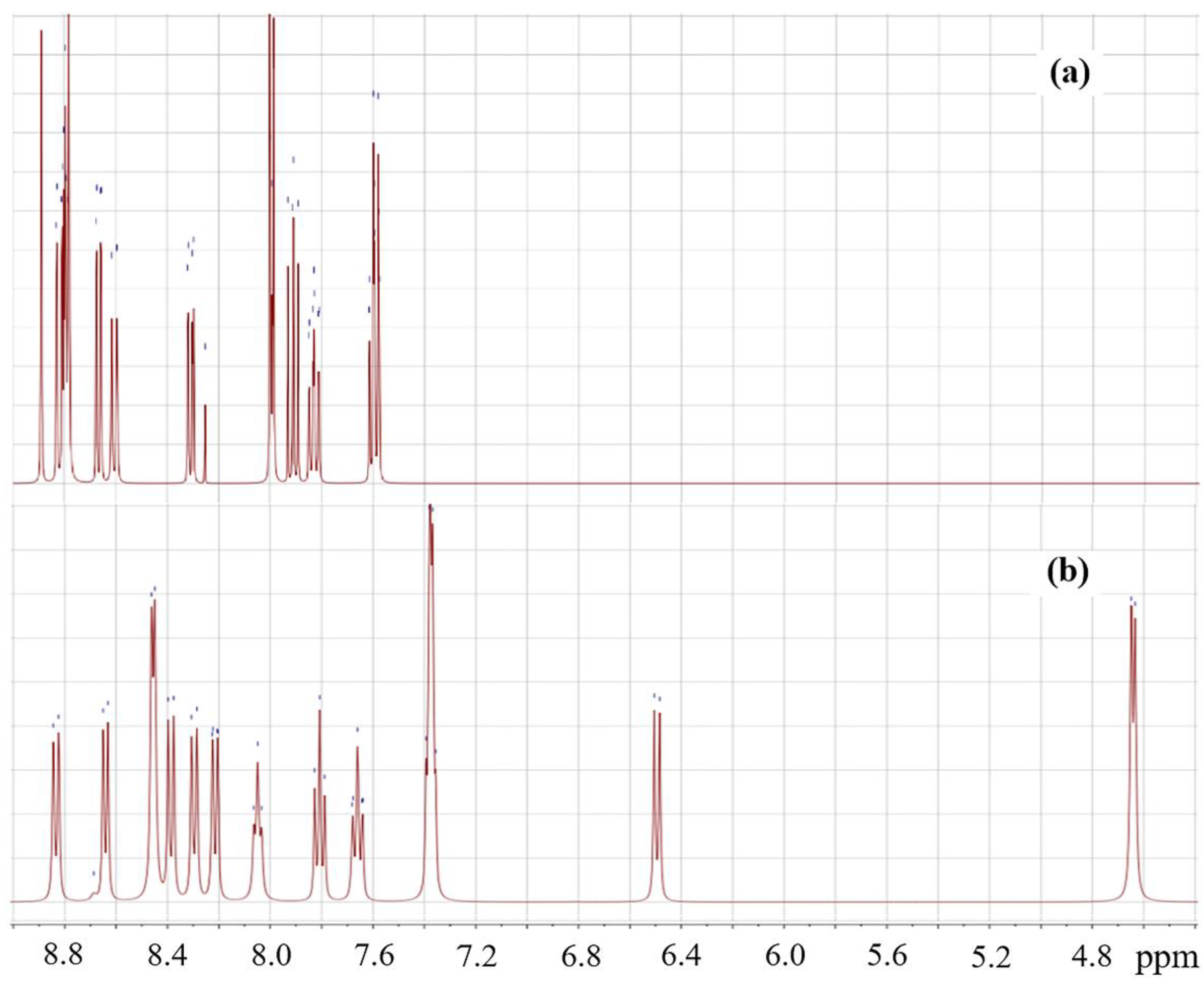
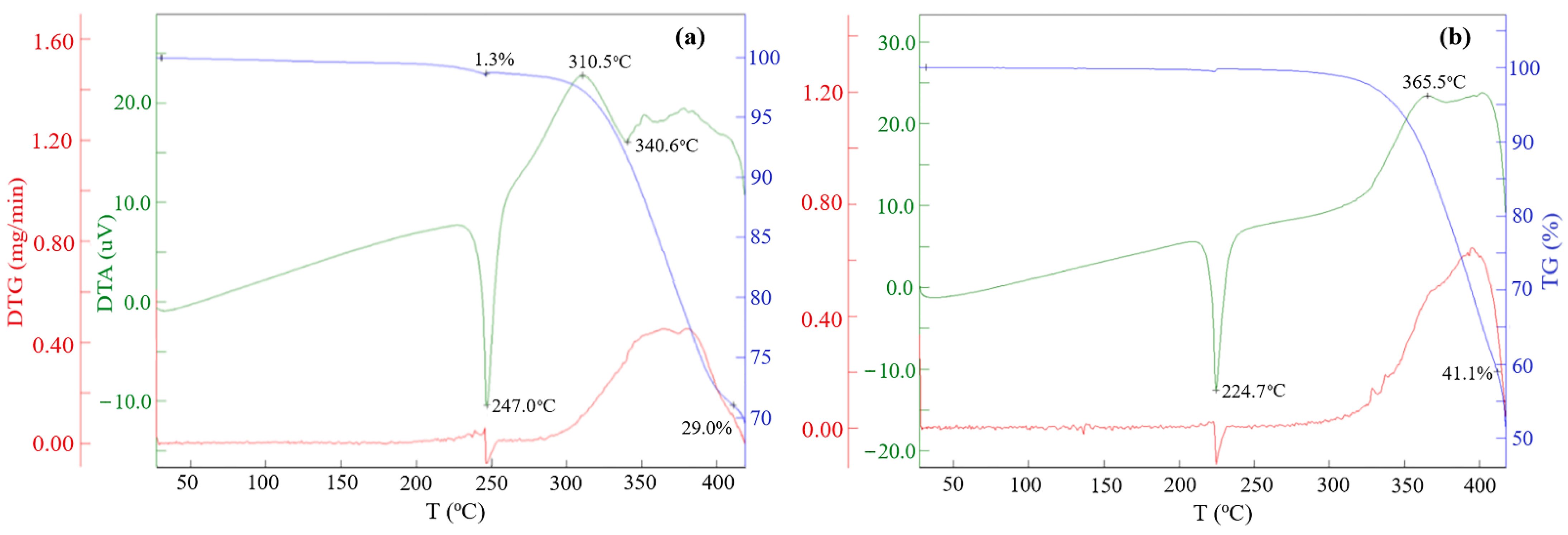
2.1. Synthesis and Characterization of Azomethines
2.2. Reduction of Obtained Azomethines
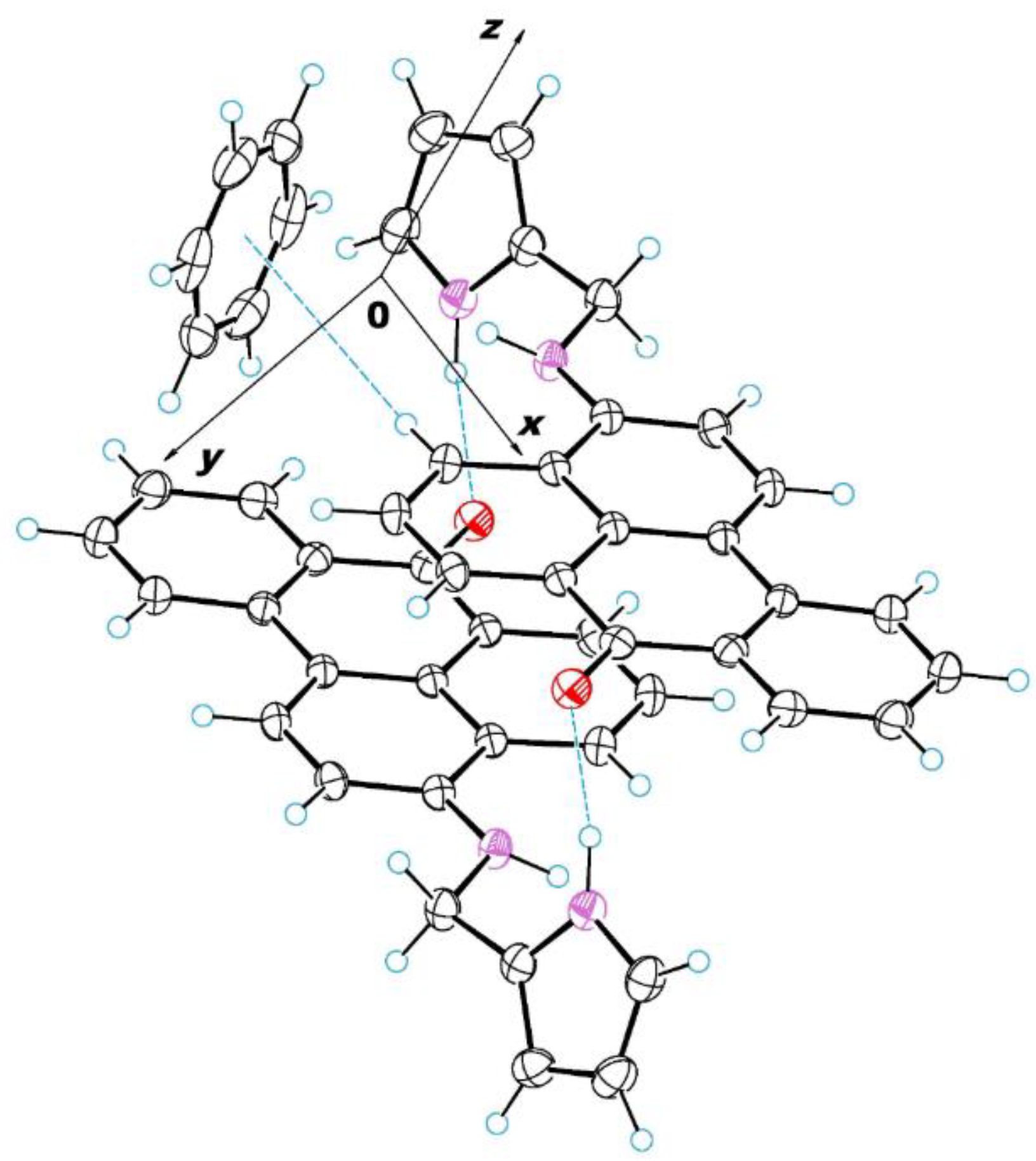
2.3. X-ray Crystallographic Study
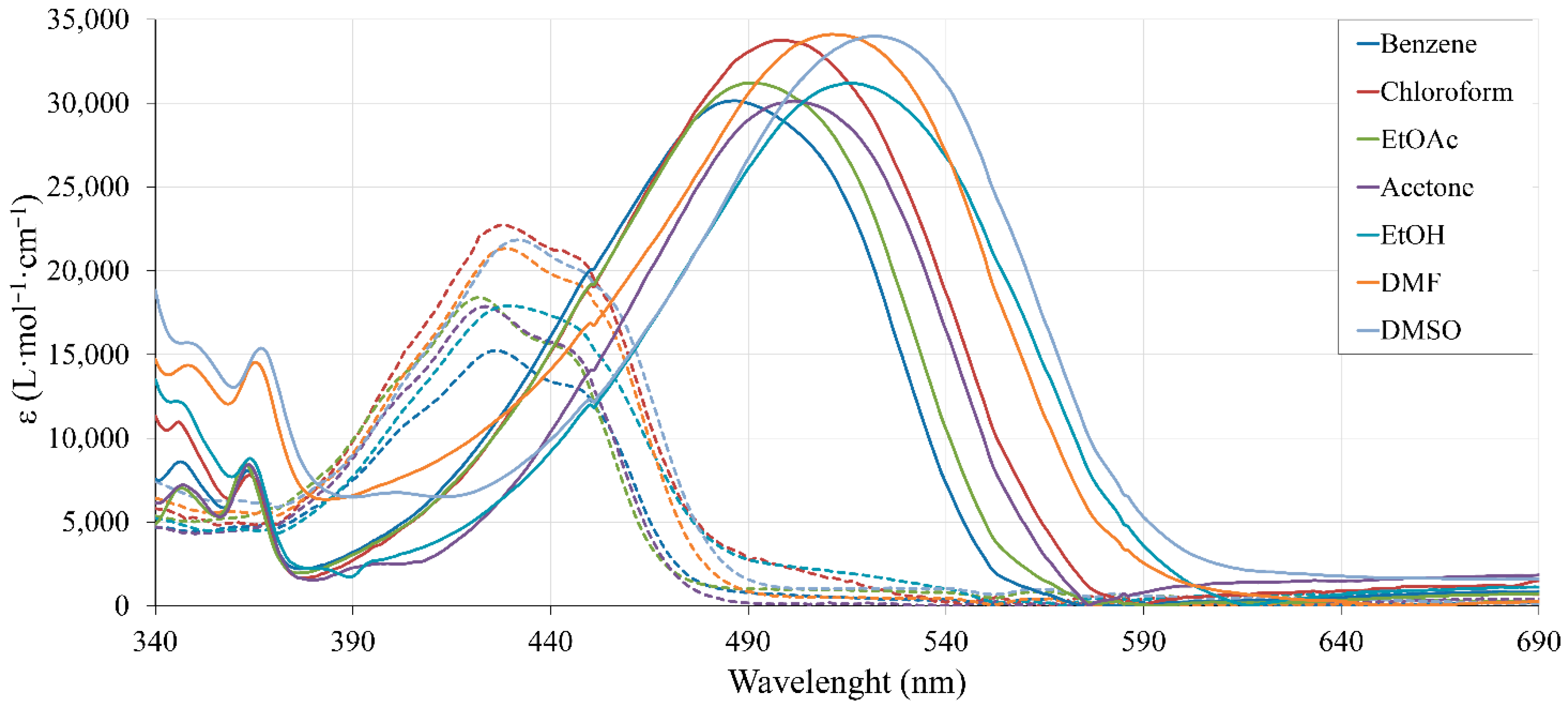
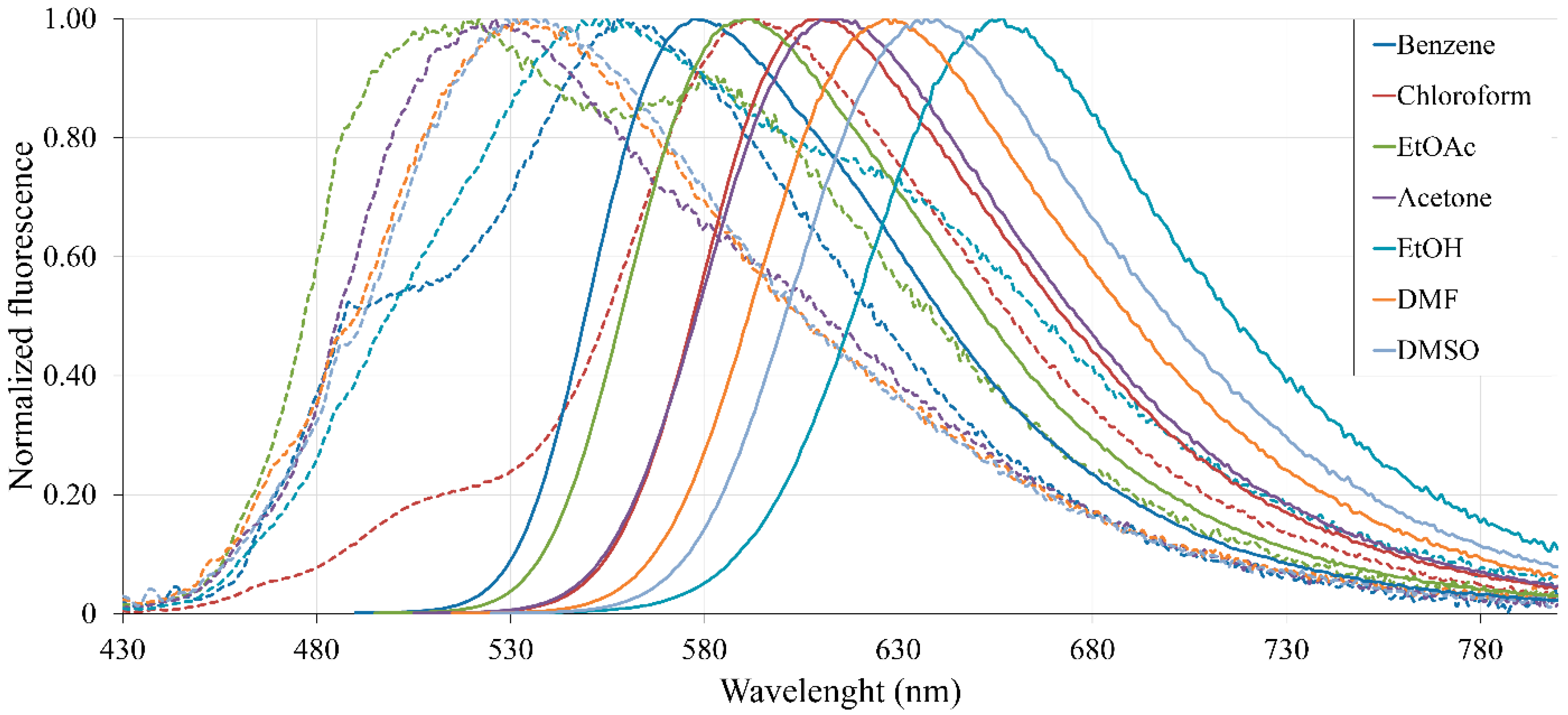
2.4. Spectroscopic Properties
3. Experimental Method
3.1. Materials and Basic Measurements
3.2. Spectroscopic Measurements
3.3. Single Crystal X-ray Diffraction Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dhar, D.N.; Taploo, C.L. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506. [Google Scholar]
- Berhanu, A.L.; Gaurav, M.I.; Malik, A.; Aulakh, J.S.; Kumar, V.; Kim, K.H. A review of the applications of Schiff bases as optical chemical sensors. Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Al-Kahraman, Y.M.S.A.; Madkour, H.M.F.; Ali, D.; Yasinzai, M. Antileishmanial, antimicrobial and antifungal activities of some new aryl azomethines. Molecules 2010, 15, 660–671. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.-Y.; Zhang, Q.-L.; Yang, X.-J.; Huang, Y.-L.; Redshaw, C.; Xu, H. A simple turn-off Schiff base fluorescent sensor for copper (II) ion and its application in water analysis. Molecules 2021, 26, 1233. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.W. The chemistry of imines. Chem. Rev. 1963, 63, 489–510. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, A.; Rivier, L.; Dufresne, S.; Skene, W.G. Spectral investigation of conjugated azomethines: A large palette of colors possible with acid and oxidant doping. Mater. Chem. Phys. 2012, 132, 722–728. [Google Scholar] [CrossRef]
