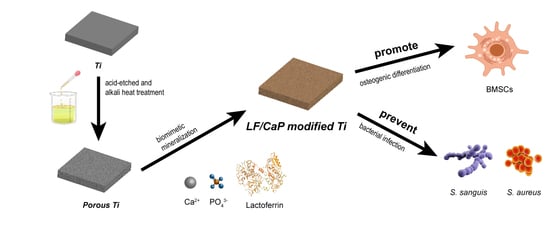
Lactoferrin/Calcium Phosphate-Modified Porous Ti by Biomimetic Mineralization: Effective Infection Prevention and Excellent Osteoinduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation and Characterization
2.1.1. Preparation of Alkali- and Heat-Treated Porous Ti
2.1.2. Pre-Calcification and LF/CaP Fabrication
2.1.3. Characterization of Samples
2.1.4. In Vitro Release Kinetics of LF
2.2. Antibacterial Evaluation
2.2.1. Bacterial Strains and Culture Conditions
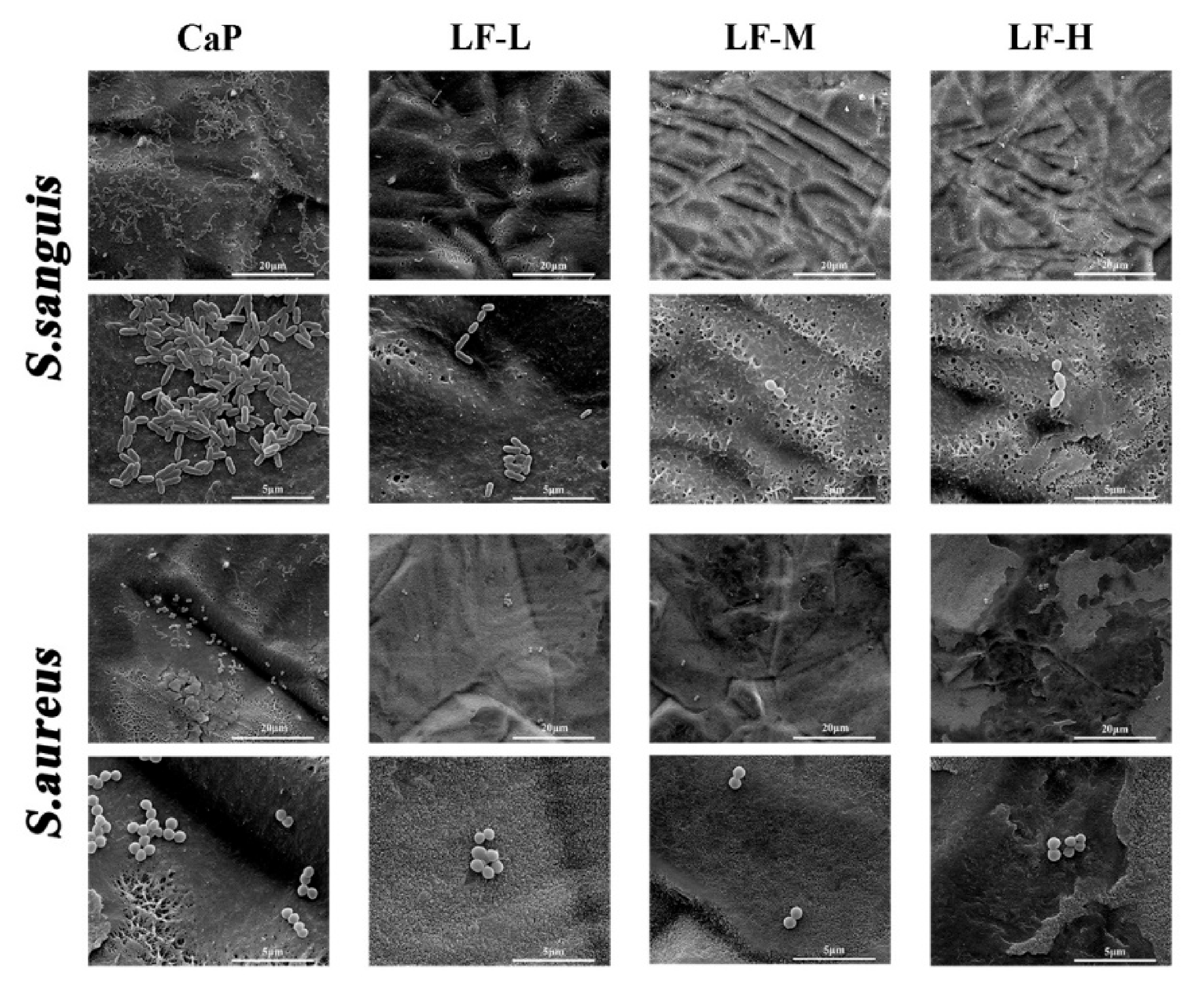
2.2.2. Bacterial Adhesion
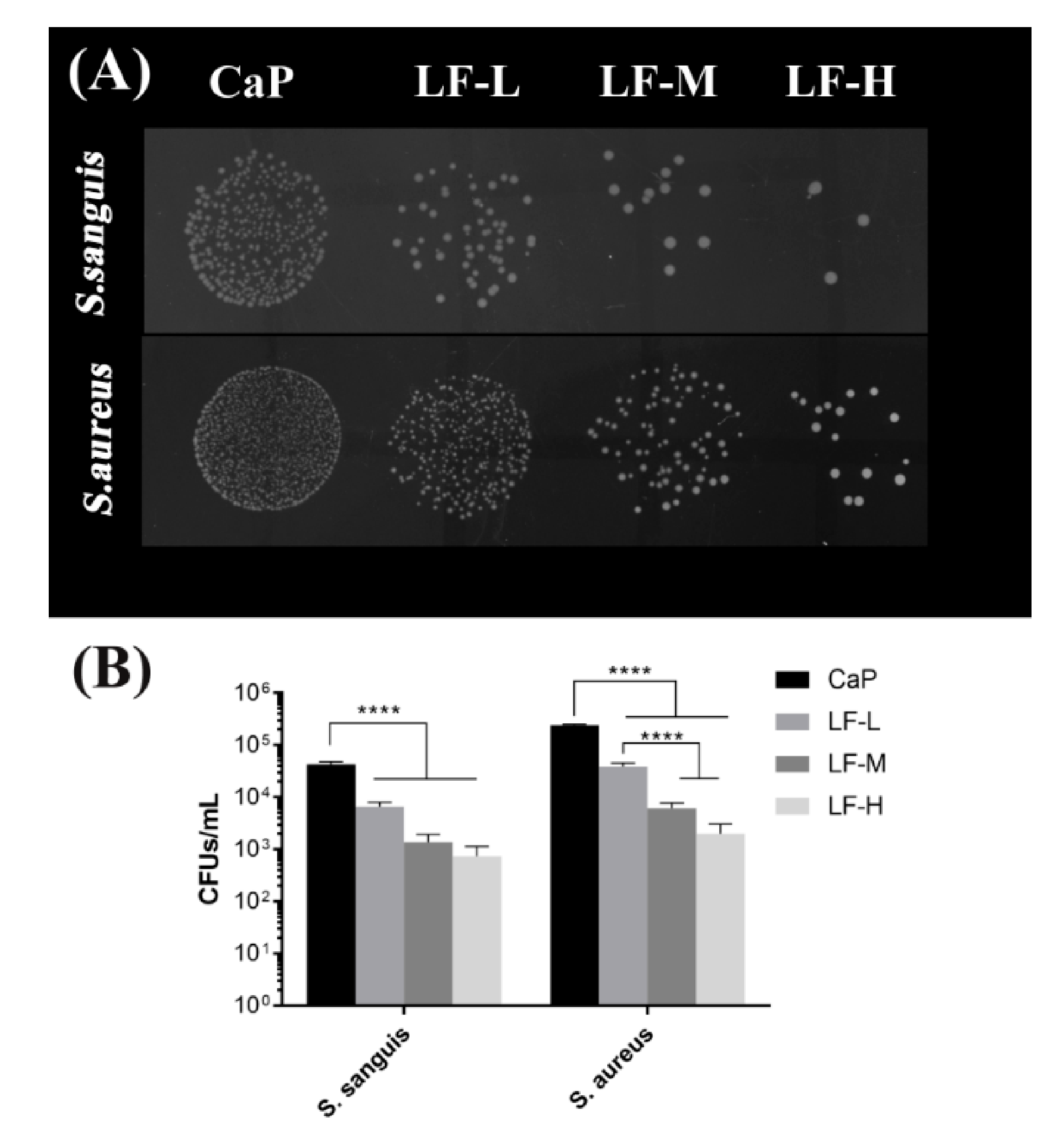
2.2.3. Bacterial Proliferation
2.3. In Vitro Cellular Assessment
2.3.1. Cell Culture
2.3.2. Cell Adhesion
2.3.3. Alkaline Phosphatase Activity
2.3.4. Extracellular Matrix Mineralization
2.3.5. Cell Toxicity
2.4. Statistical Analysis
3. Results
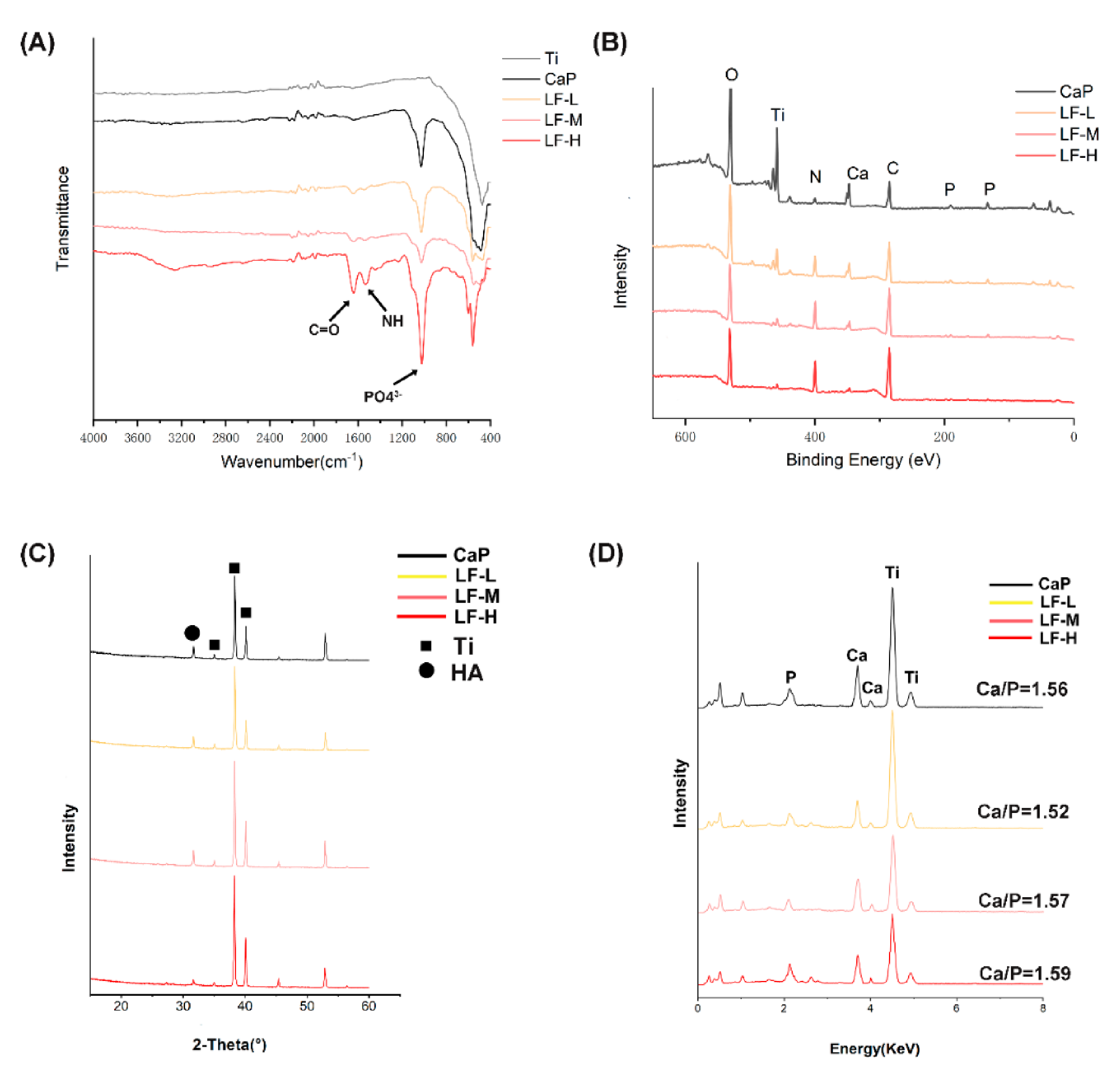
3.1. Surface Characterization and LF Release Profile
3.1.1. Surface Characterization
3.1.2. In Vitro Release Kinetics of LF
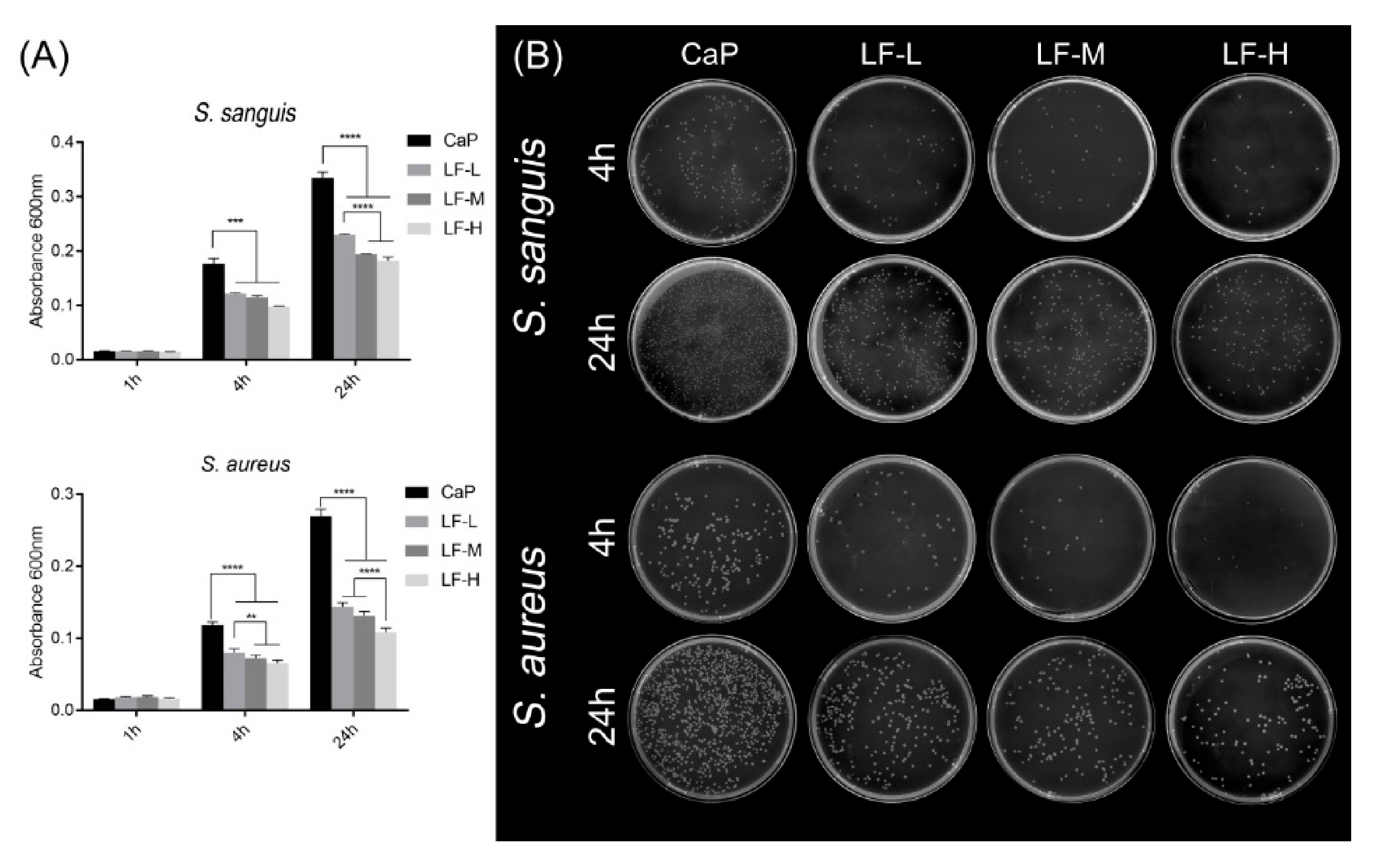
3.2. Antibacterial Abilities
3.3. In Vitro Cell Behavior
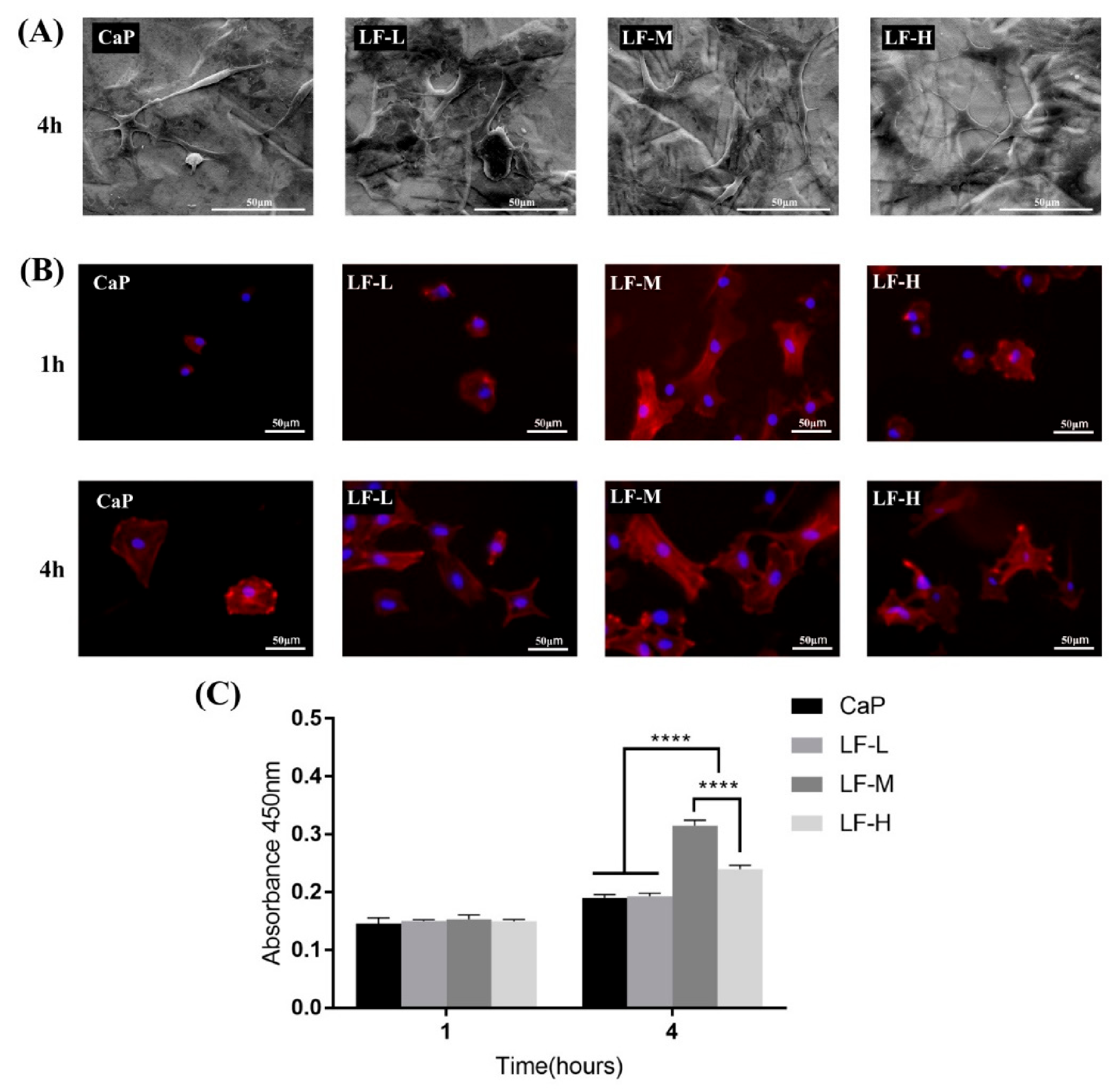
