Effect of Organic and Conventional Production Methods on Fruit Yield and Nutritional Quality Parameters in Three Traditional Cretan Grape Varieties: Results from a Farm Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Farm Survey Strategy
2.2. Sugar and Dry Matter Content
2.3. Chemical Reagents
2.4. Total Phenolic Content (TPC)
2.5. Total Antioxidant Activity (TAA)
2.6. Total Anthocyanin Content (TAC)
2.7. Identification and Quantification of Individual Anthocyanins by High Performance Liquid Chromatography (HPLC)
2.8. Identification of Individual Anthocyanins by LC–MS
2.9. Statistical Analysis
3. Results
3.1. Yields, Dry Matter and Sugar Content in Grapes
3.2. Antioxidant Activity and Phenolic and Anthocyanin Content in Grapes
3.3. Associations between Variety and Agronomic Drivers and Grape Yield and Composition
4. Discussion
4.1. Effect of Variety and Production System on Grape Yields
4.2. Effect of Year, Variety, and Production System on Table Grape Quality Parameters
4.3. Potential Nutritional Impacts of Contrasting Antioxidant Levels in Grapes
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulero, J.; Pardo, F.; Zafrilla, P. Antioxidant activity and phenolic composition of organic and conventional grapes and wines. J. Food Compost. Anal. 2010, 23, 569–574. [Google Scholar] [CrossRef]
- Vian, M.A.; Tomao, V.; Coulomb, P.O.; Lacombe, J.M.; Dangles, O. Comparison of the anthocyanin composition during ripening of Syrah grapes grown using organic or conventional agricultural practices. J. Agric. Food Chem. 2006, 54, 5230–5235. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: An update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Fruits and fruit products. Food Chem. 2004, 806–861. [Google Scholar] [CrossRef]
- da Silva Haas, I.C.; Toaldo, I.M.; de Gois, J.S.; Borges, D.L.; de Oliveira Petkowicz, C.L.; Bordignon-Luiz, M.T. Phytochemicals, monosaccharides and elemental composition of the non-pomace constituent of organic and conventional grape juices (Vitis labrusca L.): Effect of drying on the bioactive content. Plant Foods Hum. Nutr. 2016, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Margraf, T.; Brotzakis, I.; Capuano, E.; van Ruth, S.M. Characterization of conventional, biodynamic, and organic purple grape juices by chemical markers, antioxidant capacity, and instrumental taste profile. J. Food Sci. 2015, 80, C55–C65. [Google Scholar] [CrossRef]
- Lockshin, L.; Corsi, A.M. Consumer behaviour for wine 2.0: A review since 2003 and future directions. Wine Econ. Policy 2012, 1, 2–23. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E. The on-farm conservation of grapevine (Vitis vinifera L.) landraces assures the habitat diversity in the viticultural agro-ecosystem. Vitis 2015, 54, 265–269. [Google Scholar]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J. Phenolic content and antioxidant activities of white and purple juices manufactured with organically-or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef]
- Laureati, M.; Gaeta, D.; Pagliarini, E. Qualitative and sensory evaluation of Sangiovese red wine obtained from organically and conventionally grown grapes. Ital. J. Food Sci. 2014, 26, 355–362. [Google Scholar]
- Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Seal, C.; Rosa, E.A.S.; Iversen, P.O.; Vigar, V.; Barkla, B.; et al. Effects of Production Region, Production Systems and Grape Type/Variety on Nutritional Quality Parameters of Table Grapes; Results from a UK Retail Survey. Foods 2020, 9, 1874. [Google Scholar] [CrossRef] [PubMed]
- Bois, B.; Zito, S.; Calonnec, A. Climate vs grapevine pests and diseases worldwide: The first results of a global survey. OENO One 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar] [CrossRef]
- Corrales, M.; Fernandez, A.; Pinto, M.G.V.; Butz, P.; Franz, C.M.; Schuele, E.; Tauscher, B. Characterization of phenolic content, in vitro biological activity, and pesticide loads of extracts from white grape skins from organic and conventional cultivars. Food Chem. Toxicol. 2010, 48, 3471–3476. [Google Scholar] [CrossRef]
- Wines of Crete. Varieties. 2019. Available online: www.winesofcrete.gr (accessed on 19 February 2021).