- Yousif, E.; Salih, N.; Salimon, J. Improvement of photo stabilisation of PVC in the presence of 2N-salisylidine-5-(substituted)-1,3,4-triadiazole. J. Appl. Polym. Sci. 2011, 120, 2207–2214. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Bejan, A.-E.; Damaceanu, M.-D. New heterocyclic conjugated azomethines containing triphenylamine units with optical and electrochemical responses towards the acid environment. Synth. Met. 2020, 268, 116498. [Google Scholar] [CrossRef]
- Temizkan, K.; Kaya, İ. Fluorescence quantum yields and chromatic properties of poly(azomethine)s containing pyridine ring. Mater. Sci. Eng. B 2020, 252, 114483. [Google Scholar] [CrossRef]
- Popova, O.; Revinskii, Y.V.; Tkachev, V.V.; Utenyshev, A.N.; Karlutova, O.Y.; Starikov, A.G.; Dubonosov, A.D.; Bren, V.; Aldoshin, S.; Minkin, V. Structure, spectral-luminescent and ionochromic properties of hydroxyaryl(hetaryl)idene azomethine imines. J. Mol. Struct. 2020, 1199, 127013. [Google Scholar] [CrossRef]
- Khan, S.A.; Ullah, Q.; Almalki, A.S.A.; Kumar, S.; Obaid, R.J.; Alsharif, M.A.; Alfaifi, S.Y.; Hashmi, A.A. Synthesis and photophysical investigation of (BTHN) Schiff base as off-on Cd2+ fluorescent chemosensor and its live cell imaging. J. Mol. Liq. 2021, 328, 115407. [Google Scholar] [CrossRef]
- Shanty, A.A.; Philip, J.E.; Sneha, E.J.; Kurup, P.M.R.; Balachandran, S.; Mohanan, P.V. Synthesis, characterization and biological studies of Schiff bases derived from heterocyclic moiety. Bioorg. Chem. 2017, 70, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Prabhakara, C.T.; Halasangi, B.M.; Toragalmath, S.S.; Badami, P.S. DNA cleavage, antibacterial, antifungal and anthelmintic studies of Co (II), Ni (II) and Cu (II) complexes of coumarin Schiff bases: Synthesis and spectral approach. Spectrochim. Acta A 2015, 137, 641–651. [Google Scholar] [CrossRef]
