Optimization of Gum Arabic and Starch-Based Edible Coatings with Lemongrass Oil Using Response Surface Methodology for Improving Postharvest Quality of Whole “Wonderful” Pomegranate Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Solutions
2.3. Coating Application and Storage
2.4. Weight Loss
2.5. Respiration Rate
2.6. Total Soluble Solids and Titratable Acidity
2.7. Antioxidant Activity
2.7.1. Radical-Scavenging Activity
2.7.2. Ferric Ion Reducing Antioxidant Power
2.8. Experimental Design and Statistical Analysis
3. Results
3.1. Weight Loss
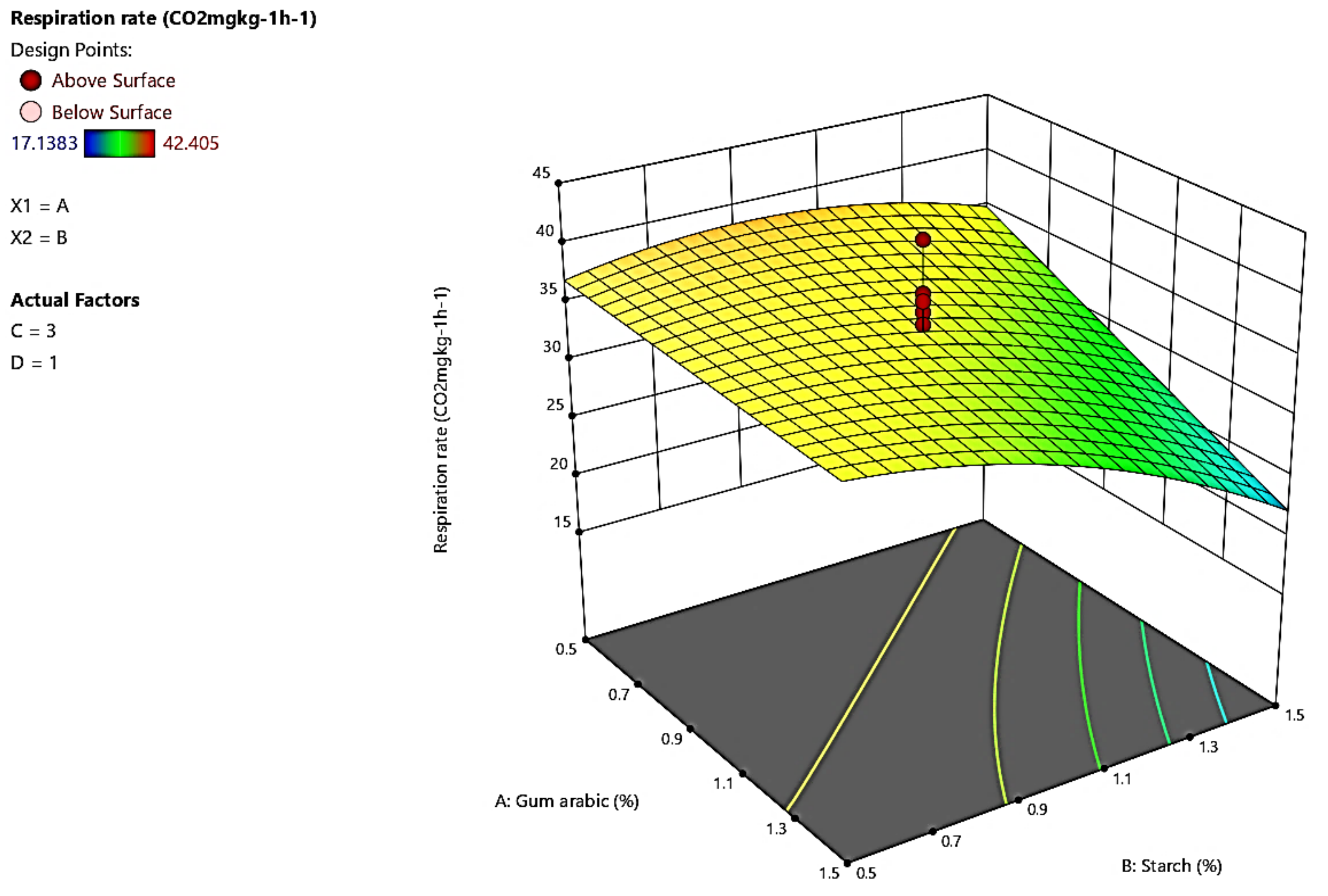
3.2. Respiration Rate
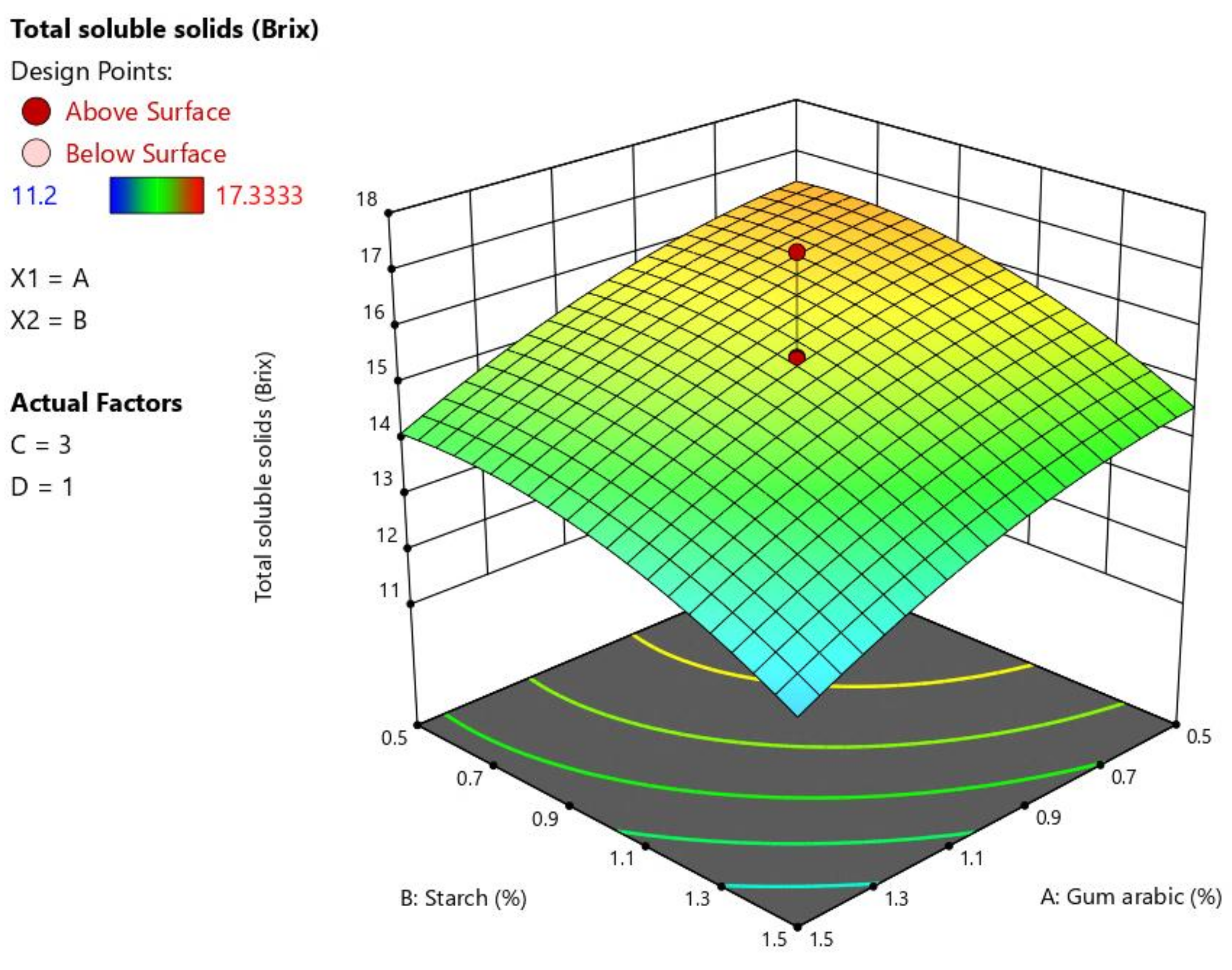
3.3. Total Soluble Solids
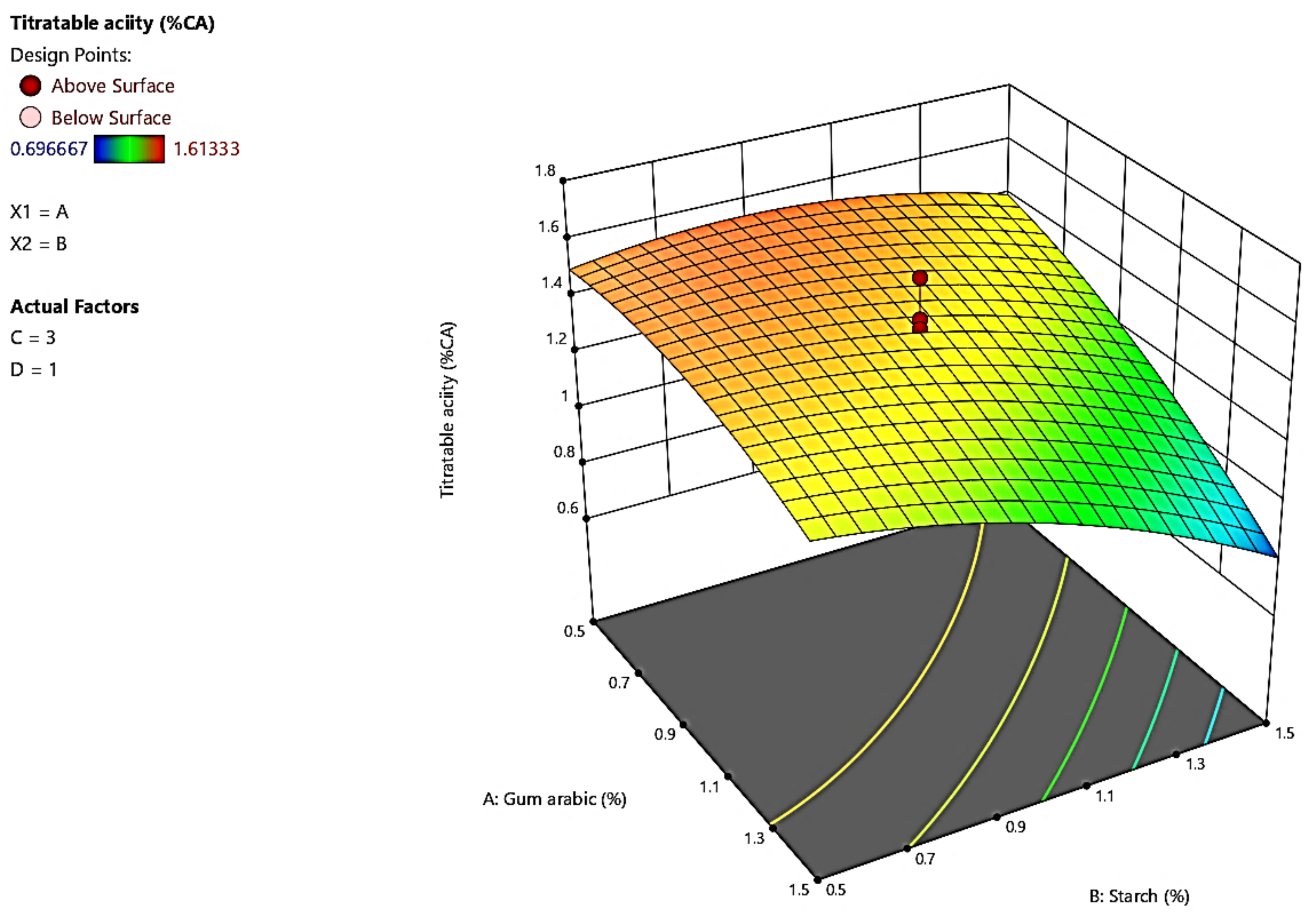
3.4. Titratable Acidity
3.5. Radical Scavenging Activity
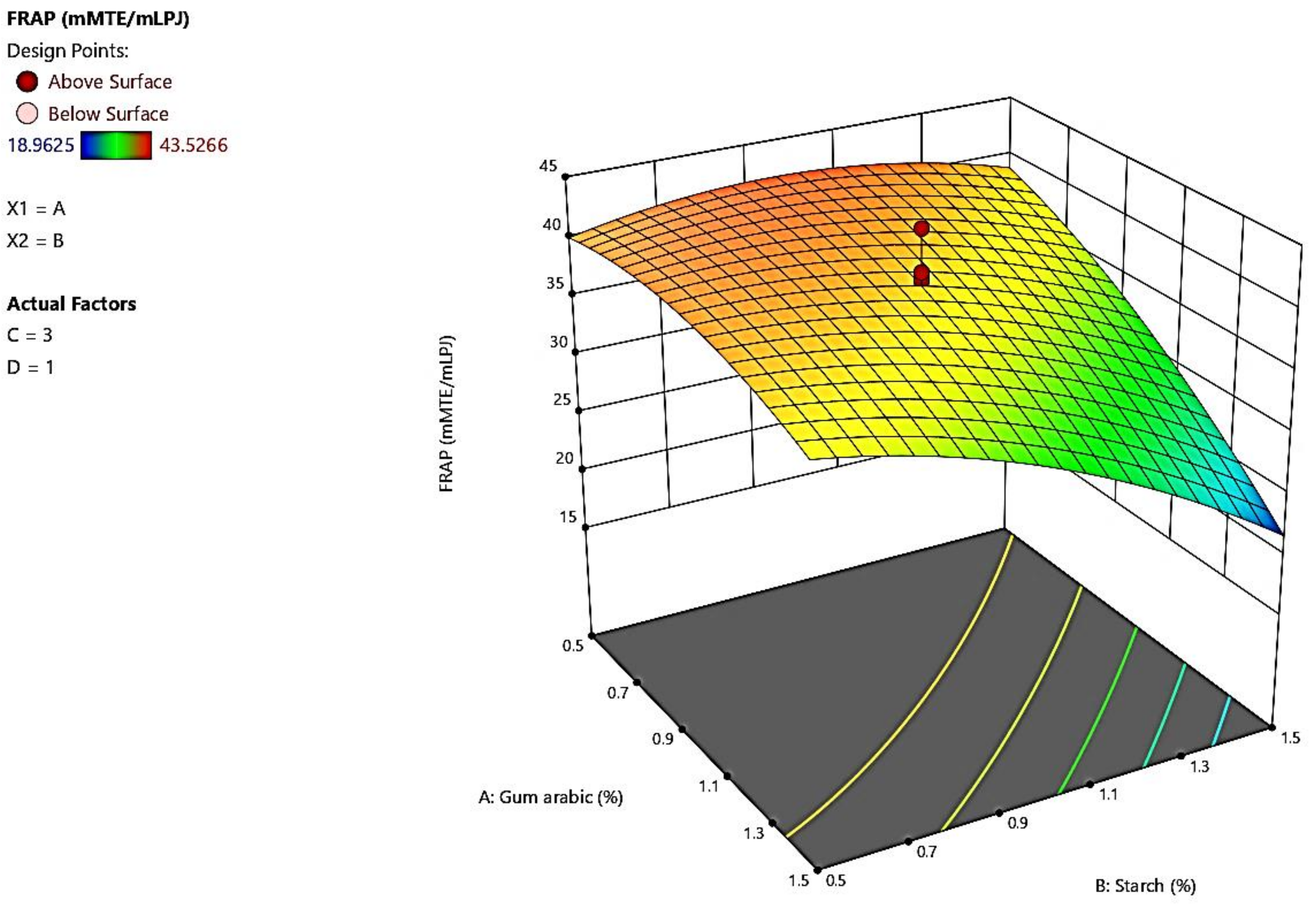
3.6. Ferric Reducing Antioxidant Power
3.7. Optimization
4. Discussion
4.1. Weight Loss
4.2. Respiration Rate
4.3. Total Soluble Solids
4.4. Titratable Acidity
4.5. Radical Scavenging Activity
4.6. Ferric Reducing Antioxidant Power
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Ng, P.K.W. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid-based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 1–38. [Google Scholar]
- Ibrahim, M.S.; Hamza, Y.M.; Fazal-ur-Rehman, M.; Zaharadeen, I.M.; Sirajo, I.I. Biopolymer materials, an alternative to synthetic polymer materials. Int. Invent. Sci. J. 2018, 2, 286–295. [Google Scholar]
- Radev, R.; Pashova, S. Application of edible films and coatings for fresh fruit and vegetables. Qual. Access Success 2020, 21, 108–112. [Google Scholar]
- Islam, A.M.; Phillips, G.O.; Sljivo, A.; Snowden, M.J.; Williams, P.A. A review of recent developments on the regulatory, structural and functional aspects of gum arabic. Food Hydrocoll. 1997, 11, 493–505. [Google Scholar] [CrossRef]
- Dror, Y.; Cohen, Y.; Yerushalmi-Rozen, R. Structure of gum arabic in aqueous solution. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3265–3271. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol. Technol. 2011, 62, 71–76. [Google Scholar] [CrossRef]