- Cunniff, P. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists (AOAC): Washington DC, USA, 1996. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Tassoni, A.; Tango, N.; Ferri, M. Comparison of biogenic amine and polyphenol profiles of grape berries and wines obtained following conventional, organic and biodynamic agricultural and oenological practices. Food Chem. 2013, 139, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidnat capacity in popular antioxidnat-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Intl. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Identification of (poly)phenolic compounds in concord grape juice and their metabolites in human plasma and urine after juice consumption. J. Agric. Food Chem. 2011, 59, 9512–9522. [Google Scholar] [CrossRef]
- Revilla, I.; Pérez-Magariño, S.; González-SanJosé, M.L.; Beltrán, S. Identification of anthocyanin derivatives in grape skin extracts and red wines by liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 1999, 847, 83–90. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer Science & Business Media: New York, NY, USA, 2006. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 19 February 2021).
- Ter Braak, C.J.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. 1H NMR-based metabonomics for the classification of Greek wines according to variety, region, and vintage. Comparison with HPLC data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef]
- Tassoni, A.; Tango, N.; Ferri, M. Polyphenol and biogenic amine profiles of Albana and Lambrusco grape berries and wines obtained following different agricultural and oenological practices. Food Sci. Nutr. 2014, 5, 8. [Google Scholar] [CrossRef]
- Siminis, H. Vine and Wine Products. Georgia and Ktinotrofia (Crop and Livestock Production) Magazine (10(2012)). 2012. Available online: https://agrotypos.gr/magazine/ (accessed on 19 February 2021).
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; et al. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem. 2018, 66, 10369–10379. [Google Scholar] [CrossRef]
- Bunea, C.I.; Pop, N.; Babeş, A.C.; Matea, C.; Dulf, F.V.; Bunea, A. Carotenoids, total polyphenols and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chem. Cent. J. 2012, 6, 66. [Google Scholar] [CrossRef]