3.3.1. Cell Adhesion
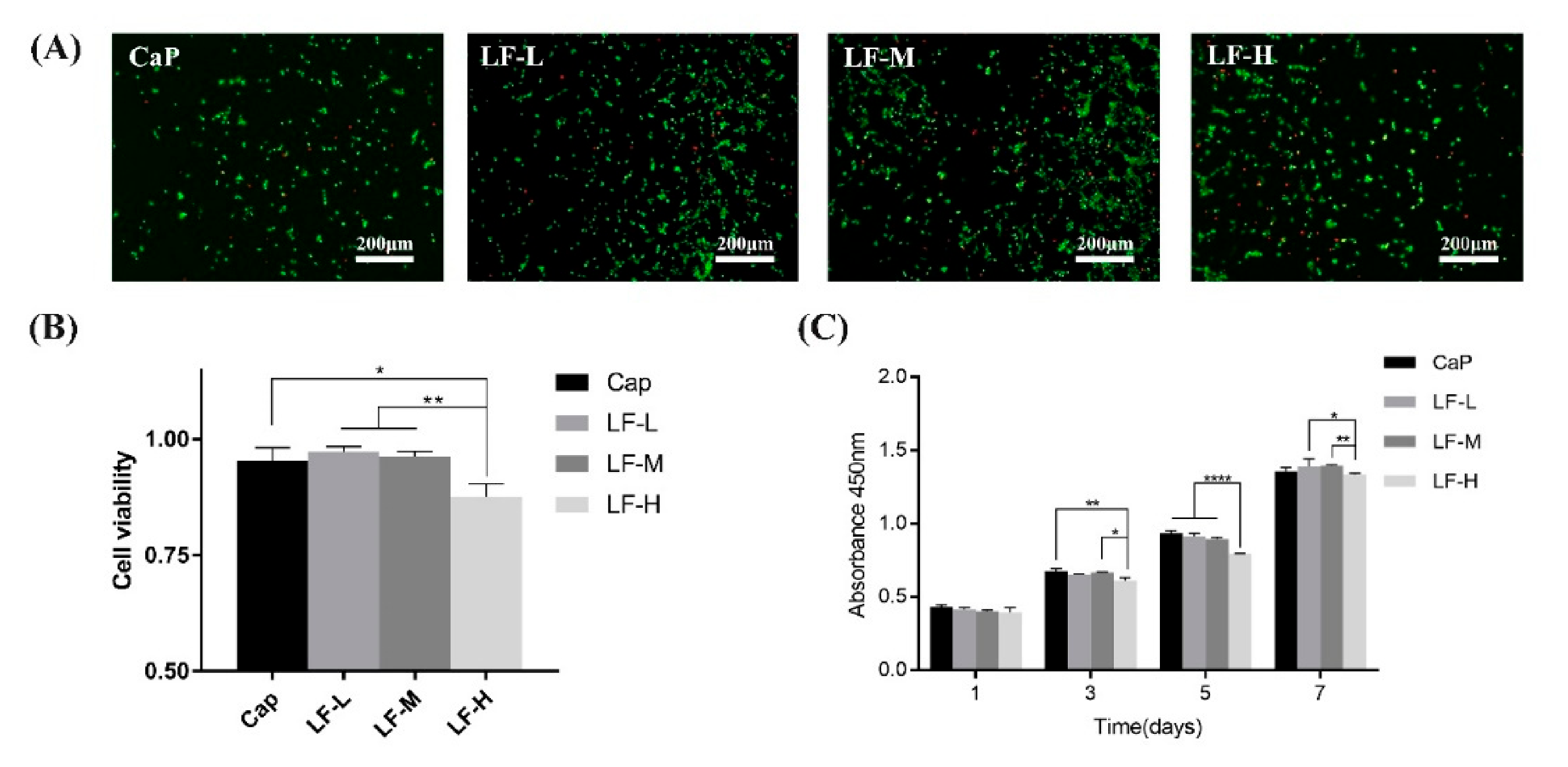
3.3.2. Osteogenic-Related Assay
3.3.3. Cell Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, N. Oral Implants: The Paradigm Shift in Restorative Dentistry. J. Dent. Res. 2019, 98, 1287–1293. [Google Scholar] [CrossRef]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K.X. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed. Res. Int. 2016, 2016, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Schissler, A.; Chantrjaroen, W.; Beecher, C.; Razzak, A.; Urak, R.; Alas, S. Corrosion behaviour and biocompatibility of boron containing titanium alloys in simulated physiological environments. Corros. Eng. Sci. Technol. 2012, 47, 383–387. [Google Scholar] [CrossRef]
- Han, H.-J.; Kim, S.; Han, D.-H. Multifactorial evaluation of implant failure: A 19-year retrospective study. Int. J. Oral. Maxillofac. Implant. 2014, 29, 303–310. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T.; Linder, E.; Lang, N.P.; Lindhe, J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin. Oral. Implant. Res. 2004, 15, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Bortolin, M.; De