Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review
Abstract
1. Introduction
1.1. Methods to Reduce the Growth of the Microbes
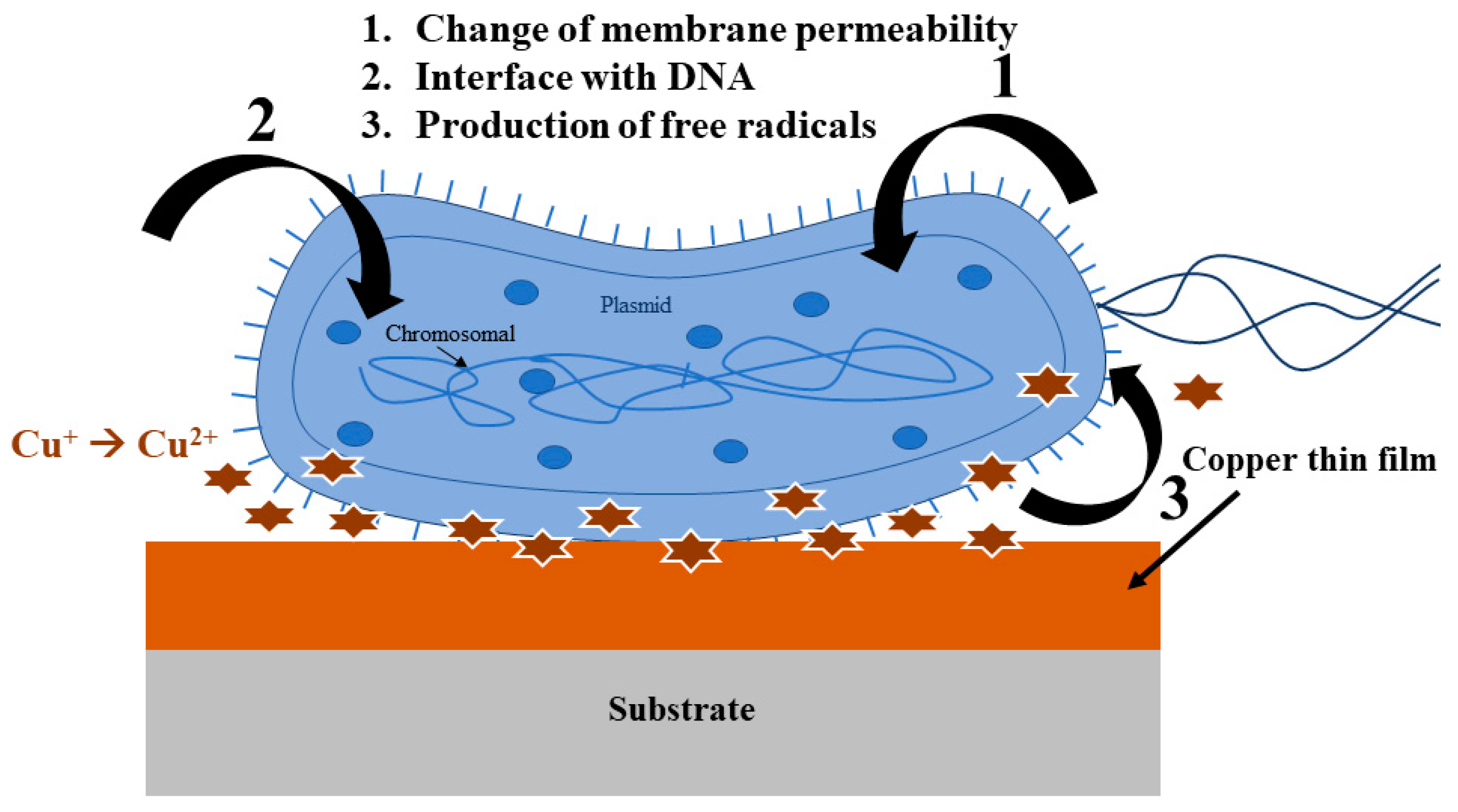
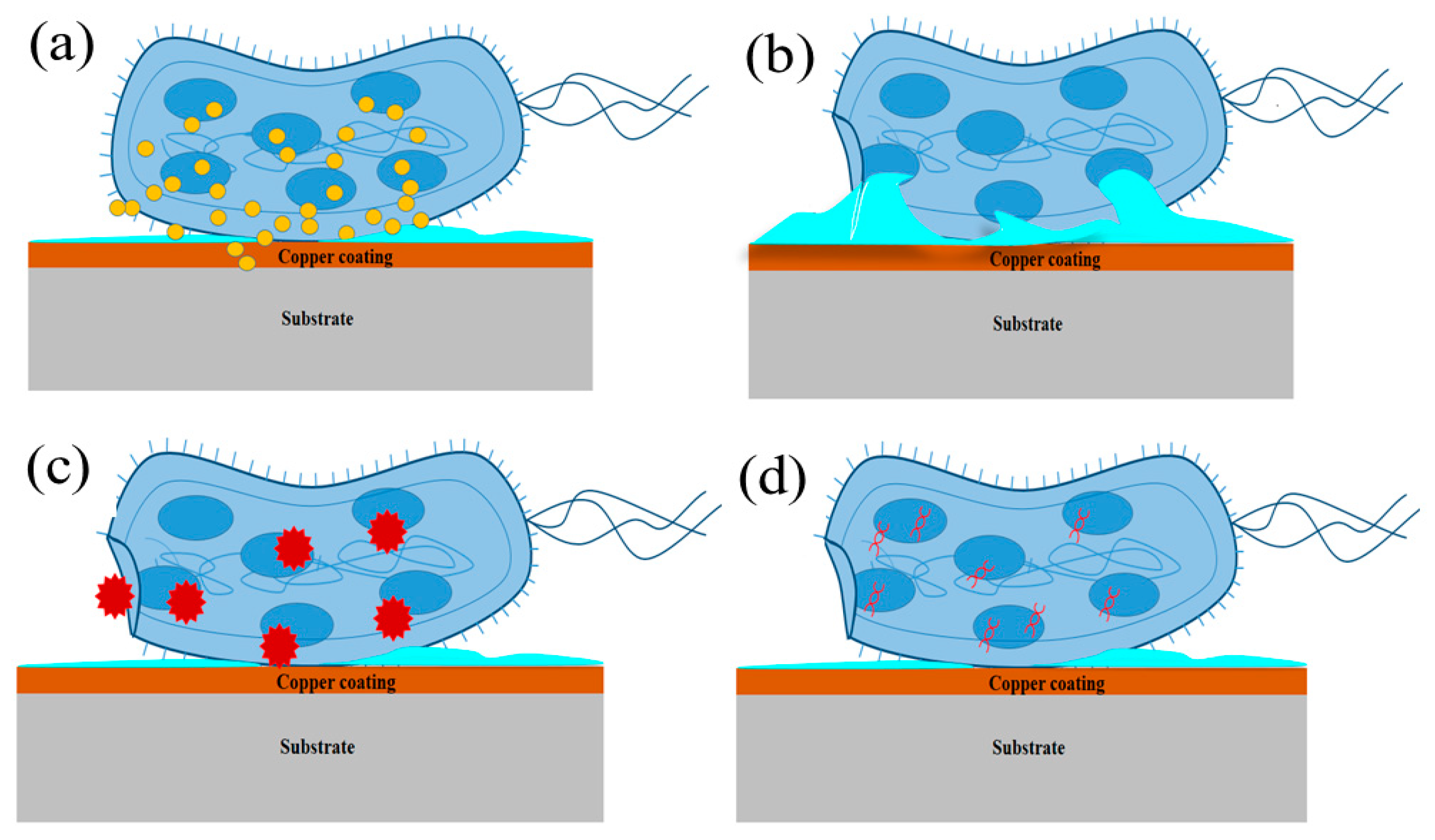
1.2. Mechanism of Microbe Killing
- Copper is a stable metal and it can be used in different forms, such as particles, ions absorbed or exchanged, in carrier salts, hybrid structures, composites, and alloys.
- Copper can encapsulate nanoparticles as a thin sheath on a variety of metals as a coating. These copper nanostructures or alloys can be easily prepared and handled.