- Phillips, A.O.; Phillips, G.O. Biofunctional behaviour and health benefits of a specific gum arabic. Food Hydrocoll. 2011, 25, 165–169. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Emulsification and microencapsulation properties of gum arabic. J. Agric. Food Chem. 1998, 46, 4551–4555. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Application of gum arabic and methyl cellulose coatings enriched with thyme oil to maintain quality and extend shelf life of ‘Acco’ pomegranate arils. Plants 2020, 9, 1690. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Alderson, P.G.; Zahid, N. Effect of gum arabic as an edible coating on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. Postharvest Biol. Technol. 2013, 76, 119–124. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-based coatings for preservation of fruits and vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Cuq, B.; Gontard, N.; Guilbert, S. Edible films and coatings as active layers. In Active Food Packaging; Rooney, M.L., Ed.; Springer: Boston, MA, USA, 1995; Volume 1, pp. 111–142. [Google Scholar]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Starch-based coatings: Effect on refrigerated strawberry (Fragaria ananassa) quality. J. Sci. Food Agric. 1998, 76, 411–420. [Google Scholar] [CrossRef]
- García, M.A.; Martino, N.; Zaritzky, N.E. Composite starch-based coatings applied to strawberries (Fragaria ananassa). Mol. Nutr. Food Res. 2001, 45, 267–272. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Aguilar-Palazuelos, E.; Vega-García, M.O.; Zazueta-Morales, J.J.; Calderón-Castro, A.; Montoya-Rodríguez, A.; Delgado-Nieblas, C.I.; Camacho-Hernández, I.L. Effect of a corn starch coating obtained by the combination of extrusion process and casting technique on the postharvest quality of tomato. Rev. Mex. Ing. Quim. 2019, 18, 789–801. [Google Scholar] [CrossRef]
- Ghosh, A.; Dey, K.; Bhowmick, N. Effect of corn starch coating on storage life and quality of Assam lemon (Citrus limon Burn.). J. Crop Weed 2015, 11, 101–107. [Google Scholar]
- Kathiresan, S.; Lasekan, O. Effects of glycerol and stearic acid on the performance of chickpea starch-based coatings applied to fresh-cut papaya. J. Food 2019, 17, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Jagannath, J.H.; Nanjappa, C.; das Gupta, D.K.; Bawa, A.S. Mechanical and barrier properties of edible starch-protein-based films. J. Appl. Polym. Sci. 2003, 88, 64–71. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C.; Bifani, V. Natural additives in bioactive edible films and coatings: Functionality and applications in foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Sharoba, A.M.; El Waseif, K.H.; El Mansy, H.A.; El Tanahy, H. Effect of edible coating by chitosan with lemongrass and thyme oils on strawberry quality and shelf life during storage. J. Food Technol. Nutr. Sci. 2017, 3, 1–11. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Kader, A.A.; Ben-Yehoshua, S. Effects of super atmospheric oxygen levels on postharvest physiology and quality of fresh fruits and vegetables. Postharvest Biol. Technol. 2000, 20, 1–13. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Stability, solubility, mechanical and barrier properties of cassava starch-carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll. 2012, 28, 59–67. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Ghazali, F.M. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. Optimization of the arabic gum based edible coating formulations with sodium caseinate and ‘Tulsi’ extract for guava. LWT Food Sci. Technol. 2017, 80, 271–279. [Google Scholar] [CrossRef]
- Chelladurai, S.J.S.; Murugan, K.; Ray, A.P.; Upadhyaya, M.; Narasimharaj, V.; Gnanasekaran, S. Optimization of process parameters using response surface methodology: A review. Mater. Today Proc. 2020, 37, 1301–1304. [Google Scholar] [CrossRef]
- Nandane, A.S.; Dave, R.K.; Rao, T.V.R. Optimization of edible coating formulations for improving postharvest quality and shelf life of pear fruit using response surface methodology. J. Food Sci. Technol. 2017, 54, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. Effect of chitosan and carrageenan-based edible coatings on post-harvested longan (Dimocarpus longan) fruits. CYTA J. Food 2018, 16, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Jadhav, V.T.; Sharma, J. Global scenario of pomegranate (Punica granatum L.) culture with special reference to India. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 7–18. [Google Scholar]
- Elyatem, S.M.; Kader, A.A. Postharvest physiology and storage behaviour of pomegranate fruits. Sci. Hortic. 1983, 24, 287–298. [Google Scholar] [CrossRef]
- Meighani, H.; Ghasemnezhad, M.; Bakhshi, D. Effect of different coatings on postharvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2014, 52, 4507–4514. [Google Scholar] [CrossRef]
- Saba, M.K.; Amini, R. Nano-ZnO/carboxymethyl cellulose-based active coating impact on ready-to-use pomegranate during cold storage. Food Chem. 2017, 232, 721–726. [Google Scholar] [CrossRef]