Vecchi, E.; Agrappi, S.; Weinstein, R.L.; Mattina, R.; Francetti, L. Antibiofilm activity of sandblasted and laser-modified titanium against microorganisms isolated from peri-implantitis lesions. J. Chemother. 2016, 28, 383–389. [Google Scholar] [CrossRef]
- Wang, X.; Yan, L.; Ye, T.; Cheng, R.; Tian, J.; Ma, C.; Wang, Y.; Cui, W. Osteogenic and antiseptic nanocoating by in situ chitosan regulated electrochemical deposition for promoting osseointegration. Mater. Sci. Eng. C 2019, 102, 415–426. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 1–22. [Google Scholar] [CrossRef]
- Xie, X.; Mao, C.; Liu, X.; Zhang, Y.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Chu, P.K.; Wu, S. Synergistic Bacteria Killing through Photodynamic and Physical Actions of Graphene Oxide/Ag/Collagen Coating. ACS Appl. Mater. Interfaces 2017, 9, 26417–26428. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Changes in Lactoferrin and Lysozyme Levels in Human Milk During the First Twelve Weeks of Lactation. Bioact. Compon. Hum. Milk 2001, 241–247. [Google Scholar]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nat. Cell Biol. 2002, 417, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Okamoto, K.; Haing, S.; Nguyen, P.T.; Imanaka, H.; Takata, T. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2018, 507, 142–147. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. Physico-chemical properties of different forms of bovine lactoferrin. Food Chem. 2013, 141, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z. Formation of lactoferrin/sodium caseinate complexes and their adsorption behaviour at the air/water interface. Food Chem. 2017, 232, 697–703. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Drying and denaturation characteristics of three forms of bovine lactoferrin. Dry. Technol. 2016, 35, 606–615. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, H.; Li, H.; Zhang, T.; Zhou, Q.; Liu, N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J. Dairy Sci. 2010, 93, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Legostaeva, E.V.; Kulyashova, K.S.; Komarova, E.G.; Epple, M.; Sharkeev, Y.P.; Khlusov, I.A. Physical, chemical and biological properties of micro-arc deposited calcium phosphate coatings on titanium and zirconium-niobium alloy. Mater. Werkst. 2013, 44, 188–197. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2014, 103, 224–234. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Coupling Hydroxyapatite Nanocrystals with Lactoferrin as a Promising Strategy to Fine Regulate Bone Homeostasis. PLoS ONE 2015, 10, e0132633. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Lai, B.F.; Ding, C.; Kizhakkedathu, J.N.; Hancock, R.E.; Wang, R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 5969–5977. [Google Scholar] [CrossRef]
- Escada, A.L.A.; Machado, J.P.B.; Schneider, S.G.; Rezende, M.C.R.A.; Claro, A.P.R.A. Biomimetic calcium phosphate coating on Ti-7.5Mo alloy for dental application. J. Mater. Sci. Mater. Electron. 2011, 22, 2457–2465. [Google Scholar] [CrossRef]
- Liang, F.; Zhou, L.; Wang, K. Apatite formation on porous titanium by alkali and heat-treatment. Surf. Coatings Technol. 2003, 165, 133–139. [Google Scholar] [CrossRef]
- Yuan, Z.; Tao, B.; He, Y.; Liu, J.; Lin, C.; Shen, X.; Ding, Y.; Yu, Y.; Mu, C.; Liu, P.; et al. Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials 2019, 217, 119290. [Google Scholar] [CrossRef]