- Ongaratti, B.R.; Machado, F.D.S.; Medeiros, N.D.S.; Destri, C.; da Silva, E.R.; Quincozes-Santos, A.; Dani, C.; Funchal, C. Antioxidant and neuroprotective effect of organic and conventional white grape juices on oxidative stress induced by sodium azide in cerebral cortex of rats. Eur. J. Nutr. Food Saf. 2014, 4, 592–603. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Response of grapevine cv. ‘Tempranillo’to timing and amount of irrigation: Water relations, vine growth, yield and berry and wine composition. Irrig. Sci. 2010, 28, 113. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; dos Santos, M.T.; Fank, B.; Dani, C.; Vanderlinde, R.; Henriques, J.A.; Coitinho, A.S.; Salvador, M. Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats. Nutr. Res. 2013, 33, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L) grape berries during ripening. J. Sci. Food Agric. 2001, 81, 409–420. [Google Scholar] [CrossRef]
- Conradie, W.J.; Carey, V.A.; Bonnardot, V.; Saayman, D.; Schoor, L.H. Effect of different environmental factors on the performance of Sauvignon blanc grapevines in the Stellenbosch/Durbanville districts of South Africa. I. Geology, soil, climate, phenology and grape composition. S. Afr. J. Enol. Vitic. 2002, 23, 78–91. [Google Scholar]
- Margraf, T.; Santos, É.N.T.; de Andrade, E.F.; van Ruth, S.M.; Granato, D. Effects of geographical origin, variety and farming system on the chemical markers and in vitro antioxidant capacity of Brazilian purple grape juices. Food Res. Int. 2016, 82, 145–155. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Cruz, F.A.; de Lima Alves, T.; de Gois, J.S.; Borges, D.L.; Cunha, H.P.; da Silva, E.L.; Bordignon-Luiz, M.T. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: Phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem. 2015, 173, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Hao, F.; Mo, H.; Jiang, J.; Wang, H.; Liu, C.; Fan, X.; Zhang, Y. Phenol profiles and antioxidant properties of white skinned grapes and their coloured genotypes during growth. Biotech. Biotechnol. Equip. 2017, 31, 58–67. [Google Scholar] [CrossRef]