- Chinnasamy, R.P.; Sundararajan, R.; Govindaraj, S. Synthesis, characterization, and analgesic activity novel schiff base of isatin derivatives. J. Adv. Pharm. Technol. Res. 2010, 1, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sztanke, K.; Maziarka, A.; Osinka, A.; Sztanke, M. An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg. Med. Chem. 2013, 21, 3648–3666. [Google Scholar] [CrossRef] [PubMed]
- Carlini, F.; Pafoni, C.; Bofa, G. New daylight fluorescent pigments. Dye. Pigments 1982, 3, 59–69. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I. Synthesis and properties of benzanthrone derivatives as luminophore dyes for liquid crystals. Dye. Pigments 1998, 37, 155–164. [Google Scholar] [CrossRef]
- Grabchev, I.; Bojinov, V.; Moneva, I. Functional properties of azomethine substituted benzanthrone dyes for use in nematic liquid cristals. J. Mol. Struct. 1998, 471, 19–25. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Wolarz, E.; Bauman, D.; Stoyanov, S. Spectral properties of 3-benzanthrone derivative dyes in isotropic solvents, polymer film and liquid crystal. Z. Naturforschung A 2001, 56, 291–296. [Google Scholar] [CrossRef]
- Refat, M.S.; Aqeel, S.M.; Grabtchev, I.K. Spectroscopic and physicochemical studies of charge-transfer complexes of some benzanthrone derivatives “luminophore dyes” with iodine as δ-acceptor. Can. J. Anal. Sci. Spectrosc. 2004, 49, 258–265. [Google Scholar]
- Iwan, A.; Sek, D. Processible polyazomethines and polyketanils: From aerospace to light-emitting diodes and other advanced applications. Prog. Polym. Sci. 2008, 33, 289–345. [Google Scholar] [CrossRef]
- Chen, L.X.; Niu, C.G.; Xie, Z.M.; Long, Y.Q.; Song, X.R. Fiber-Optic sensor for iodine based on a covalently immobilized aminobenzanthrone Schiff base. Anal. Sci. 2006, 22, 977–981. [Google Scholar] [CrossRef]