- Öz, A.T.; Eker, T. Effects of edible coating on minimally processed pomegranate fruits. J. Process Energy Agric. 2017, 4487, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; do Nascimento, P.; Fontes, E.A.F.; Mauro, M.A.; Kimura, M. Edible coatings from native and modified starches retain carotenoids in pumpkin during drying. Food Sci. Technol. 2013, 50, 420–425. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.O. Effects of bruising and storage duration on physiological response and quality attributes of pomegranate fruit. Sci Hortic. 2020, 267, 1–7. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Opara, U.L.; Witthuhn, C.R. Modelling the respiration rates of pomegranate fruit and arils. Postharvest Biol. Technol. 2012, 64, 49–54. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L.; Theron, K.I. Chemical and phytochemical properties and antioxidant activities of three pomegranate cultivars grown in South Africa. Food Bioprocess. Technol. 2012, 5, 2934–2940. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S.; Chern, B.H. Characterization of the influence of main emulsion components on the physicochemical properties of orange beverage emulsion using response surface methodology. Food Hydrocoll. 2009, 23, 271–280. [Google Scholar] [CrossRef]
- Fawole, O.A.; Riva, S.C.; Opara, U.L. Efficacy of edible coatings in alleviating shrivel and maintaining quality of Japanese plum (Prunus salicina Lindl.) during export and shelf life conditions. Agronomy 2020, 10, 1023. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; de Luca Sarantópoulos, C.I.; Hubinger, M.D. Effect of antimicrobial starch edible coating on shelf-life of fresh strawberries. Packag. Technol. Sci. 2012, 25, 413–425. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during postharvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Banks, N.H.; Dadzie, B.K.; Cleland, D.J. Reducing gas exchange of fruits with surface coatings. Postharvest Biol. Technol. 1993, 3, 269–284. [Google Scholar] [CrossRef]
- Barman, K.; Asrey, R.; Pal, R.K. Putrescine and carnauba wax pretreatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci. Hortic. 2011, 130, 795–800. [Google Scholar] [CrossRef]
- Varasteh, F.; Arzani, K.; Barzegar, M.; Zamani, Z. Pomegranate (Punica granatum L.) fruit storability improvement using pre-storage chitosan coating technique. J. Agr. Sci. Tech. 2017, 19, 389–400. [Google Scholar]