2. Strategies for Designing Antimicrobial Coatings
2.1. Antimicrobial Agent Release Coatings
2.2. Contact Killing
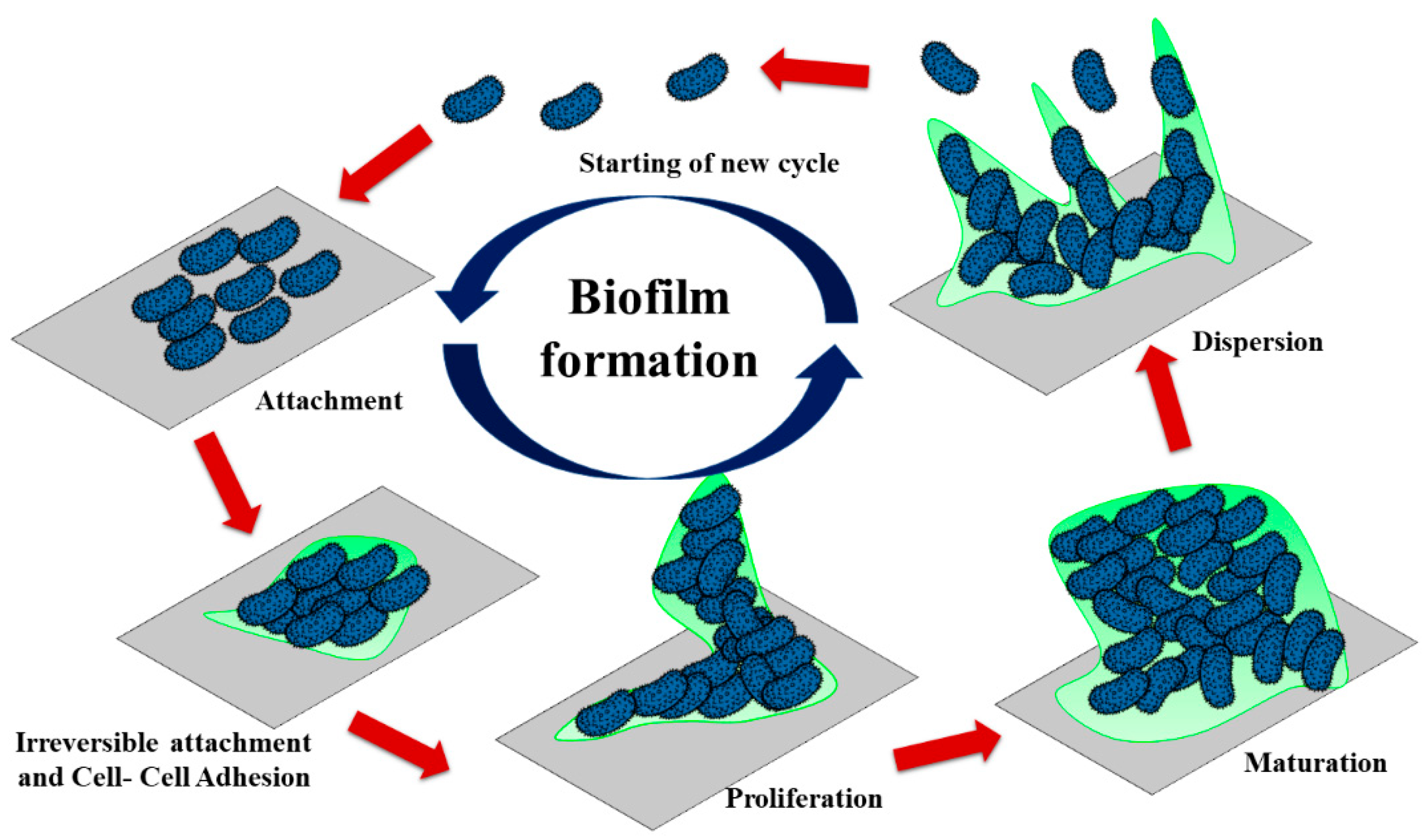
2.3. Anti-Adhesion/Bacterial Repelling
3. Copper Coatings as Antimicrobial Surfaces
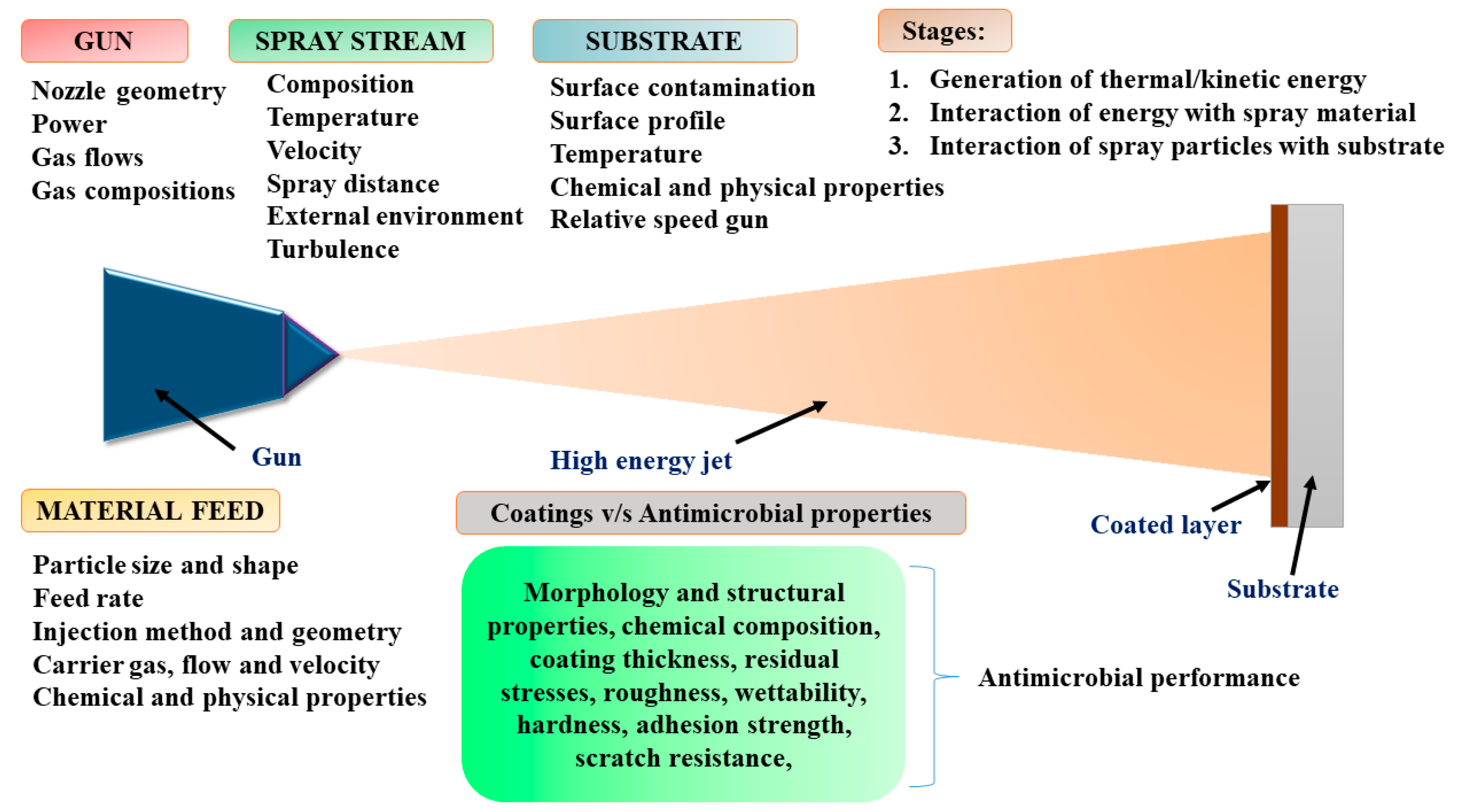
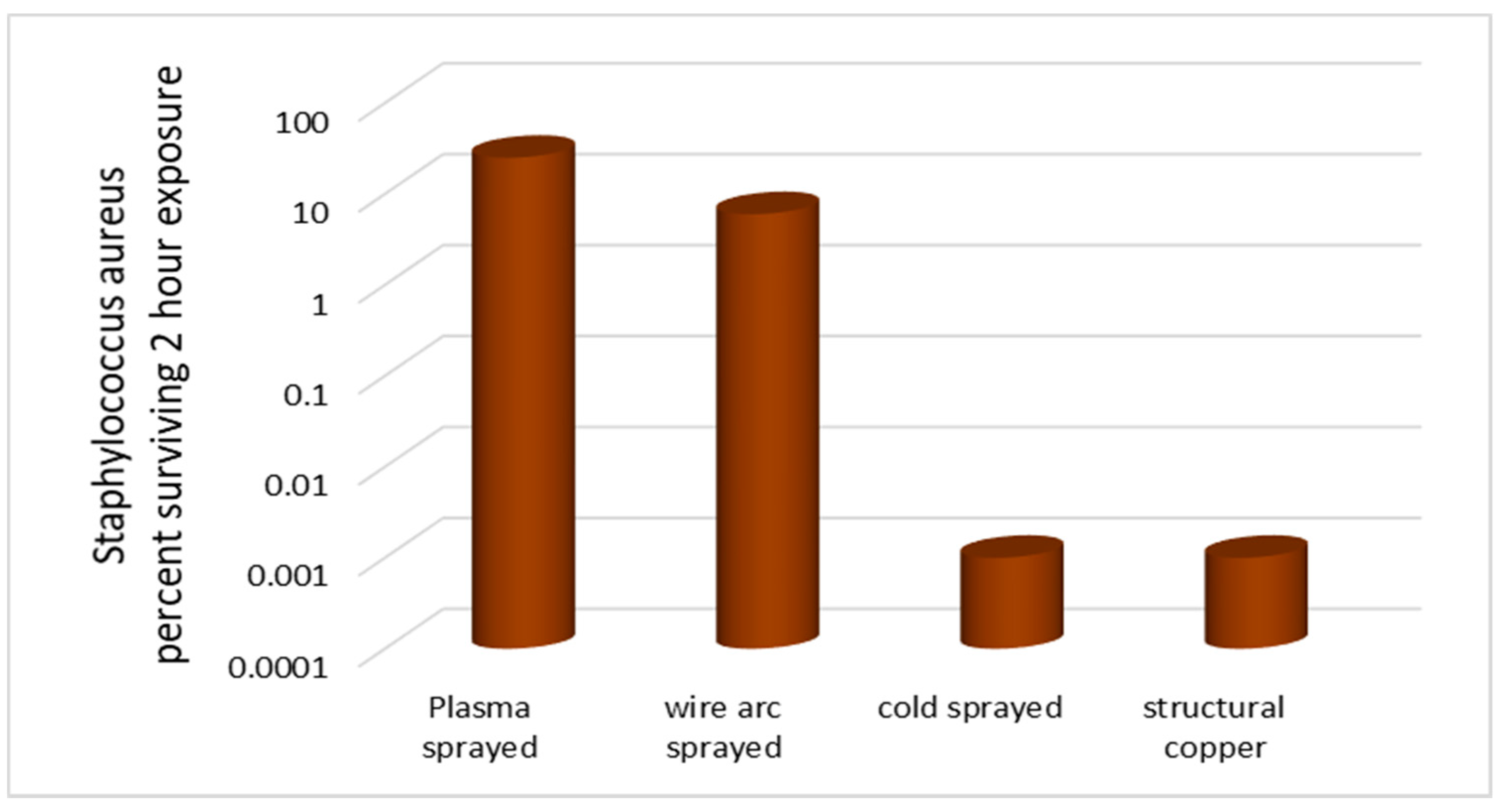
3.1. Thermal Spray Techniques
- (a)
- The particles must be in a fully molten state.