- Gonta, S.; Utinans, M.; Kirilov, G.; Belyakov, S.; Ivanova, I.; Fleisher, M.; Savenkov, V.; Kirilova, E. Fluorescent substituted amidines of benzanthrone: Synthesis, spectroscopy and quantum chemical calculations. Spectrochim. Acta A 2013, 101, 325–334. [Google Scholar] [CrossRef]
- Ryzhova, O.; Vus, K.; Trusova, V.; Kirilova, E.; Kirilov, G.; Gorbenko, G.; Kinnunen, P. Novel benzanthrone probes for membrane and protein studies. Methods Appl. Fluoresc. 2016, 4, 034007. [Google Scholar] [CrossRef]
- Kirilova, E.M.; Puckins, A.I.; Romanovska, E.; Fleisher, M.; Belyakov, S.V. Novel amidine derivatives of benzanthrone: Effect of bromine atom on the spectral parameters. Spectrochim. Acta A 2018, 202, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shivraj; Siddlingeshwar, B.; Kirilova, E.M.; Belyakov, S.V.; Divakar, D.D.; Alkheraif, A.A. Photophysical properties of benzanthrone derivatives: Effect of substituent, solvent polarity and hydrogen bonding. Photochem. Photobiol. Sci. 2018, 17, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.A.; Altalhi, T.A.; El-Megharbel, S.M.; Saad, H.A.; Refat, M.S.; Grabchev, I.; Althobaiti, R.A. Capturing of environment polluting metal ions Co2+, Ni2+, Cu2+, and Zn2+ using a 3-azomethine benzanthrone-based fluorescent dye: Its synthesis, structural, and spectroscopic characterizations. Russ. J. Gen. Chem. 2020, 90, 2394–2399. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Grabchev, I.K. Synthesis of ethyl 3-aryl-1-methyl-8-oxo- 8H-anthra(9,1-gh)-quinoline-2-carboxylates as dyes for potential application in liquid crystal displays. Org. Lett. 2003, 12, 2185–2187. [Google Scholar] [CrossRef]
- Nashimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; Wiley: New York, NY, USA, 2001; pp. 288–290. [Google Scholar]