- Fagundes, C.; Carciofi, B.A.M.; Monteiro, A.R. Estimate of respiration rate and physicochemical changes of fresh-cut apples stored under different temperatures. Food Sci. Technol. 2013, 33, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Yaman, Ö.; Bayoindirli, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. Food Sci. Technol. 2002, 35, 146–1450. [Google Scholar] [CrossRef]
- Daisy, L.L.; Nduko, J.M.; Joseph, W.M.; Richard, S.M. Effect of edible gum Arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Technol. 2020, 57, 79–85. [Google Scholar] [CrossRef]
- Oluwaseun, A.C.; Arowora, K.A.; Bolajoko, F.O.; Bunmi, A.J.; Olagbaju, A.R. Effect of edible coatings of carboxy methyl cellulose and corn starch on cucumber stored at ambient temperature. Asian J. Agric. Biol. 2013, 1, 133–140. [Google Scholar]
- Maqbool, M.; Ali, A.; Ramachandran, S.; Smith, D.R.; Alderson, P.G. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Prot. 2010, 29, 1136–1341. [Google Scholar] [CrossRef]
- Pigozzi, M.T.; Silva, V.M.; Mendes, F.Q.; de Oliveira, I.R.N.; Moraes, A.R.F.; Lopes, E.A. Post-harvest quality of papaya coated with polivinilic alcohol and maize starch. Ciencia e Agrotecnologia 2020, 45, 1–8. [Google Scholar]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef]
- Rohani, M.; Zaipun, M.; Norhayati, M. Effect of modified atmosphere on the storage life and quality of ‘Eksotika’ papaya. J. Trop. Agric. Food Sci. 1997, 25, 103–114. [Google Scholar]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality control of fresh-cut apples after coating application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Ghasemnezhad, M.; Zareh, S.; Rassa, M.; Sajedi, R.H. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J. Sci. Food Agric. 2013, 93, 368–374. [Google Scholar] [CrossRef]
- Hernández-Guerrero, S.E.; Balois-Morales, R.; Palomino-Hermosillo, Y.A.; López-Guzmán, G.G.; Berumen-Varela, G.; Bau-tista-Rosales, P.U.; Alejo-Santiago, G. Novel edible coating of starch-based stenospermocarpic mango prolongs the shelf life of mango ‘Ataulfo’ fruit. J. Food Qual. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effects of edible coatings on quality maintenance of fresh-cut nectarines. Emir. J. Food Agric. 2016, 28, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Dávila-Aviña, J.E.; Villa-Rodríguez, J.A. Effect of edible coatings on bioactive compounds and antioxidant capacity of tomatoes at different maturity stages. J. Food Sci. Technol. 2014, 51, 2706–2712. [Google Scholar] [CrossRef] [Green Version]