- Arapitsas, P.; Oliveira, J.; Mattivi, F. Do white grapes really exist? Food Res. Int. 2015, 69, 21–25. [Google Scholar] [CrossRef]
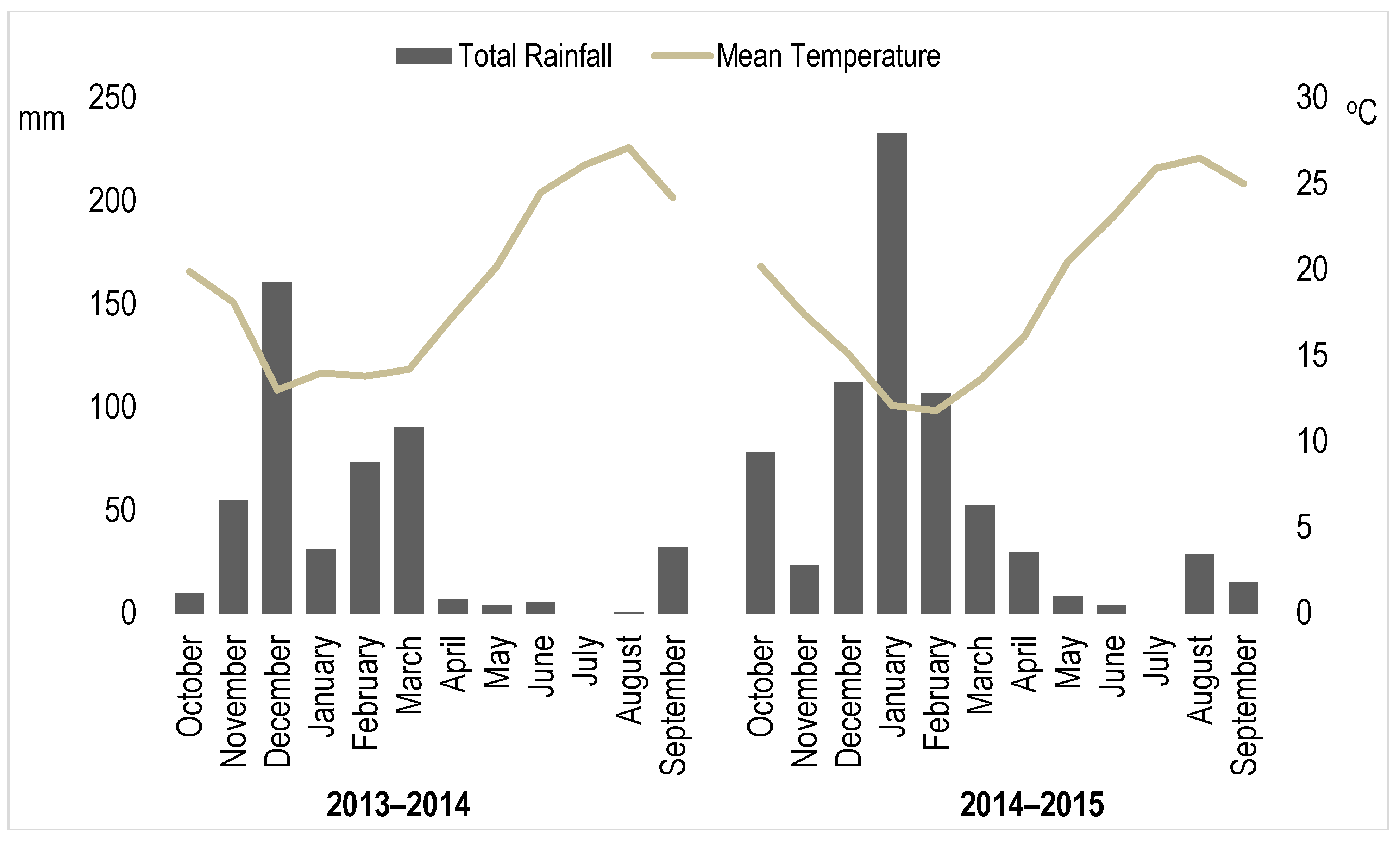
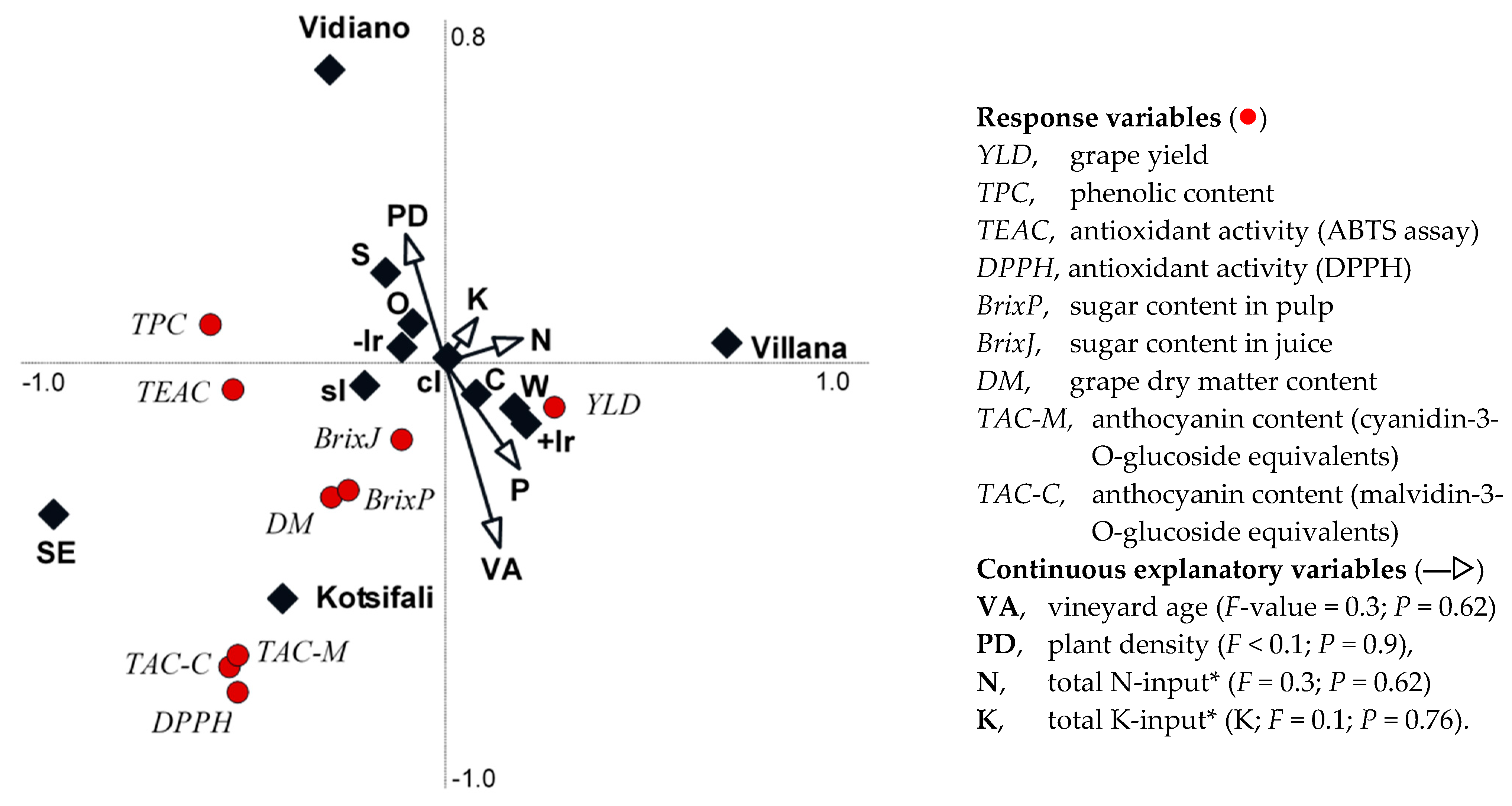
| Factors | Yield (t/ha) | DM (%) | SC Pulp (°Brix) | SC Juice (°Brix) | TPC (mg GAE·kg−1) | TAA (DPPH) (µmol TE·g−1) | TAA (TEAC) (µmol TE·g−1) | TAC (mg cyan·kg−1) | TAC (mg mal·kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Year (Yr) | |||||||||
| 2014 (n = 22) | 14.6 ± 1.1 | 23.0 ± 0.6 | 21.5 ± 0.7 | 21.5 ± 0.5 | 2037 ± 150 | 84 ± 6 | 14.3 ± 1.1 | 432 ± 47 | 456 ± 50 |
| 2015 (n = 26) | 14.2 ± 1.1 | 21.3 ± 0.5 | 18.5 ± 0.4 | 19.1 ± 0.5 | 1423 ± 94 | 75 ± 5 | 7.3 ± 0.6 | 296 ± 23 | 313 ± 25 |
| Production System (PS) | |||||||||
| ORG (n = 24) | 14.0 ± 1.3 | 21.6 ± 0.6 | 19.2 ± 0.5 | 19.7 ± 0.6 | 1770 ± 139 | 79 ± 6 | 11.3 ± 1.2 | 341 ± 48 | 360 ± 51 |
| CONV (n = 24) | 14.8 ± 0.9 | 22.5 ± 0.6 | 20.5 ± 0.7 | 20.7 ± 0.5 | 1638 ± 133 | 79 ± 6 | 9.7 ± 1.0 | 372 ± 34 | 392 ± 36 |
| Variety (Va) | |||||||||
| Kotsifali (n = 18) | 14.6 ± 1.3 | 23.5 ± 0.5 | 20.7 ± 0.6 | 21.6 ± 0.6 | 1906 ± 131 a | 114 ± 2 a | 12.9 ± 1.2 a | 356 ± 29 a | 376 ± 31 a |
| Villana (n = 16) | 15.7 ± 7.0 | 21.1 ± 0.7 | 19.3 ± 0.8 | 19.2 ± 0.7 | 1222 ± 113 b | 57 ± 1 b | 6.7 ± 0.8 b | 9 ± 2 b | 10 ± 2 b |
| Vidiano (n = 14) | 12.5 ± 1.0 | 21.3 ± 0.6 | 19.4 ± 0.8 | 19.6 ± 0.6 | 1996 ± 192 a | 59 ± 1 b | 11.7 ± 1.6 a | 21 ± 4 b | 23 ± 4 b |
| ANOVA results | |||||||||
| (p-values) | |||||||||
| Main effects | |||||||||
| Yr | NS | 0.0201 | 0.0028 | 0.0078 | 0.0022 | <0.0001 | <0.0001 | T | T |
| PS | NS | NS | T | NS | NS | NS | 0.0456 | NS | NS |
| Va | NS | T | NS | T | 0.0014 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Interactions | |||||||||
| Yr × PS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Yr × Va | NS | NS | NS | NS | NS | 0.00672 | NS | 0.02752 | 0.02752 |
| PS × Va | NS | NS | NS | NS | NS | NS | 0.01601 | NS | NS |
| Yr × PS × Va | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Factor 1 | Factor 2 | ||
|---|---|---|---|
| Farming System | |||
| Parameter | Variety | Organic | Conventional |