- Byung, T.C.; Sang, K.K. Direct and indirect reductive amination of aldehydes and ketones with solid acid-activated sodium borohydride under solvent-free conditions. Tetrahedron 2005, 61, 5725–5734. [Google Scholar]
- Lukevics, E.; Ignatovich, L.; Belyakov, S. Disordering in the crystal structures of thienyl-germatranes. Chem. Heterocycl. Compd. 2007, 43, 243–249. [Google Scholar] [CrossRef]
- Kapusta, P.; Machalicky, O.; Hrdina, R.; Nepras, M.; Zimmt, B.M.; Fidler, V. Photophysics of 3-substituted benzanthrones: Substituent and solvent control of intersystem crossing. J. Phys. Chem. 2003, 107, 9740–9746. [Google Scholar] [CrossRef]
- Krasovitskii, B.M.; Bolotin, B.M. Organic Luminescent Materials; Wiley-VCH: New York, NY, USA, 1988; pp. 149–152. [Google Scholar]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, E.M.; Nikolaeva, I.D.; Romanovska, E.; Puckins, A.I.; Belyakov, S.V. The synthesis of novel heterocyclic 3-acetamide derivatives of benzanthrone. Chem. Heterocycl. Compd. 2020, 56, 192–198. [Google Scholar] [CrossRef]
- Bentley, P.; McKellar, J.F.; Phillips, G.O. The photochemistry of benz[de]anthracen-7-ones. Part I. Electronic absorption and emission spectroscopy. J. Chem. Soc. Perkin Trans. 2 1974, 5, 523–526. [Google Scholar] [CrossRef]
- Sednev, M.V.; Belov, V.N.; Hell, S.W. Fluorescent dyes with large stokes shifts for super-resolution optical microscopy of biological objects: A review. Methods Appl. Fluoresc. 2015, 3, 042004. [Google Scholar] [CrossRef]
- Czaplińska, B.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Slodek, A.; Korzec, M.; Musiol, R. Theoretical and experimental investigations of large stokes shift fluorophores based on a quinoline scaffold. Molecules 2020, 25, 2488. [Google Scholar] [CrossRef]