- Hmid, I.; Elothmani, D.; Hanine, H.; Oukabli, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, 2675–2684. [Google Scholar] [CrossRef] [Green Version]


| Point Type | A: Gum Arabic (%) | B: Maize Starch (%) | C: Lemongrass Oil (%) | D: Glycerol (%) |
|---|---|---|---|---|
| Factorial | 0.5 | 0.5 | 2 | 0.5 |
| Factorial | 1.5 | 0.5 | 2 | 0.5 |
| Factorial | 0.5 | 1.5 | 2 | 0.5 |
| Factorial | 1.5 | 1.5 | 2 | 0.5 |
| Factorial | 0.5 | 0.5 | 4 | 0.5 |
| Factorial | 1.5 | 0.5 | 4 | 0.5 |
| Factorial | 0.5 | 1.5 | 4 | 0.5 |
| Factorial | 1.5 | 1.5 | 4 | 0.5 |
| Factorial | 0.5 | 0.5 | 2 | 1.5 |
| Factorial | 1.5 | 0.5 | 2 | 1.5 |
| Factorial | 0.5 | 1.5 | 2 | 1.5 |
| Factorial | 1.5 | 1.5 | 2 | 1.5 |
| Factorial | 0.5 | 0.5 | 4 | 1.5 |
| Factorial | 1.5 | 0.5 | 4 | 1.5 |
| Factorial | 0.5 | 1.5 | 4 | 1.5 |
| Factorial | 1.5 | 1.5 | 4 | 1.5 |
| Axial | 0 | 1 | 3 | 1 |
| Axial | 2 | 1 | 3 | 1 |
| Axial | 1 | 0 | 3 | 1 |
| Axial | 1 | 2 | 3 | 1 |
| Axial | 1 | 1 | 1 | 1 |
| Axial | 1 | 1 | 5 | 1 |
| Axial | 1 | 1 | 3 | 0 |
| Axial | 1 | 1 | 3 | 2 |
| Center | 1 | 1 | 3 | 1 |
| Center | 1 | 1 | 3 | 1 |
| Center | 1 | 1 | 3 | 1 |
| Center | 1 | 1 | 3 | 1 |
| Center | 1 | 1 | 3 | 1 |
| Center | 1 | 1 | 3 | 1 |
| Independent Variable | Levels | ||
|---|---|---|---|
| Low (−1) | Center (0) | High (+1) | |
| A-Gum arabic (% w/v) | 0.5 | 1 | 1.5 |
| B-Maize starch (% w/v) | 0.5 | 1 | 0.5 |
| C-Lemongrass oil (% w/v) | 2 | 3 | 4 |
| D-Glycerol (% w/v) | 0.5 | 1 | 1.5 |
| Regression Coefficients | WL (%) | RR (CO2 mg·kg−1·h−1) | TSS (°Brix) | TA (% CA) | RSA (mMAAE/ mLPJ) | FRAP (mMTE/mL PJ) |
|---|---|---|---|---|---|---|
| A-Gum arabic | −0.6816 * | −3.55 * | −1.15 * | −0.1861 * | −801.18 * | −497.25 * |
| B-Starch | −0.5087 * | −3.81 * | −0.9222 * | −0.1586 * | −631.83 * | −420.19 * |
| C-Lemongrass oil | −0.0215 | - | −0.0722 | −0.0031 | 7.14 | −32.75 |
| D-Glycerol | −0.1431 | - | −0.2556 | −0.0150 | −68.61 | −17.18 |
| AB | −0.4258 * | −2.81 * | −0.2958 | −0.1104 * | −502.59 * | −362.16 * |
| AC | 0.1024 | - | 0.4750 | 0.0521 | 189.43 * | 139.82 * |
| AD | 0.2156 * | - | 0.1792 | 0.0300 | 172.56 * | 76.00 |