- (b)
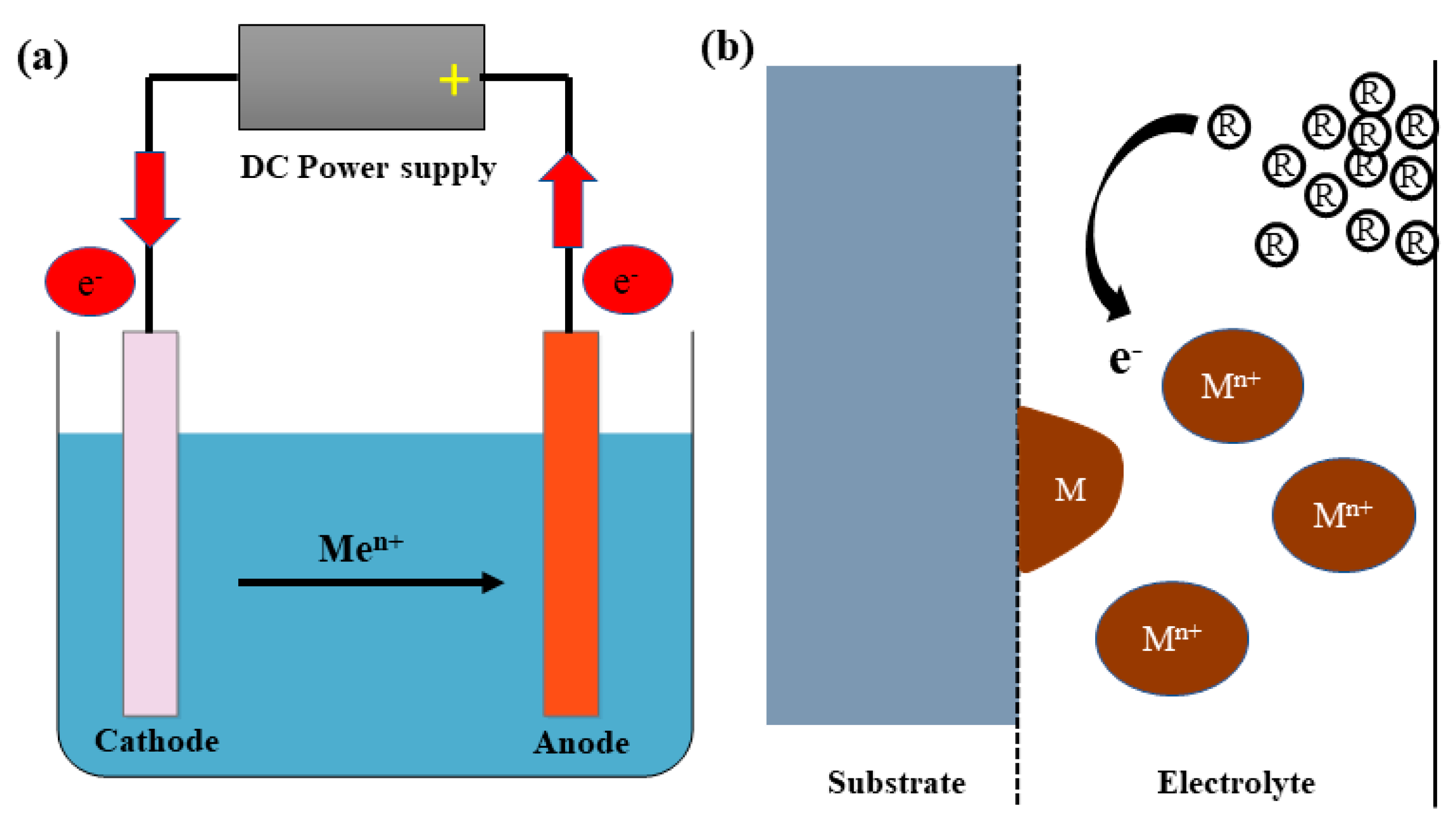
3.2. Electrodeposition Techniques
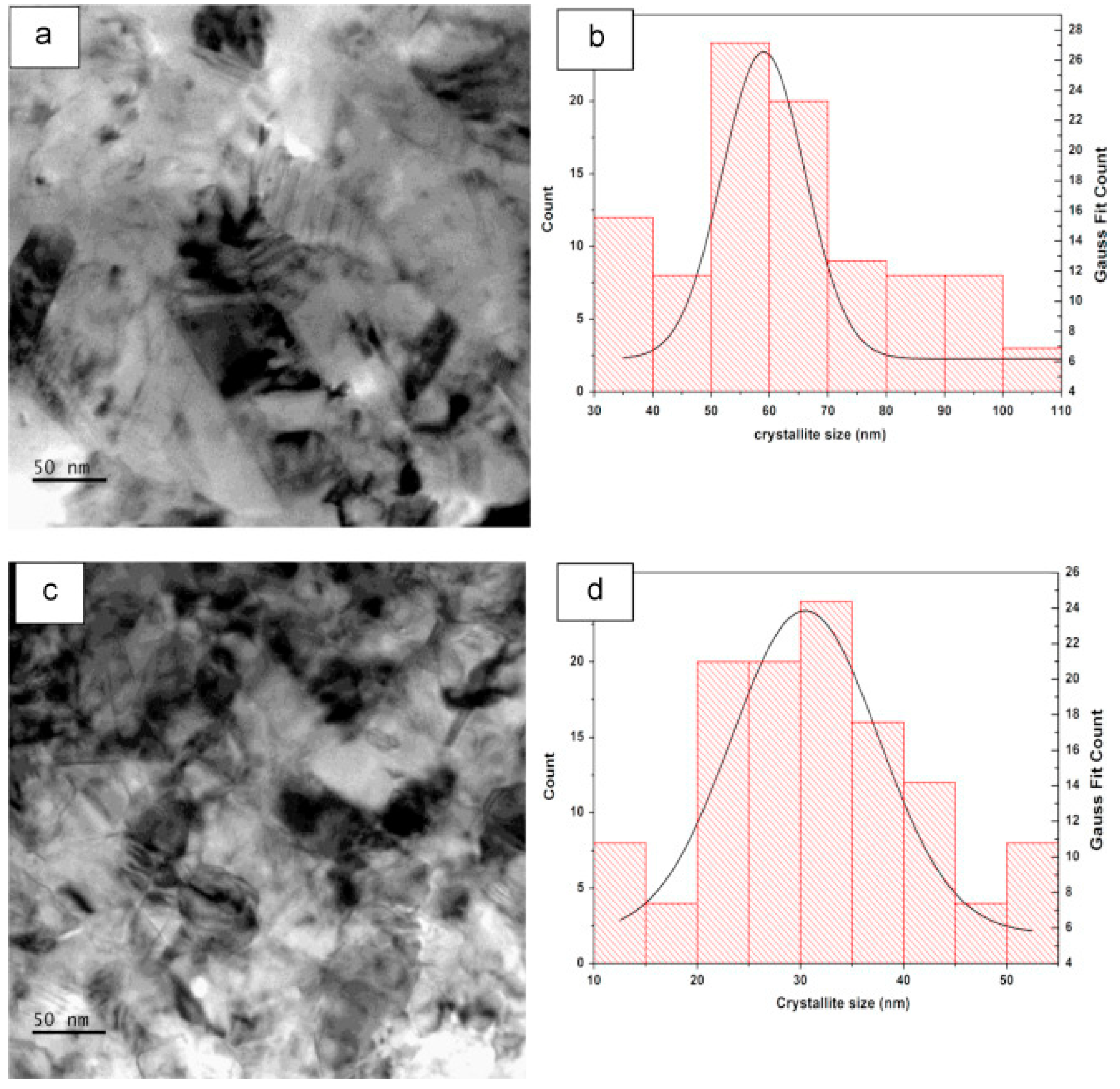
3.3. Electroless Plating
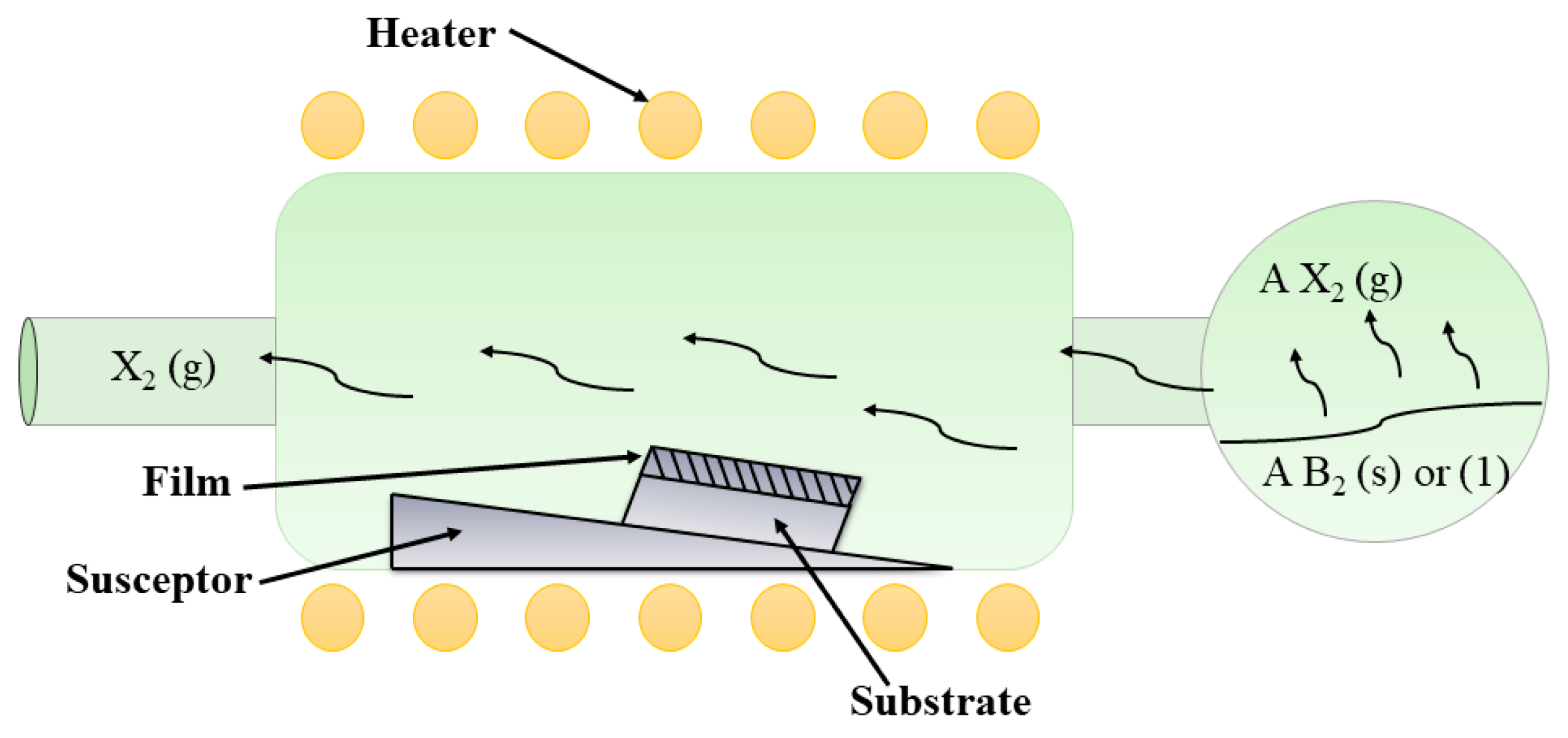
3.4. Chemical Vapor Deposition
3.5. Physical Vapor Deposition
3.6. Sputtering Technique
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; World Health Organization: Geneva, Switzerland, 2011; pp. 1–40. [Google Scholar]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 2010, 38, S25–S33. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Platt, R.; Yokoe, D.S.; Huang, S.S. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch. Intern. Med. 2011, 171, 491–494. [Google Scholar] [CrossRef] [PubMed]
- EPA. U.S. Environmental Protection Agency. Available online: https://www3.epa.gov (accessed on 28 August 2020).
- Dollwet, H.H.A.; Sorenson, J.R.J. Historic uses of copper compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Dick, R.J.; Wray, J.A.; Johnston, H.N. A Literature and Technology Search on the Bacteriostatic and Sanitizing Properties of Copper and Copper Alloy Surfaces; International Copper Research Association: New York, NY, USA, 1973; Volume 212. [Google Scholar]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yasuyuki, M.; Kunihiro, K.; Kurissery, S.; Kanavillil, N. Antibacterial properties of nine pure metals: A laboratory study using Staphylococcus aureus and Escherichia coli. J. Bio Adhes. Biofilm Res. 2010, 26, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Introduction to Antimicrobial Copper. Cu+, Antimicrobial Copper. 2014. Available online: http://www.antimicrobialcopper.org/uk/introduction-to-antimicrobial-copper (accessed on 5 October 2016).
- European Copper Institute. Mechanical Properties of Copper. 2016. Available online: http://www.copperalliance.eu/about-copper/properties/mechanical-properties (accessed on 2 April 2020).
- Xu, F.F.; Imlay, J.A. Silver (I), mercury (II), cadmium (II), and zinc (II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, X.; Huang, X.; Lu, K. Revealing the Maximum Strength in Nanotwinned Copper. Science 2009, 323, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh Strength and High Electrical Conductivity in Copper. Science 2004, 304, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.A.; Weertman, J.R. Elastic and tensile behavior of nano- crystalline copper and palladium. Acta Mater. 1997, 45, 4019–4025. [Google Scholar]
- Warnes, S.L.; Caves, V.; Keevil, C.W. Mechanism of copper surface toxicity in Escherichia coli O157: H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observe. Environ. Microbiol. 2012, 14, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Marais, F.; Mehtar, S.; Chalkley, L. Antimicrobial efficacy of copper touch surfaces in reducing environmental bioburden in a South African community healthcare facility. J. Hosp. Infect. 2010, 74, 80–82. [Google Scholar] [CrossRef]
- Norziehana, N.; Isa, C.; Mohd, Y.; Hafizudden, M.; Zaki, M. Characterization of copper coating electrodeposited on stainless steel substrate. Int. J. Electrochem. Sci. 2017, 12, 6010–6021. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Ksirova, P.; Zietz, C.; Drache, S.; Cada, M.; Hubicka, Z.; Bader, R.; Tichy, M.; Helm, C.A.; et al. Ionized vapor deposition of antimicrobial Ti-Cu films with controlled copper release. Thin Solid Films 2014, 550, 389–394. [Google Scholar] [CrossRef]
- Michels, H.; Moran, W.; Michel, J. Antimicrobial Properties of Copper Alloy Surfaces, With a Focus on Hospital-Acquired Infections. Int. J. Metalcast. 2008, 2, 47–56. [Google Scholar] [CrossRef]
- Von Recum, H.A.; Cyphert, E.L. Emerging technologies for long-term antimicrobial device coatings: Advantages and limitations. Exp. Biol. Med. 2017, 242, 788–798. [Google Scholar]
- Longano, D.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Cioff, N. Synthesis and Antimicrobial Activity of Copper Nanomaterials. In Nano-Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2012; pp. 85–117. [Google Scholar]
- Salgado, C.D.; Sepkowitz, K.A.; John, J.F.; Cantey, J.R.; Attaway, H.H.; Freeman, K.D.; Sharpe, P.A.; Michels, H.T.; Schmidt, M.G. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect. Control Hosp. Epidemiol. 2013, 34, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Montavani, D.; Rosei, F. Antimicrobial Coatings: Challenges, Perspectives and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Donskey, J. Does improving surface cleaning and disinfection reduce health care-associated infections? Am. J. Infect. Control 2013, 41, S12–S19. [Google Scholar]
- Shepherd, S.J.; Beggs, C.B.; Smith, C.F.; Kerr, K.G.; Noakes, C.J.; Sleigh, P.A. Effect of negative air ions on the potential for bacterial contamination of plastic medical equipment. BMC Infect. Dis. 2010, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Pangule, R.C.; Brooks, S.J.; Dinu, C.Z.; Bale, S.S.; Salmon, S.L.; Zhu, G.; Metzger, D.W.; Kane, R.S.; Dordick, J.S. Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates. ACS Nano 2010, 4, 3993–4000. [Google Scholar] [CrossRef]
- Diaz-Tena, E.; Rodriguez-Ezquerro, A.; Lopez de Lacalle Marcaide, L.N.; Gurtubay Bustinduy, L.; Elias Saenz, A. A sustainable process for material removal on pure copper by use of extremophile bacteria. J. Clean. Prod. 2014, 84, 752–760. [Google Scholar] [CrossRef]
- Diaz-Tena, E.; Gallastegui, G.; Hipperdinger, M.; Donati, E.R.; Rojo, N.; Santaolalla, A.; Ramierz, M.; Barona, A.; Elias, A. Simultaneous Culture and Biomachining of Copper in MAC Medium: A comparison between Acidithiobacillus ferrooxidans and Sulfobacillus thermosulfidooxidans. ACS Sustain. Chem. Eng. 2018, 6, 17026–17034. [Google Scholar] [CrossRef]