- Wang, G.; Weng, D.; Chen, C.; Chen, L.; Wang, J. Influence of TiO2 nanostructure size and surface modification on surface wettability and bacterial adhesion. Colloid Interface Sci. Commun. 2020, 34, 100220. [Google Scholar] [CrossRef]
- Li, X.; Gan, J.; Cao, P.; Guo, W.; Wang, R.; Song, P.; He, Y. Preparation of blackberry-shape cationic copolymer particles for highly effective antibacterial coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126202. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2017, 59, 580–596. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Lin, J.; Hodgson, P.D.; Wen, C. Effect of heat-treatment atmosphere on the bond strength of apatite layer on Ti substrate. Dent. Mater. 2008, 24, 1549–1555. [Google Scholar] [CrossRef]
- Li, Y.H.; Sun, Z.Q.; Chen, R.B.; Fang, W.; Deng, Z.Y. Deposition of hydroxyapatite coating on biocompatible porous titanium by biomimetic method. Optoelectron. Adv. Mater. Rapid Commun. 2013, 7, 541–544. [Google Scholar]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding Adsorption-Desorption Dynamics of BMP-2 on Hydroxyapatite (001) Surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef]
- Xu, A.-W.; Ma, Y.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2006, 17, 415–449. [Google Scholar] [CrossRef]