| Antioxidant activity (TEAC) µmol TE·g−1 | Kotsifali | 12.6 ± 1.7 a A | 13.1 ± 1.7 a A |
| Villana | 7.1 ± 1.1 b B | 6.4 ± 1.2 b B | |
| Vidiano | 14.3 ± 2.4 a A | 9.1 ± 1.6 b B | |
| Parameter Assessed. | Production System | Country | |||
|---|---|---|---|---|---|
| Grape Type Grape Variety | Organic | Conventional | ANOVA Results | or Region (Study Type) | Reference |
| TAATEAC (mM TE·L−1) | |||||
| Villana | 7 ± 1.1 | 6 ± 1.2 | NS | GR (FS) | this study |
| Vidiano | 14 ± 2.4 | 9 ± 1.6 | * | GR (FS) | this study |
| Early Sweet | 5 ± 1.0 | 8 ± 1.4 | NS | SA (RS) | [15] 1 |
| Prime | 6 ± 0.3 | 5 ± 0.5 | NS | SA (RS) | [15] 1 |
| Sugarlone | 7 ± 1.3 | 4 ± 0.3 | NS | SA (RS) | [15] 1 |
| Thompson | 6 ± 1.1 | 5 ± 0.6 | NS | SA (RS) | [15] 1 |
| TAADPPH (mM TE·L−1) | |||||
| Villana | 59 ± 1.7 | 58 ± 1.4 | NS | GR (FS) | this study |
| Vidiano | 57 ± 1.4 | 57 ± 1.4 | NS | GR (FS) | this study |
| Early Sweet | 54 ± 2.7 | 42 ± 0.4 | NS | SA (RS) | [15] 1 |
| Prime | 53 ± 2.6 | 57 ± 2.0 | NS | SA (RS) | [15] 1 |
| Sugarlone | 61 ± 1.6 | 60 ± 1.2 | NS | SA (RS) | [15] 1 |
| Thompson | 61 ± 1.7 | 56 ± 1.8 | NS | SA (RS) | [15] 1 |
| Pignoletto | 7 ± 0.8 | 8 ± 0.3 | NS | IT (FS) | [22] |
| Albana | 6 ± 0.1 | 7 ± 0.4 | * | IT (FS) | [34] |
| Muscat Ottonel | 16 ± 0.4 | 14 ± 0.2 | NS | RO (FS) | [37] |
| Aromat de Iaşi | 3 ± 0.2 | 2 ± 0.6 | * | RO (FS) | [37] |
| Traminer roz | 7 ± 0.3 | 5 ± 0.2 | * | RO (FS) | [37] |
| Riesling italian | 9 ± 0.7 | 8 ± 0.4 | * | RO (FS) | [37] |
| Feteasca regală | 10 ± 0.2 | 12 ± 0.9 | * | RO (FS) | [37] |
| Timpuriu de Cluj | 12 ± 1.1 | 16 ± 1.9 | * | RO (FS) | [37] |
| TPC (mg GAE·kg−1) | |||||
| Villana | 1201 ± 146 | 243 ± 182 | NS | GR (FS) | this study |
| Vidiano | 2243 ± 250 | 1748 ± 194 | NS | GR (FS) | this study |
| Early Sweet | 1180 ± 19 | 1328 ± 142 | NS | SA (RS) | [15] 1 |
| Prime | 1088 ± 72 | 1388 ± 35 | * | SA (RS) | [15] 1 |
| Sugarlone | 1845 ± 87 | 1556 ± 109 | * | SA (RS) | [15] 1 |
| Thompson | 943 ± 113 | 861 ± 49 | NS | SA (RS) | [15] 1 |
| Muscat Ottonel | 631 ± 21 | 41 ± 32 | * | RO (FS) | [37] |
| Aromat de Iaşi | 220 ± 14 | 228 ± 6 | NS | RO (FS) | [37] |
| Traminer roz | 219 ± 4 | 330 ± 2 | NS | RO (FS) | [37] |