- Diaz-Tena, E.; Barona, A.; Gallastegui, G.; Rodriguez, A.; Norberto Lopez de Lacalle, L.; Elias, A. Biomachining: Metal etching via microorgaisms. Crit. Rev. Biotechnol. 2015, 37, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–491. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Gregor, G.; Rensing, C.; Solioz, M. Metallic Copper as Antimicrobial Surfaces. Am. Soc. Microbiol. 2011, 77, 1541–1547. [Google Scholar]
- Thiele, J.; Richard, A.; Dennis, F. Copper: An essential metal in biology. Curr. Biol. 2011, 21, 877–883. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Shaobin, W. A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes Pigment. 2008, 76, 714–720. [Google Scholar]
- Macomber, L.; Rensing, C.; Imlay, J.A. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 2007, 189, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Massa, M. Membrane-associated redox cycling of copper mediates hydroperoxide toxicity in Escherichia coli. Biochim. Biophys. Acta Bioenerg. 1993, 1144, 77–84. [Google Scholar]
- Borkow, G.; Okon-Levy, N.; Gabbay, J. Copper oxide impregnated wound dressing: Biocidal and safety studies. Wounds 2010, 22, 301–310. [Google Scholar] [PubMed]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y.; Kitamoto, K.; Anraku, Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 1988, 170, 2676–2682. [Google Scholar] [CrossRef]
- Warnes, S.L. Laboratory Studies to Investigate the Efficacy and Mechanism of Action of Copper Alloys to Kill a Range of Bacterial Pathogens. Ph.D. Thesis, University of Southampton, Southampton, UK, 2014. [Google Scholar]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterization of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2003, 33, 327–335. [Google Scholar] [CrossRef]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Elguindi, J.; Wagner, J.; Rensing, C. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 2009, 106, 1448–1455. [Google Scholar] [CrossRef]
- Michels, H.T.; Noyce, J.O.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett. Appl. Microbiol. 2009, 49, 191–195. [Google Scholar] [CrossRef]
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as a Disinfectant. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 23–45. [Google Scholar]
- Hans, M.; Mathews, S.; Mucklich, F.; Solioz, M. Physicochemical properties of copper important for its antibacterial activity and development of unified model. Biointerphases 2016, 11, 018902. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, R.; Espirito Santo, C.; Madayiputhiya, N.; Grass, G. Quantitative proteomic profiling of the Escherichia coli response to metallic copper surfaces. Biometals 2011, 24, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Green, S.M.; Michels, H.T.; Keevil, C.W. Biocidal Efficacy of Copper Alloys against Pathogenic Enterococci Involves Degradation of Genomic and Plasmid DNAs. Appl. Environ. Microbiol. 2010, 76, 5390–5401. [Google Scholar] [CrossRef]
- Dunne, W.M. Bacterial Adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Ismail, S.; Pratten, J.; Parkin, I.P. An investigation into bacterial attachment to an elastomeric super hydrophobic surface prepared via aerosol assisted deposition. Thin Solid Films 2011, 519, 3722–3727. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levanen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Nie, Y.; Kalapos, C.; Nie, X.; Murphy, M.; Hussein, R.; Zhang, L. Superhydrophilicity and Antibacterial property of a Cu dotted oxide coating surface. Ann. Clin. Microbial. 2010, 9, 1–10. [Google Scholar]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcona, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Elizabeth, E.; Baranwal, G.; Krishnan, A.G.; Menon, D.; Nair, M. ZnO Nanoparticle incorporated nanostructured metallic titanium for increased mesenchymal stem cell response and antibacterial activity. Nanotechnology 2014, 25, 115101. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Erbe, A.; Mathewas, S.; Chen, Y.; Solioz, M.; Mucklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef] [PubMed]
- Holinka, J.; Pilz, M.; Kubista, B.; Presterl, E.; Windhager, R. Effects of selenium coating of orthopedic implant surfaces on bacterial adherence and osteoblastic cell growth. Bone Jt. J. 2013, 95, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Koseki, H.; Asahara, T.; Shida, T.; Yoda, I.; Horiuchi, H.; Baba, K.; Osaki, M. Clinical and histomorphometrical study on titanium dioxide-coated external fixation pins. Int. J. Nanomed. 2013, 8, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J. Biomed. Mater. Res. 2014, 102, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Thallinger, B.; Prasetyo, E.N.; Nyalnhongo, G.S.; Guebitz, G.M. Antimicrobial enzymes: An emerging strategy to fight microbes and microbial biofilms. Biotechnol. J. 2013, 8, 97–109. [Google Scholar] [CrossRef]
- Li, B.; McKeague, A.L. Emerging ideas: Interleukin-12 nanocoating’s prevent open fracture-associated infections. Clin. Orthop. Relat. Res. 2011, 469, 3262–3265. [Google Scholar] [CrossRef] [PubMed]
- Rapsch, K.; Bier, F.F.; Tadros, M.; Nickisch-Rosenegk, M.V. Identification of antimicrobial peptides and immobilization strategy suitable for a covalent surface coating with biocompatible properties. Bioconjug. Chem. 2014, 25, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant. Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V.; Adams, C.S.; Parvizi, J.; Ducheyne, P.; Shapiro, I.M.; Hickok, N.J. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin. Orthop. Relat. Res. 2007, 461, 81–87. [Google Scholar] [CrossRef]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef]