- Ribeiro, A.; Balestra, R.; Rocha, M.; Peripolli, S.; Andrade, M.; Pereira, L.; Oliveira, M. Dense and porous titanium substrates with a biomimetic calcium phosphate coating. Appl. Surf. Sci. 2013, 265, 250–256. [Google Scholar] [CrossRef]
- Jager, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Di Foggia, M.; Bonora, S.; Prat, M.; Roveri, N. Adsorption and spectroscopic characterization of lactoferrin on hydroxyapatite nanocrystals. Dalton. Trans. 2010, 40, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Robinson, C.; Söderling, E.; Vallittu, P. Early plaque formation on fibre-reinforced composites in vivo. Clin. Oral Investig. 2005, 9, 154–160. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, T.; Gan, Y.; Yang, J.; Zhu, H.; Wang, A.; Wang, Y.; Xi, W. Effect of micropore/microsphere topography and a silicon-incorporating modified titanium plate surface on the adhesion and osteogenic differentiation of BMSCs. Artif. Cells, Nanomed. Biotechnol. 2019, 48, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, X.G.; Chen, Y. Graphene accelerates osteoblast attachment and biomineralization. Carbon Lett. 2017, 22, 42–47. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; He, Y.; Zhong, L.; Xie, W.; Xue, Y.; Wang, J. Lactoferrin/Calcium Phosphate-Modified Porous Ti by Biomimetic Mineralization: Effective Infection Prevention and Excellent Osteoinduction. Materials 2021, 14, 992. https://doi.org/10.3390/ma14040992
Chen S, He Y, Zhong L, Xie W, Xue Y, Wang J. Lactoferrin/Calcium Phosphate-Modified Porous Ti by Biomimetic Mineralization: Effective Infection Prevention and Excellent Osteoinduction. Materials. 2021; 14(4):992. https://doi.org/10.3390/ma14040992
Chicago/Turabian StyleChen, Song, Yuanli He, Linna Zhong, Wenjia Xie, Yiyuan Xue, and Jian Wang. 2021. "Lactoferrin/Calcium Phosphate-Modified Porous Ti by Biomimetic Mineralization: Effective Infection Prevention and Excellent Osteoinduction" Materials 14, no. 4: 992. https://doi.org/10.3390/ma14040992
APA StyleChen, S., He, Y., Zhong, L., Xie, W., Xue, Y., & Wang, J. (2021). Lactoferrin/Calcium Phosphate-Modified Porous Ti by Biomimetic Mineralization: Effective Infection Prevention and Excellent Osteoinduction. Materials, 14(4), 992. https://doi.org/10.3390/ma14040992