- Luttringhaus, A.; Neresheimer, H. Zur kenntnis des benzanthrons. Justus Liebigs Ann. Chem. 1929, 473, 259–289. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Bond, Angle | 2a | 2e | 3a |
|---|---|---|---|
| N18−C3 | 1.402(1) | 1.405(2) | 1.376(1) |
| N18−C19 | 1.281(2) | 1.277(2) | 1.458(1) |
| C19−C20 | 1.428(2) | 1.439(2) | 1.489(1) |
| C20−C21 | 1.383(2) | 1.363(2) | 1.372(2) |
| C21−C22 | 1.404(2) | 1.410(2) | 1.418(2) |
| C22−C23 | 1.364(2) | 1.348(3) | 1.362(2) |
| C23−S24 | - | 1.715(2) | - |
| C23−N24 | 1.349(2) | - | 1.368(1) |
| S24−C20 | - | 1.721(1) | - |
| N24−C20 | 1.376(2) | - | 1.369(2) |
| C3−N18−C19 | 119.8(1) | 119.7(1) | 120.44(9) |
| N18−C19−C20 | 122.2(1) | 121.8(2) | 111.51(9) |
| C19−C20−C21 | 128.9(1) | 128.9(1) | 130.6(1) |
| C20−C21−C22 | 106.9(1) | 113.3(2) | 107.4(1) |
| C21−C22−C23 | 107.7(2) | 112.2(2) | 107.3(1) |
| C22−C23−S24 | - | 112.3(1) | - |
| C22−C23−N24 | 108.8(1) | - | 108.3(1) |
| C23−S24−C20 | - | 91.34(9) | - |
| C23−N24−C20 | 109.1(1) | - | 109.4(1) |
| S24−C20−C19 | - | 121.6(1) | - |
| N24−C20−C19 | 123.3(1) | - | 121.7(1) |
| Solvent | 1 | 2a | 3a | 2c | 3c | 2e | 3e | 2f | 3f |
|---|---|---|---|---|---|---|---|---|---|
| Benzene | 465 | 426 | 478 | 426 | 487 | 437 | 479 | 435 | 475 |
| CHCl3 | 475 | 430 | 481 | 442 | 499 | 441 | 489 | 443 | 486 |
| EtOAc | 486 | 422 | 487 | 422 | 490 | 433 | 488 | 432 | 484 |
| Acetone | 495 | 424 | 489 | 424 | 502 | 437 | 498 | 435 | 495 |
| EtOH | 519 | 430 | 517 | 430 | 515 | 444 | 520 | 439 | 514 |
| DMF | 514 | 428 | 514 | 429 | 511 | 443 | 510 | 442 | 510 |
| DMSO | 524 | 432 | 524 | 432 | 522 | 447 | 522 | 446 | 519 |
| Solvent | 1 | 2a | 3a | 2c | 3c | 2e | 3e | 2f | 3f |
|---|---|---|---|---|---|---|---|---|---|