| Riesling italian | 423 ± 10 | 436 ± 11 | NS | RO (FS) | [37] |
| Feteasca regală | 579 ± 11 | 575 ± 9 | NS | RO (FS) | [37] |
| Timpuriu de Cluj | 331 ± 4 | 380 ± 23 | NS | RO (FS) | [37] |
| Niagara | 22 ± 1 | 7 ± 1 | * | BR (RS) | [38] |
| Niagara | 524 ± 7 | 339 ± 7 | * | BR (RS) | [13] |
| Parameter Assessed | Production System | Country | |||
|---|---|---|---|---|---|
| Grape Type Grape Variety | Organic | Conventional | ANOVA Results | or Region (Study Type) | Reference |
| TAATEAC (mM TE·L−1) | |||||
| Kotsifali | 13 ± 1.7 | 13 ± 1.7 | NS | GR (FS) | this study |
| Allison | 5 ± 0.4 | 4 ± 0.1 | NS | SA (RS) | [15] 1 |
| Crimson | 9 ± 1.6 | 5 ± 0.6 | NS | SA (RS) | [15] 1 |
| Flame | 3 ± 0.2 | 2 ± 0.3 | NS | SA (RS) | [15] 1 |
| Sweet Celebration | 12 ± 2.0 | 15 ± 0.9 | NS | SA (RS) | [15] 1 |
| Allison | 11 ± 0.1 | 7 ± 2.6 | NS | MED (RS) | [15] 2 |
| Crimson | 9 ± 1.3 | 9 ± 0.9 | NS | MED (RS) | [15] 1 |
| Flame | 14 ± 3.4 | 16 ± 4.9 | NS | MED (RS) | [15] 2 |
| Scarlotta | 6 ± 0.8 | 5 ± 1.0 | NS | MED (RS) | [15] 1 |
| Autumn Royal | 16 ± 3.2 | 13 ± 1.5 | NS | MED (RS) | [15] 1 |
| Midnight Beauty | 30 ± 3.9 | 17 ± 2.6 | * | MED (RS) | [15] 1 |
| Allison | 7 ± 0.7 | 6 ± 0.4 | NS | MED (RS) | [15] 2 |
| Crimson | 5 ± 0.8 | 6 ± 0.6 | NS | MED (RS) | [15] 2 |
| Bordo + Isabel | 52 ± 0.3 | 31 ± 0.2 | * | BR (RS) | [39] |
| Bordo | 131 ± 1.7 | 131 ± 1.7 | NS | BR (FS) | [7] |
| TAADPPH (mM TE·L−1) | |||||
| Kotsifali | 113 ± 2.7 | 115 ± 2.7 | NS | GR (FS) | this study |
| Sangioves | 25 ± 0.6 | 31 ± 3.2 | * | IT (FS) | [22] |
| Allison | 100 ± 3.4 | 102 ± 0.1 | NS | SA (RS) | [15] 1 |
| Crimson | 97 ± 2.0 | 96 ± 1.5 | NS | SA (RS) | [15] 1 |
| Flame | 51 ± 0.6 | 52 ± 2.4 | NS | SA (RS) | [15] 1 |
| Sweet Celebration | 109 ± 6.4 | 108 ± 1.3 | NS | SA (RS) | [15] 1 |
| Allison | 97 ± 3.5 | 102 ± 12.0 | NS | MED (RS) | [15] 2 |
| Crimson | 103 ± 4.9 | 106 ± 6.4 | NS | MED (RS) | [15] 1 |
| Flame | 157 ± 19.5 | 144 ± 28.3 | NS | MED (RS) | [15] 2 |
| Scarlotta | 98 ± 4.4 | 90 ± 8.1 | NS | MED (RS) | [15] 1 |
| Autumn Royal | 114 ± 8.3 | 105 ± 7.4 | NS | MED (RS) | [15] 1 |
| Midnight Beauty | 160 ± 12.9 | 123 ± 14.2 | * | MED (RS) | [15] 1 |
| Allison | 144 ± 2.7 | 142 ± 3.3 | NS | MED (RS) | [15] 2 |