- He, T.; Chan, V. Covalent layer-by-layer assembly of polyethyleneimine multilayer for antibacterial applications. J. Biomed. Mater. Res. Part A 2010, 95, 454–464. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2013, 7, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Grijpma, D.W.; Feihen, J.; Nieuwenhuis, P.; Busscher, H.J. Positively charged biomaterials exert antimicrobial effects on gram-negative bacilli in rats. Biomaterials 2003, 24, 2707–2710. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Stavrakis, A.I.; Billi, F.; Cho, J.S.; Kremen, T.J.; Simon, S.I.; Cheung, A.L.; Finerman, G.A.; Lieberman, J.R.; Adams, J.S.; et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS ONE 2010, 5, e12580. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on super hydrophobic surfaces. Chem. Commun. 2014, 18, 3900–3913. [Google Scholar] [CrossRef]
- Shida, T.; Koeski, H.; Yoda, I.; Horiuchi, H.; Sakoda, H.; Osaki, M. Adherence ability of Staphylococcus epidermidis on prosthetic biomaterials: An in vitro study. Int. J. Nanomed. 2013, 8, 3955–3961. [Google Scholar]
- Pandit, V.; Zuidema, J.M.; Venuto, K.N.; Macione, J.; Dai, G.; Gilbert, R.J.; Kotha, S.P. Evaluation of multifunctional polysaccharide hydrogels with varying stiffness for bone tissue engineering. Tissue Eng. A 2013, 19, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cho, J.; Shivapooja, P.; Ista, L.K.; Lopez, G.P. Nanopatterned smart polymer surfaces for controlled attachment, killing, and release of bacteria. ACS Appl. Master Interfaces 2013, 5, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Antoci, V., Jr.; Hickok, N.J.; Shapiro, I.M. Self-protective smart orthopedic implants. Expert Rev. Med. Devices 2007, 4, 55–64. [Google Scholar] [CrossRef]
- Bhat, U.; Augustin, A.; Bhat, S.; Udupa, K.R. Preparation and characterization of copper thin films for Antimicrobial applications. In Microscopy Applied to Materials Sciences and Life Sciences; Apple Academic Press Inc.: Waretown, NJ, USA, 2018; pp. 91–110. [Google Scholar]
- Singh, H.; Sidhu, T.S.; Kalsi, S.B.S.; Karthikeyan, J. Development of cold spray from innovation to emerging future coating technology. J. Braz. Soc. Mech. Sci. Eng. 2013, 35, 231–245. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Borchers, C.; Gartner, F.; Stoltenhof, T.; Assadi, H.; Kreye, H. Microstructural and macroscopic properties of cold sprayed copper coatings. J. Appl. Phys. 2003, 93, 10064. [Google Scholar] [CrossRef]
- Crawmer, D.E. Thermal Spray Processes. In Handbook of Thermal Spray Technology; ASM International: Russell Township, OH, USA, 2013; pp. 54–76. [Google Scholar]
- Smith, M.F.; Hall, A.C.; Fleetwood, J.D.; Meyer, P. Very Low-Pressure Plasma Spray—A Review of an Emerging Technology in the Thermal Spray Community. Coatings 2011, 1, 117–132. [Google Scholar] [CrossRef]
- Jandin, G.; Liao, H.; Feng, Z.Q.; Coddet, C. Correlations between operating conditions, microstructure and mechanical properties of twin wire arc sprayed steel coatings. Mater. Sci. Eng. 2003, 349, 298–305. [Google Scholar] [CrossRef]
- Wang, X.; Heberlein, J.; Pfender, E.; Gerberich, W. Effect of Nozzle Configuration, Gas Pressure, and Gas Type on Coating Properties in Wire Arc Spray. J. Therm. Spray Technol. 1999, 8, 565–575. [Google Scholar] [CrossRef]
- Pourmousa, A.; Mostaghimi, J.; Abedini, A.; Chandra, S. Particle size distribution in a wire-arc spraying system. J. Therm. Spray Technol. 2005, 14, 502–510. [Google Scholar] [CrossRef]
- Davis, J.R. Cold Spray Process. In Thermal Spray Technology; ASM International: Russell Township, OH, USA, 2004; pp. 77–84. [Google Scholar]
- Champagne, V.; Sundberg, K.; Helfritch, D. Kinetically deposited copper antimicrobial surfaces. Coatings 2019, 9, 257. [Google Scholar] [CrossRef]
- Sundberg, K.L. Application of Materials Characterization, Efficacy Testing, and Modelling Methods on Copper Cold Spray Coatings for Optimized Antimicrobial Properties. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2019. [Google Scholar]
- Da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, I.; Guilemany, J.; Caires, C.; Lima, A.; Silva, C.; Oliveira, S.; Caires, A.; et al. Corrosion resistance and antibacterial properties of copper coatings deposited by cold gas spray. Surf. Coat. Technol. 2019, 361, 292–301. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Salimijazi, H.R.; Fathi, M.H.; Mostaghimi, J.; Pershin, L. Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings. Surf. Coat. Technol. 2013, 233, 74–79. [Google Scholar] [CrossRef]
- Worna, A.; Bilewska, K.; Lis, M.; Kaminska, M.; Olszewski, T.; Pajzderski, P.; Więcław, G.; Jaśkiewicz, M.; Kamysz, W. Antimicrobial properties of protective coatings produced by plasm spraying technique. Surf. Coat. Technol. 2017, 318, 332–340. [Google Scholar]
- Ibrahim, M.; Al-Athel, K.; Fazal, A.; Arif, M. Strength and Hardness Assessment of Copper and Copper Alloy Coatings on Stainless Steel Substrates. In Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition, Phoenix, AZ, USA, 11–17 November 2016. [Google Scholar]
- Champagne, V.K.; Helfritch, D.J. A Demonstration of the antimicrobial effectiveness of various copper surfaces. J. Biol. Eng. 2013, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharifahmadian, O.; Salimijazi, H.R.; Fathi, M.H.; Mostaghimi, J.; Pershin, L. Study of the Antibacterial Behavior of Wire Arc Sprayed Copper Coatings. J. Therm. Spray Technol. 2013, 22, 371–379. [Google Scholar] [CrossRef]
- Tailor, S.; Modi, A.; Modi, S.C. Thermally Sprayed Thin Copper Coatings by W-HVOF. J. Therm. Spray Technol. 2019, 28, 273–282. [Google Scholar] [CrossRef]
- Augustin, A.; Thaira, H.; Bhat, K.U.; Udupa, K.R. Effect of Electrodeposited Copper Thin Film on the Morphology and Cell Death of E. coli; Electron Microscopic Study. In Biotechnology and Biochemical Engineering; Springer: Singapore, 2016; pp. 227–232. [Google Scholar]