| Benzene | 570 | 563 | 577 | 560 | 577 | 506 | 567 | 508 | 564 |
| CHCl3 | 590 | 530 | 608 | 593 | 609 | 540 | 602 | 508;583 | 597 |
| EtOAc | 592 | 516;576 | 592 | 516;580 | 592 | 497 | 586 | 509;579 | 584 |
| Acetone | 625 | 530 | 615 | 526 | 615 | 518 | 609 | 526;587 | 607 |
| EtOH | 659 | 516 | 657 | 553 | 657 | 560 | 657 | 553 | 655 |
| DMF | 622 | 530 | 627 | 533 | 627 | 528 | 621 | 533 | 629 |
| DMSO | 634 | 531 | 640 | 534 | 637 | 568 | 634 | 534 | 632 |
| Solvent | 1 | 2a | 3a | 2c | 3c | 2e | 3e | 2f | 3f |
|---|---|---|---|---|---|---|---|---|---|
| Benzene | 0.60 | 0.01 | 0.51 | 0.02 | 0.55 | 0.03 | 0.57 | 0.02 | 0.56 |
| CHCl3 | 0.37 | 0.01 | 0.35 | 0.03 | 0.38 | 0.03 | 0.42 | 0.03 | 0.40 |
| EtOAc | 0.34 | 0.03 | 0.52 | 0.04 | 0.57 | 0.04 | 0.61 | 0.03 | 0.58 |
| Acetone | 0.12 | 0.02 | 0.36 | 0.04 | 0.36 | 0.03 | 0.40 | 0.03 | 0.37 |
| EtOH | 0.08 | 0.01 | 0.09 | 0.02 | 0.09 | 0.02 | 0.11 | 0.02 | 0.09 |
| DMF | 0.21 | 0.02 | 0.20 | 0.03 | 0.22 | 0.03 | 0.24 | 0.04 | 0.23 |
| DMSO | 0.26 | 0.02 | 0.24 | 0.03 | 0.25 | 0.03 | 0.27 | 0.03 | 0.25 |
| Parameter | 2a | 2e | 3a |
|---|---|---|---|
| Empirical formula | C22H14N2O | C22H13NOS | C22H16N2O·½C6H6 |
| Formula weight | 322.37 | 339.41 | 363.42 |
| Diffractometer | Rigaku, XtaLAB Synergy, Dualflex, HyPix | Bruker-Nonius KappaCCD | Rigaku, XtaLAB Synergy, Dualflex, HyPix |
| Radiation | Cu-Kα (λ = 1.54184 Å) | Mo-Kα (λ = 0.71073 Å) | Cu-Kα (λ = 1.54184 Å) |
| Temperature (K) | 130 | 173 | 150 |
| Crystal size (mm3) | 0.11 × 0.07 × 0.02 | 0.32 × 0.27 × 0.11 | 0.19 × 0.03 × 0.02 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/a | P21/c |
| a (Å) | 11.4365(3) | 12.9304(3) | 11.8920(1) |
| b (Å) | 8.1050(2) | 9.2304(3) | 8.19563(8) |