| Crimson | 141 ± 4.2 | 140 ± 1.6 | NS | MED (RS) | [15] 2 |
| Lambrusco | 26 ± 1.0 | 20 ± 1.0 | * | IT (FS) | [22] |
| Bordo + Isabel | 54 ± 0.2 | 41 ± 0.7 | * | BR (RS) | [39] |
| Bordo | 77 ± 3.4 | 102 ± 1.7 | NS | BR (FS) | [7] |
| Bordo | 146 ± 1 | 126 ± 2 | * | BR (RS) | [40] |
| Napoca | 32 ± 1.4 | 25 ± 1.3 | NS | RO (FS) | [37] |
| Muscat Hamburg | 23 ± 0.1 | 23 ± 0.5 | NS | RO (FS) | [37] |
| Parameter Assessed | Production System | Country | |||
|---|---|---|---|---|---|
| Grape Type Grape Variety | Organic | Conventional | ANOVA Results | or Region (Study Type) | Reference |
| TPC (mg GAE·kg−1) | |||||
| Kotsifali | 1938 ± 187 | 1903 ± 94 | NS | GR (FS) | this study |
| Allison | 1838 ± 83 | 1866 ± 87 | NS | SA (RS) | [15] 1 |
| Crimson | 1416 ± 101 | 1296 ± 47 | NS | SA (RS) | [15] 1 |
| Flame | 2083 ± 299 | 1784 ± 243 | NS | SA (RS) | [15] 1 |
| Sweet Celebration | 1824 ± 104 | 1804 ± 219 | NS | SA (RS) | [15] 1 |
| Allison | 1768 ± 676 | 2058 ± 29 | NS | MED (RS) | [15] 1 |
| Crimson | 2012 ± 113 | 1876 ± 109 | NS | MED (RS) | [15] 1 |
| Flame | 2769 ± 462 | 2511 ± 347 | NS | MED (RS) | [15]2 |
| Scarlotta | 2159 ± 292 | 1494 ± 419 | * | MED (RS) | [15] 1 |
| Autumn Royal | 2213 ± 559 | 1925 ± 535 | NS | MED (RS) | [15] 1 |
| Midnight Beauty | 3173 ± 261 | 2435 ± 108 | NS | MED (RS) | [15] 1 |
| Allison | 2154 ± 230 | 1914 ± 41 | NS | MED (RS) | [15] 2 |
| Crimson | 1942 ± 188 | 2356 ± 178 | NS | MED (RS) | [15] 2 |
| Bord±sabel | 3378 ± 50 | 2015 ± 22 | * | BR (RS) | [44] |
| Bordo | 2724 ± 56 | 3636 ± 72 | * | BR (FS) | [7] |
| Bordo | 3346 ± 17 | 1985 ± 56 | * | BR (RS) | [13] |
| Bordo | 146 ± 1 | 126 ± 2 | * | BR (RS) | [40] |
| Napoca | 1341 ± 21 | 1231 ± 21 | NS | RO (FS) | [37] |
| Muscat Hamburg | 978 ± 13 | 953 ± 10 | NS | RO (FS) | [37] |
| TAC (mg cyan·L−1) | |||||
| Kotsifali | 341 ± 48 | 372 ± 34 | NS | GR (FS) | this study |
| Allison | 109 ± 35 | 174 ± 15 | NS | SA (RS) | [15] 1 |
| Crimson | 72 ± 12 | 131 ± 16 | NS | SA (RS) | [15] 1 |
| Flame | 128 ± 28 | 75 ± 16 | NS | SA (RS) | [15] 1 |
| Sweet Celebration | 97 ± 13 | 94 ± 8 | NS | SA (RS) | [15] 1 |
| Allison | 49 ± 5 | 118 ± 58 | NS | MED (RS) | [15] 1 |