| BC | −0.1627 | - | −0.3833 | −0.0325 | −84.29 | −40.83 |
| BD | 0.1407 | - | −0.2292 | −0.0104 | −0.1777 | −18.99 |
| CD | −0.0857 | - | 0.0833 | 0.0012 | −47.85 | 24.31 |
| A2 | −0.3164 * | −2.44 * | −0.3882 * | −0.0754 * | −276.08 * | −206.33 * |
| B2 | −0.3560 * | - | −0.5757 * | −0.0887 * | −340.54 * | −245.99 * |
| C2 | −0.1768 * | - | −0.3632 * | −0.0437 * | −154.82 * | −119.53 * |
| D2 | −0.0225 | - | 0.0243 | −0.0029 | 0.9567 | −22.92 |
| Mean | 5.25 | 32.92 | 14.27 | 1.26 | 5122.93 | 3443.71 |
| R2 | 0.8603 | 0.7011 | 0.7284 | 0.9095 | 0.9490 | 0.9506 |
| Model F value | 20.85 | 14.66 | 12.11 | 37.43 | 35.37 | 50.50 |
| Point Type | A: GA | B: MS | C: LO | D: GC | WL | RR | TSS | TA | RSA | FRAP |
|---|---|---|---|---|---|---|---|---|---|---|
| - | (%) | (%) | (%) | (%) | (%) | (CO2 mg·kg−1·h−1) | (°Brix) | (%) | (mMAAE/mLPJ) | (mMTE/ mLPJ) |
| Factorial | 0.5 | 0.5 | 2 | 0.5 | 6.22 | 37.83 | 16.13 | 1.48 | 61.58 | 40.44 |
| Factorial | 1.5 | 0.5 | 2 | 0.5 | 5.28 | 32.55 | 13.60 | 1.24 | 49.35 | 34.70 |
| Factorial | 0.5 | 1.5 | 2 | 0.5 | 6.36 | 39.81 | 16.60 | 1.54 | 62.19 | 42.14 |
| Factorial | 1.5 | 1.5 | 2 | 0.5 | 3.10 | 18.44 | 12.63 | 0.72 | 27.38 | 19.03 |
| Factorial | 0.5 | 0.5 | 4 | 0.5 | 6.39 | 37.90 | 15.90 | 1.46 | 59.77 | 38.16 |
| Factorial | 1.5 | 0.5 | 4 | 0.5 | 5.62 | 35.22 | 14.90 | 1.38 | 58.12 | 37.59 |
| Factorial | 0.5 | 1.5 | 4 | 0.5 | 5.46 | 35.20 | 14.20 | 1.31 | 57.66 | 35.76 |
| Factorial | 1.5 | 1.5 | 4 | 0.5 | 3.49 | 17.14 | 11.37 | 0.70 | 26.48 | 18.96 |
| Factorial | 0.5 | 0.5 | 2 | 1.5 | 5.22 | 38.24 | 16.30 | 1.52 | 61.61 | 41.33 |
| Factorial | 1.5 | 0.5 | 2 | 1.5 | 5.13 | 32.18 | 13.37 | 1.22 | 50.93 | 34.84 |
| Factorial | 0.5 | 1.5 | 2 | 1.5 | 5.73 | 34.81 | 14.47 | 1.33 | 54.51 | 36.44 |
| Factorial | 1.5 | 1.5 | 2 | 1.5 | 3.98 | 18.90 | 11.20 | 0.76 | 31.02 | 20.45 |
| Factorial | 0.5 | 0.5 | 4 | 1.5 | 5.23 | 36.22 | 14.90 | 1.39 | 55.66 | 36.65 |
| Factorial | 1.5 | 0.5 | 4 | 1.5 | 5.22 | 30.20 | 14.83 | 1.37 | 54.37 | 38.28 |
| Factorial | 0.5 | 1.5 | 4 | 1.5 | 4.94 | 30.20 | 12.77 | 1.17 | 49.34 | 34.70 |
| Factorial | 1.5 | 1.5 | 4 | 1.5 | 3.29 | 20.47 | 11.57 | 0.78 | 32.56 | 21.48 |
| Axial | 0 | 1 | 3 | 1 | 6.22 | 38.57 | 16.17 | 1.51 | 61.71 | 40.83 |
| Axial | 2 | 1 | 3 | 1 | 3.26 | 38.57 | 11.23 | 0.79 | 31.62 | 21.30 |
| Axial | 1 | 0 | 3 | 1 | 5.64 | 34.56 | 14.70 | 1.36 | 54.43 | 36.44 |