| c (Å) | 16.5061(3) | 14.4560(4) | 18.9101(2) |
| β (°) | 94.054(2) | 112.567(1) | 96.8959(9) |
| Unit cell volume (Å3) | 1526.17(6) | 1593.26(8) | 1829.69(3) |
| Molecular multiplicity | 4 | 4 | 4 |
| Calculated density (g/cm3) | 1.403 | 1.415 | 1.319 |
| Absorption coefficient (mm−1) | 0.691 | 0.212 | 0.636 |
| F(000) | 672 | 704 | 764 |
| 2θmax (°) | 155.0 | 57.0 | 155.0 |
| Reflections collected | 16174 | 4376 | 19931 |
| Number of independent reflections | 3200 (Rint = 0.064) | 4089 (Rint = 0.024) | 3879 (Rint = 0.034) |
| Reflections with I > 2σ(I) | 2888 | 3157 | 3543 |
| Number of refined parameters | 242 | 238 | 262 |
| Goodness of fit | 1.029 | 1.047 | 1.074 |
| R-factors (R1 for I > 2σ(I), and wR2 for all data) | 0.0491, 0.1490 | 0.0497, 0.1242 | 0.0414, 0.1209 |
| Δρmax, Δρmin (e Å−3) | 0.247 −0.311 | 0.217 −0.256 | 0.280 −0.182 |
| CCDC deposition number | 2062655 | 2062656 | 2062657 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, N.; Nikolajeva, I.; Pučkins, A.; Belyakov, S.; Kirilova, E. Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy. Molecules 2021, 26, 2570. https://doi.org/10.3390/molecules26092570
Orlova N, Nikolajeva I, Pučkins A, Belyakov S, Kirilova E. Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy. Molecules. 2021; 26(9):2570. https://doi.org/10.3390/molecules26092570
Chicago/Turabian StyleOrlova, Natalja, Irena Nikolajeva, Aleksandrs Pučkins, Sergey Belyakov, and Elena Kirilova. 2021. "Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy" Molecules 26, no. 9: 2570. https://doi.org/10.3390/molecules26092570
APA StyleOrlova, N., Nikolajeva, I., Pučkins, A., Belyakov, S., & Kirilova, E. (2021). Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy. Molecules, 26(9), 2570. https://doi.org/10.3390/molecules26092570