- Augustin, A.; Huilgol, P.; Udupa, K.R.; Bhat, K.U. Effect of current density during electrodeposition on microstructure and hardness of textured Cu coating in the application Al touch surface. J. Mech. Behav. Biomed. Mater. 2016, 63, 352. [Google Scholar] [CrossRef]
- Schwarzacher, W. Electrodeposition: A technology for the future. Electrochem. Soc. Interface 2016, 15, 32–35. [Google Scholar] [CrossRef]
- Rasmussen, A.A.; Jensen, J.A.D.; Horsewell, A.; Somers, M.A. Microstructure in electrodeposited copper layers; the role of the substrate. Electrochim. Acta. 2001, 47, 67–74. [Google Scholar] [CrossRef]
- Nikolic, N.D.; Popov, K.I.; Pavlovic, L.J.; Pavlovic, M.G. Morphologies of copper deposits obtained by the electrodeposition at high overpotentials. Surf. Coat. Technol. 2006, 201, 560–566. [Google Scholar] [CrossRef]
- Milchev, A.; Zapryanova, T. Nucleation and growth of copper under combined charge transfer and diffusion limitations. Electrochim. Acta Part I 2006, 51, 433–438. [Google Scholar] [CrossRef]
- Ibañez, A.; Fatás, E. Mechanical and structural properties of electrodeposited copper and their relation with the electrodeposition parameters. Surf. Coat. Technol. 2005, 191, 7–16. [Google Scholar] [CrossRef]
- Shekar, R. Synergistic effect of additives on electrodeposition of copper from cyanide free electrolytes and its structural and morphological characteristics. Trans. Nonferrous Met. Soc. China 2017, 27, 1665–1676. [Google Scholar] [CrossRef]
- Shao, W.; Pattanaik, G.; Zangari, G. Influence of chloride anions on the mechanism of copper electrodeposition from acidic sulfate electrolytes. J. Electrochem. Soc. 2007, 154, D201–D207. [Google Scholar] [CrossRef]
- Norziehana, N.; Isa, C.; Mohd, Y.; Mohamad, S.A.S.; Zaki, M.H.M. Antibacterial Activity of Copper Coating Electrodeposited on 304 stainless steel. AIP Conf. Proc. 2017, 1901, 020009. [Google Scholar]
- Ivshin, Y.V.; Shaikhutdinova, F.N.; Sysoev, V.A. Electrodeposition of Copper on Mild Steel: Peculiarities of the Process. Surf. Eng. Appl. Electrochem. 2017, 54, 452–458. [Google Scholar]
- Haider, F.I.; Ani, M.H.; Mahmood, M.H. Modelling and Optimization of Copper Electroplating Adhesion Strength. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 5th International Conference on Nanomaterials and Materials Engineering, Bali, Indonesia, 1–3 April 2017; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 204. [Google Scholar]
- Zeiger, M.; Solioz, M.; Edongue, H.; Arzt, E.; Schneider, A.S. Surface structure influences contact killing of bacteria by copper. Microbiol. Open 2014, 3, 327–332. [Google Scholar] [CrossRef]
- Augustin, A.; Bhat, U.K.; Rajendra Udupa, K.K.; Hegade, C.A. Electron microscopic study of nodules formed during electrodeposition of copper on aluminium. In Materials Science Forum; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2015; pp. 371–374. [Google Scholar]
- Scholz, F. Active sites of heterogeneous nucleation understood as chemical reaction sites. Electrochem. Commun. 2011, 13, 932–933. [Google Scholar] [CrossRef]
- Kumar, S.K.; Biswas, K. Effect of thiourea on grain refinement and defect structure of the pulsed electrodeposited nanocrystalline copper. Surf. Coat. Technol. 2013, 214, 8–18. [Google Scholar] [CrossRef]
- Banthiaa, S.; Sengupta, S.; Mallik, M.; Das, S.; Das, K. Substrate effect on electrodeposited copper morphology and crystal shapes. Surf. Eng. 2018, 34, 485–492. [Google Scholar] [CrossRef]
- Chandrasekar, M.S.; Pushpavanam, M. Pulse and pulse reverse plating—Conceptual, advantages and applications. Electrochim. Acta 2008, 53, 3313–3322. [Google Scholar] [CrossRef]
- Deckert, C.A. Electroless Copper Plating A Review: Part, I. Plat. Surf. Eng. 1995, 82, 48–55. [Google Scholar]
- Ghosh, S. Electroless copper deposition: A critical review. Thin Solid Films 2019, 669, 641–658. [Google Scholar] [CrossRef]
- Li, J.; Hayden, H.; Kohl, P.A. The influence of 2,2′-dipyridyl on non-formaldehyde electroless copper plating. Electrochem. Acta 2004, 49, 1789–1795. [Google Scholar] [CrossRef]
- Gan, X.; Wu, Y.; Liu, L.; Hu, W. Effects of K4Fe(CN)6 on electroless copper plating using hypophosphite as reducing agent. J. Appl. Electrochem. 2007, 37, 899–904. [Google Scholar] [CrossRef]
- Luo, L.M.; Lu, Z.L.; Huang, X.M.; Tan, X.Y.; Ding, X.Y.; Cheng, J.G.; Zhu, L.; Wu, Y.-C. Electroless copper plating on PC engineering plastic with a novel palladium-free surface activation process. Surf. Coat. Technol. 2014, 251, 69–73. [Google Scholar] [CrossRef]
- Oita, M.; Matsuoka, M.; Iwakura, C. The deposition rate and morphology of electroless copper film from solutions containing 2,2′-dipyridyl. Electrochim. Acta 1997, 42, 1435–1440. [Google Scholar] [CrossRef]
- Argueta–Figueroa, L.; Morales-Luckie, R.A.; Scougall-Vilchis, R.J.; Olea-Mejia, O.F. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu–Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014, 24, 321–328. [Google Scholar] [CrossRef]
- BuFaroosha, M. Precursors for Chemical Vapor Deposition of Copper. Doctoral Dissertations, Louisiana State University, Baton Rouge, LA, USA, 2002. No. 1437. [Google Scholar]
- Shima, K.; Shimizu, H.; Momose, T.; Shimogaki, Y. Comparative Study on Cu-CVD Nucleation Using -diketonato and Amidinato Precursors for Sub-10-nm-Thick Continuous Film Growth. ECS J. Solid-State Sci. Technol. 2015, 4, P305–P313. [Google Scholar] [CrossRef]