| Axial | 1 | 2 | 3 | 1 | 3.51 | 21.54 | 11.20 | 0.83 | 33.74 | 22.53 |
| Axial | 1 | 1 | 1 | 1 | 5.08 | 31.47 | 13.27 | 1.23 | 49.93 | 34.56 |
| Axial | 1 | 1 | 5 | 1 | 5.51 | 33.83 | 14.33 | 1.32 | 53.09 | 34.52 |
| Axial | 1 | 1 | 3 | 0 | 5.97 | 36.23 | 15.40 | 1.46 | 58.73 | 38.78 |
| Axial | 1 | 1 | 3 | 2 | 5.85 | 36.22 | 15.30 | 1.42 | 56.76 | 38.03 |
| Center | 1 | 1 | 3 | 1 | 5.19 | 31.54 | 15.50 | 1.27 | 50.53 | 34.52 |
| Center | 1 | 1 | 3 | 1 | 6.65 | 42.41 | 15.27 | 1.61 | 63.51 | 43.53 |
| Center | 1 | 1 | 3 | 1 | 5.93 | 36.49 | 13.40 | 1.42 | 55.24 | 39.73 |
| Center | 1 | 1 | 3 | 1 | 5.75 | 35.46 | 17.33 | 1.37 | 56.89 | 38.00 |
| Center | 1 | 1 | 3 | 1 | 6.13 | 38.04 | 15.47 | 1.47 | 60.09 | 39.34 |
| Center | 1 | 1 | 3 | 1 | 6.02 | 37.39 | 14.93 | 1.44 | 58.10 | 40.05 |
| Response Variable | Predicted Value a | Experimental Value a |
|---|---|---|
| TSS (°Brix) | 11.88 | 11.37 ± 0.67 |
| TA (% CA) | 0.78 | 0.70 ± 0.06 |
| Weight loss (%) | 3.25 | 3.49 ± 0.27 |
| RSA (mMAAE/mL PJ) | 31.26 | 26.48 ± 1.98 |
| FRAP (mMAAE/mL PJ) | 21.59 | 18.96 ± 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Optimization of Gum Arabic and Starch-Based Edible Coatings with Lemongrass Oil Using Response Surface Methodology for Improving Postharvest Quality of Whole “Wonderful” Pomegranate Fruit. Coatings 2021, 11, 442. https://doi.org/10.3390/coatings11040442
Kawhena TG, Opara UL, Fawole OA. Optimization of Gum Arabic and Starch-Based Edible Coatings with Lemongrass Oil Using Response Surface Methodology for Improving Postharvest Quality of Whole “Wonderful” Pomegranate Fruit. Coatings. 2021; 11(4):442. https://doi.org/10.3390/coatings11040442
Chicago/Turabian StyleKawhena, Tatenda Gift, Umezuruike Linus Opara, and Olaniyi Amos Fawole. 2021. "Optimization of Gum Arabic and Starch-Based Edible Coatings with Lemongrass Oil Using Response Surface Methodology for Improving Postharvest Quality of Whole “Wonderful” Pomegranate Fruit" Coatings 11, no. 4: 442. https://doi.org/10.3390/coatings11040442
APA StyleKawhena, T. G., Opara, U. L., & Fawole, O. A. (2021). Optimization of Gum Arabic and Starch-Based Edible Coatings with Lemongrass Oil Using Response Surface Methodology for Improving Postharvest Quality of Whole “Wonderful” Pomegranate Fruit. Coatings, 11(4), 442. https://doi.org/10.3390/coatings11040442