| Crimson | 67 ± 12 | 91 ± 16 | NS | MED (RS) | [15] 1 |
| Flame | 93 ± 14 | 77 ± 11 | NS | MED (RS) | [15] 2 |
| Scarlotta | 43 ± 10 | 196 ± 139 | * | MED (RS) | [15] 1 |
| Autumn Royal | 177 ± 36 | 97 ± 99 | NS | MED (RS) | [15] 1 |
| Midnight Beauty | 851 ± 110 | 499 ± 64 | * | MED (RS) | [15] 1 |
| Allison | 74 ± 21 | 208 ± 67 | NS | MED (RS) | [15] 2 |
| Crimson | 81 ± 4 | 161 ± 58 | NS | MED (RS) | [15] 2 |
| Bordo | 341 ± 1 | 255 ± 1 | * | BR (RS) | [40] |
| Monastrell | 721 ± 35 | 518 ± 26 | * | SP (FS) | [1] |
| Syrah | 897 | 1277 | NS | FR (EX) | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Pakos, P.; Seal, C.; Rosa, E.A.S.; Markellou, E.; Iversen, P.O.; et al. Effect of Organic and Conventional Production Methods on Fruit Yield and Nutritional Quality Parameters in Three Traditional Cretan Grape Varieties: Results from a Farm Survey. Foods 2021, 10, 476. https://doi.org/10.3390/foods10020476
Hasanaliyeva G, Chatzidimitrou E, Wang J, Baranski M, Volakakis N, Pakos P, Seal C, Rosa EAS, Markellou E, Iversen PO, et al. Effect of Organic and Conventional Production Methods on Fruit Yield and Nutritional Quality Parameters in Three Traditional Cretan Grape Varieties: Results from a Farm Survey. Foods. 2021; 10(2):476. https://doi.org/10.3390/foods10020476
Chicago/Turabian StyleHasanaliyeva, Gultakin, Eleni Chatzidimitrou, Juan Wang, Marcin Baranski, Nikolaos Volakakis, Panagiotis Pakos, Chris Seal, Eduardo A. S. Rosa, Emilia Markellou, Per Ole Iversen, and et al. 2021. "Effect of Organic and Conventional Production Methods on Fruit Yield and Nutritional Quality Parameters in Three Traditional Cretan Grape Varieties: Results from a Farm Survey" Foods 10, no. 2: 476. https://doi.org/10.3390/foods10020476
APA StyleHasanaliyeva, G., Chatzidimitrou, E., Wang, J., Baranski, M., Volakakis, N., Pakos, P., Seal, C., Rosa, E. A. S., Markellou, E., Iversen, P. O., Vigar, V., Willson, A., Barkla, B., Leifert, C., & Rempelos, L. (2021). Effect of Organic and Conventional Production Methods on Fruit Yield and Nutritional Quality Parameters in Three Traditional Cretan Grape Varieties: Results from a Farm Survey. Foods, 10(2), 476. https://doi.org/10.3390/foods10020476