- Chiou, J.-C.; Chen, Y.-J.; Chen, M.-C. Copper chemical vapor deposition from Cu(hexafluoroacetylacetonate)trimethylvinylsilane. J. Electron. Mater. 1994, 23, 383–390. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapor deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Aviziotis, I.G.; Cheimarios, N.; Vahlas, C.; Boudouvis, A.G. Experimental and computational investigation of chemical vapor deposition of Cu from Cu amidinate. Surf. Coat. Technol. 2013, 230, 273–278. [Google Scholar] [CrossRef]
- Quinto, D.T. Mechanical property and structure relationships in hard coatings for cutting tools. J. Vac. Sci. Technol. 1988, 6, 2149–2157. [Google Scholar] [CrossRef]
- Varghese, S.; El Fakhri, S.O.; Sheel, D.W.; Sheel, P.; Frederick, J.; Bolton, E.; Foster, H.A. Antimicrobial activity of novel nanostructured Cu-SiO2 coatings prepared by chemical vapor deposition against hospital related pathogens. AMB Express 2013, 3, 53. [Google Scholar] [CrossRef]
- Hassan, I.A.; Parkin, I.P.; Nairb, S.P.; Carmalt, C.J. Antimicrobial activity of copper and copper(i) oxide thin films deposited via aerosol-assisted CVD. J. Mater. Chem. B 2014, 2, 2855–2860. [Google Scholar] [CrossRef]
- Ozkan, E.; Crick, C.C.; Taylor, A.; Allan, E.; Parkin, I.P. Copper-based water repellent and antibacterial coatings by aerosol assisted chemical vapor deposition. R. Soc. Chem. 2016, 7, 5126–5131. [Google Scholar]
- Romig, A., Jr. A time dependent regular solution model for the thermal evaporation of an Al-Mg alloy. 2. J. Appl. Phys. 1987, 62, 503–508. [Google Scholar] [CrossRef]
- Alam, M.M.; Mia, N.H.; Hasan, R.; Shahinuzzaman, M.; Islam, M.K.; Khan, M. Nasir Uddin. Study of Structural and Morphological Properties of Vacuum coated copper (Cu) metal thin film. Mater. Sci. Appl. 2015, 6, 753–759. [Google Scholar]
- Ustinov, A.I.; Movachan, B.A.; Polishchuk. Formation of Nano quasi crystalline Al-Cu-Fe Coatings at Electron Beam Physical Vapor Deposition. Scr. Mater. 2004, 50, 533–537. [Google Scholar] [CrossRef]
- Singh, J.; Wolfe, D.E. Nano and macro-structured component fabrication by electron beam—Physical vapor deposition (EB-PVD). J. Mater. Sci. 2005, 40, 1–26. [Google Scholar] [CrossRef]
- Komalakrishna, H.; Augustin, A.; Bhat, U.K. Electron Beam Deposition of Copper Thin Film on Aluminium Substrate and Its Characterization. Am. J. Mater. Sci. 2015, 5, 9–24. [Google Scholar]
- Kelly, P.; Arnell, R. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Thornton, J.A. High-rate thick film growth. Annu. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Augustin, A.; Rajendra Udupa, K.; Bhat, U.K. Effect of Pre-Zinc Coating on the Properties and Structure of DC magnetron sputtered copper thin film on aluminium. Am. J. Mater. Sci. 2015, 5, 58–61. [Google Scholar]
- Tian, X.B.; Wang, Z.M.; Yang, S.Q.; Luo, Z.J.; Ricky, K.Y.; Fu, P.; Chu, K. Antibacterial copper containing titanium nitride films produced by dual magnetron sputtering. Surf. Coat. Technol. 2007, 201, 8606–8609. [Google Scholar] [CrossRef]
- Rodriguez-Barrero, S.; Fernandez–Larrinoa, J.; Azkona, I.; Lopez de Lacalle, L.N.; Polvorosa, R. Enhanced Performance of Nanostructred Coatings for Drilling by Droplet Ellimination. Mater. Manuf. Process 2016, 31, 593–602. [Google Scholar] [CrossRef]
- Abdullah, M.R.; Harun, N.H.; Ibrahim, S.N.; Abdul, A. Thin Film Coating of Copper Nanoparticles with DC Magnetron Sputtering via Physical Vapor Deposition. AIP Conf. Proc. 2019, 2129, 020120. [Google Scholar]
- Musil, J.; Blazek, J.; Fajfrlik, K.; Cerstvy, R.; Proksova, S. Antibacterial Cr-Cu-O films prepared by reactive magnetron sputtering. Appl. Surf. Sci. 2013, 276, 660–666. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, W.; Yu, P.; Wang, X.; He, T.; Tan, G.; Ning, C. Antibacterial nanostructured copper coatings deposited on tantalum by magnetron sputtering. Mater. Technol. 2015, 30, B120–B125. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Banfi, F.; Gavioli, L. Antimicrobial Nanostructured Coatings: A Gas Phase Deposition and Magnetron Sputtering Perspective. Materials 2020, 13, 784. [Google Scholar] [CrossRef]




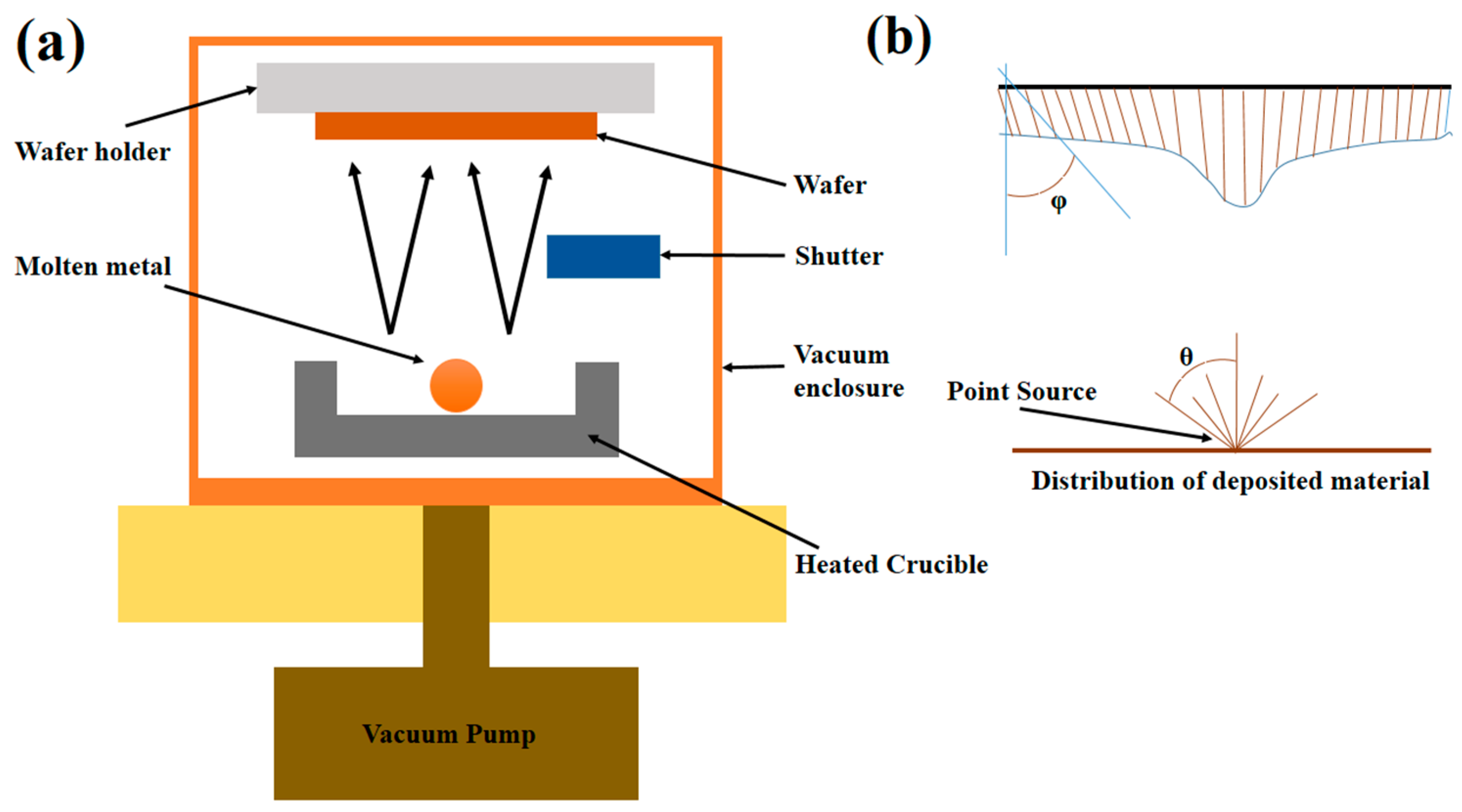
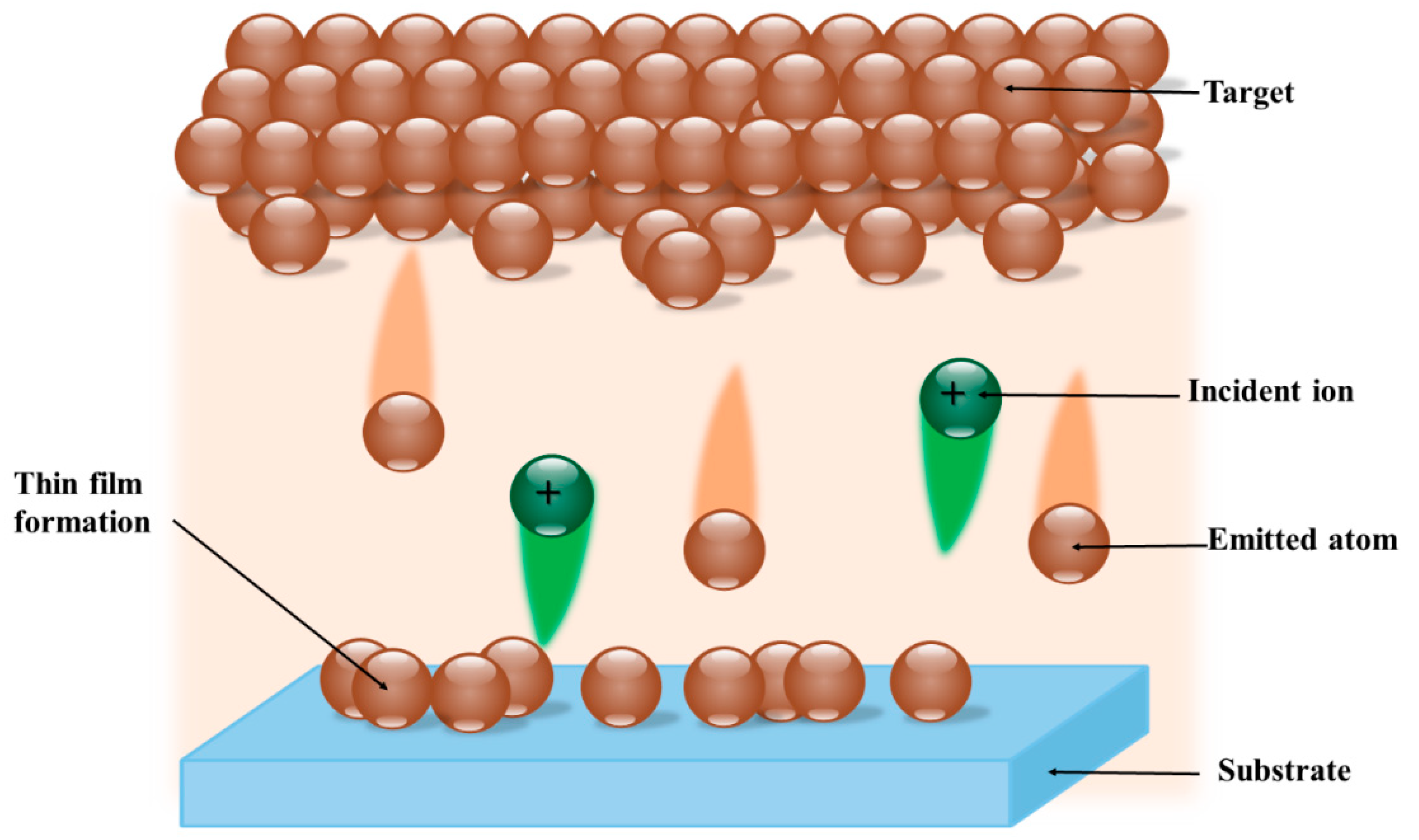
| Antibacterial Functionality | Tested Examples | Reference | |
|---|---|---|---|
| Antibacterial agent release | Nanoparticles | [33] | |
| Copper oxides | [45] | ||
| Contact killing | Inorganic | Zinc ion | [60] |
| Copper ion | [61] | ||
| Selenium ion | [62] | ||
| Titanium dioxide | [63] | ||
| Silver nanoparticles | [64] | ||
| Organic | Enzymes | [65] | |
| Cytokine | [66] | ||
| Signaling, inhibiting, and antimicrobial peptides | [67] | ||
| Chitosan derivatives | [68] | ||
| Coated or covalently linked antibiotics | [69] | ||
| Other | Non-antibiotic bactericidal substances | [70] | |
| Combined | Multilayer coatings | [71] | |
| Synergy material intensification | [72] | ||
| Positively charged polymers | [73] | ||
| Anti-adhesion/Bacterial Repelling | Anti-adhesive polymers | [74] | |
| Superhydrophobic surfaces | [75] | ||
| Nanopatterned surfaces | [76] | ||
| Hydrogels | [77] | ||
| Smart Coatings | Passive | Nanostructured “smart” material | [78] |
| Active | Concept: Sensors conjoined to Nano containers | [79] | |
| Technique | Advantages | Inconveniences |
|---|---|---|
| Thermal Spraying | Low cost High deposition rates Excellent adhesion strength | High temperature induces decomposition, oxidation of Cu could affect the antimicrobial activity. The line of sight technique leads to nonuniformity Amorphous coatings due to rapid cooling Interface separation and spalling of coating between the coating and substrate |
| Electrodeposition | Uniform distribution of coating Simple technique Can easily tune the coating morphologies Can operate at low temperature The coating is possible for highly complex objects Controlled nanoporous coatings | Low tear strength Poor adhesion Surface pretreatment required to deposit Cu on metal substrates Alkaline and acidic baths suffer environmental disposal issues |
| Electroless deposition | Simple process Low temperature Coats highly irregular objects | Coating pure Cu is difficult Limited salts for Cu plating |
| Physical vapor deposition | Less prone to crack formation Thin layers More adherent than thermal spray | Expensive method Difficulty producing nanoporous coatings |
| Chemical vapor deposition | Can easily control precursor concentrations to fabricate functionally graded coatings | Expensive method The use of volatile gases cause the formation of contamination on Cu surfaces that could reduce the rate of bacteria-killing Limited coating composition |
| Sputtering | Homogenous coating Uniform coating on flat substrates High adhesion Dense deposits | Expensive technique and time-consuming Line of sight issues Amorphous coatings |
| Deposition Method | Ideal Process Parameters for Cu Deposition | Substrate Heating (°C) and Substrate Preparation | Coating Rate and Coating Thickness | Type of Adhesion Bonding and Surface Roughness | Typical Microstructure for Antimicrobial Application |
|---|---|---|---|---|---|
| Thermal SprayTechnique [90,91,92,93,94,95,96,97,98] | Plasma Arc: Temperature: 1500–2500 °C Velocity: 100-400 m/s Porosity: ~5% Oxides: ~2% Wire Arc: Temperature: 1500–2500 °C Velocity: 50–100 m/s Porosity: ~10% Oxides: ~15% Cold Spray: Temperature: 150–400 °C Velocity: 500–100 m/s Porosity: ~10% Oxides: ~15% Flame spray: Temperature: 1010–1175 °C Porosity: ~1% Oxides: ~15% Copper particles size is less than 20 μm with 99% purity for thermally sprayed coatings. | Usually ~10% of Copper melting temperature Carbon steel, Stainless steel widely used substrates for antimicrobial applications. | >100 μm/min Approximately Coating thickness: 30 μm–300 μm | Mechanical Interlocking and Very rough coatings, higher bacterial killing rate. Good adhesion strength between substrate and coating due to high surface roughness of the substrate. | Deformed lamella |
| Electro deposition [99,100,102,103,104,105,106,108,109,112,115] | Current density range: 1 Adm−2 to 10 Adm−2 High current density will lead to formation of defects like nanotwins and staking faults regions which could improve the hardness of the coatings. pH range: For acidic bath: 1.5 to 4 for alkaline bath 12 to 14 | Cannot heat substrate while deposition. Stainless steel, Aluminium, mild steel are the good substrates for Cu electrodeposition. | <0.05 μm/min (<3 μm/h) Thickness in the range of 1 μm to few microns. | Mechanical interlocking. Surface contamination, oxide layer on the substrate decreases the adhesion Strength. Surface roughness depends on substrate pre-treatment | Cu coatings shows Columnar structures and Nanograined structures good for antimicrobial activity |
| Electroless Plating [118,122] | Coating time: Few minutes to hours. Additives are commonly used in the bath to refine the film morphology and deposition rate. Optimum bath temperature: 50 °C–80 °C Higher the pH coating quality degrades. | Cannot heat substrate while deposition. Suitable substrates: Aluminium, glass, polymers, mild steel, and stainless steel. | <0.03 μm/min (<2 μm/h) 30 min to one hour plating time required to achieve >1 μm to few microns thickness of Cu coating | Mechanical interlocking Smooth, dense, bright Cu coatings irrespective of substrate type. | Columnar-equiaxed, Nano grained, uniform thickness |
| Chemical Vapor Deposition (CVD) [124,125,128,130,131,132] | Type of precursor used for Cu deposition: Copper (I) amidinate [Cu(i-Pr-Me-AMD)]2, hexafluoroacetylacetonate (Cu (HFAc)2) Carrier gas: N2 gas. Deposition time: 1 h | 200 °C–500 °C Suitable substrates: Stainless steel, silicon, SiO2, borosilicate glass | <0.08 μm/min (<5 μm/h) Coating thickness: 80 nm to 270 nm Coating thickness: 40 nm (100% antimicrobial activity under 2 h) | Diffusion bonding | Columnar-equiaxed nodular and smooth, uniform morphology. Morphology can be tuned by substrate roughness and substrate heating Columnar structure is good for antimicrobial activity |
| Electron Beam Deposition-PVD [134,135,136,137] | Vacuum level: 1.5 × 10−5 mbar Source and substrate distance: 10 cm Coating time: 1 h | RT to 1200 °C (flexible) Suitable Substrates: Titanium, Aluminium, Stainless steel, low carbon steel | Deposition rate: 1 Å/s | Diffusion bonding | Columnar-equiaxed, Nano sized grains Increases the surface area—more beneficial for antimicrobial activity. |
| Sputtering Technique [141,142,143,144,145,146] | Base pressure: 5 × 10−5 Pa to 1.3 × 10−4 Pa Working pressure range: 0.012 mbar to 0.04 mbar Mass flow rate of Ar gas: Substrate rotation speed: Low speed (100 rpm) | <600 °C (flexible) Suitable Substrates: Titanium, Aluminium, Stainless steel, low carbon steel. | <0.08 μm/min (<5 μm/h) | Diffusion bonding | Columnar, uniform, adherent coating observed in the range of 50 W to 150 W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharadishettar, N.; Bhat K, U.; Bhat Panemangalore, D. Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review. Metals 2021, 11, 711. https://doi.org/10.3390/met11050711
Bharadishettar N, Bhat K U, Bhat Panemangalore D. Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review. Metals. 2021; 11(5):711. https://doi.org/10.3390/met11050711
Chicago/Turabian StyleBharadishettar, Naveen, Udaya Bhat K, and Devadas Bhat Panemangalore. 2021. "Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review" Metals 11, no. 5: 711. https://doi.org/10.3390/met11050711
APA StyleBharadishettar, N., Bhat K, U., & Bhat Panemangalore, D. (2021). Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review. Metals, 11(5), 711. https://doi.org/10.3390/met11050711






